Abstract
Rationale:
Recent studies have used diffusion tensor tractography (DTT) to demonstrate that central poststroke pain (CPSP) was related to spinothalamic tract (STT) injury in patients with stroke. However, few studies have been reported about delayed-onset CPSP due to degeneration of the STT following a stroke.
Patient's concerns:
A 57-year-old female patient presented with right hemiparesis after stroke. Two weeks after onset, she did not report any pain. At approximately 6 months after onset, she reported pain in the right arm and leg, and the pain slowly intensified with the passage of time. At 14 months after onset, the characteristics and severity of her pain were assessed to be continuous pain without allodynia or hyperalgesia; tingling and cold-sensational pain in her right whole arm and leg (visual analog scale score: 5).
Diagnoses:
The patient was diagnosed as the right hemiparesis due to spontaneous thalamic hemorrhage.
Interventions:
Clinical assessment and diffusion tensor imaging (DTI) were performed 2 weeks and 14 months after onset.
Outcomes:
She suffered continuous pain in her right whole arm and leg (visual analog scale score: 5). On DTT of the 2-week postonset DTI scans, the configuration of the STT was well-preserved in both hemispheres. However, in contrast to those 2-week postonset results, the 14-month postonset DTT results showed partial tearing and thinning in the left STT. Regardless, both the 2-week and 14-month postonset DTT showed that the left STT passed through the vicinity of the thalamic lesion.
Lessons:
Diagnostic importance of performing a DTT-based evaluation of the STT in patients exhibiting delayed-onset CPSP following intracerebral hemorrhage.
Keywords: central poststroke pain, diffusion tensor tractography, intracerebral hemorrhage, neural degeneration, stroke
1. Introduction
Central poststroke pain (CPSP) is neuropathic pain resulting from a brain lesion following a stroke. Although the pathophysiologic mechanisms of CPSP have not been clearly elucidated, spinothalamic tract (STT) injury has been considered a plausible mechanism.[1,2] The recent development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI) data, allows visualization and estimation of the STT.[3] Recent studies have used DTT to demonstrate that CPSP was related to STT injury in patients with stroke.[4–7] However, few studies have been reported about delayed-onset CPSP due to degeneration of the STT following a stroke.
In this study, we report a patient with thalamic hemorrhage who experienced delayed-onset CPSP due to degeneration of the STT, which was demonstrated by performing serial DTT.
2. Case report
A 57-year-old female patient presented with right hemiparesis, which occurred at the onset of a spontaneous thalamic hemorrhage. She underwent conservative management at the neurosurgery department of a university hospital. Two weeks after onset, she was transferred to the rehabilitation department of the same university hospital for treatment, during which she did not report any pain. Brain magnetic resonance (MR) images scanned at 2 weeks after onset revealed signs of hemorrhage in the left thalamus (Fig. 1A). At approximately 6 months after onset, she reported pain in the right arm and leg, and the pain slowly intensified with the passage of time. At 14 months after onset, she revisited the rehabilitation department of the same university hospital. At that time, the characteristics and severity of her pain were assessed as follows: continuous pain without allodynia or hyperalgesia; tingling and cold-sensational pain in her right whole arm and leg (visual analog scale score: 5).[8] Brain MR images scanned at 14 months after onset showed a leukomalactic lesion in the left thalamus (Fig. 1A). An electromyographic study did not show evidence of peripheral neuropathy or radiculopathy. Informed written consent was obtained from the patient and control subjects for publication of this case report and accompanying images.
Figure 1.
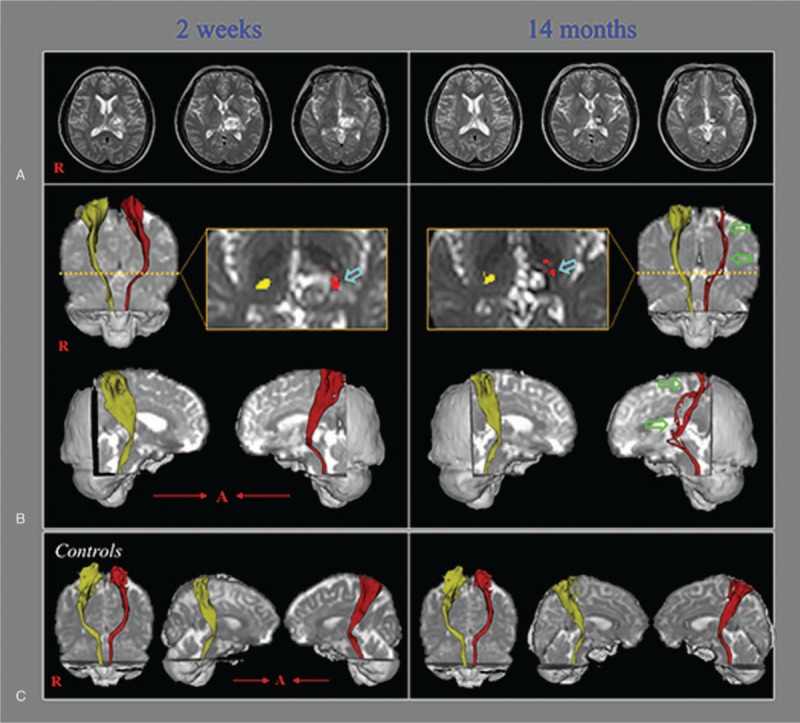
(A) T2-weighted brain magnetic resonance images showing signs of hemorrhage in the left thalamus (2 weeks after onset) and a leukomalactic lesion in the left thalamus (14 months after onset). (B) Diffusion tensor tractography (DTT) results for the spinothalamic tract (STT). On 2-week postonset DTT, the STT configuration is well-preserved in both hemispheres. However, on 14-month postonset DTT, the left STT reveals partial tearing and thinning (green arrows). The left STT may be seen passing through the area of the thalamic lesion (sky-blue arrows) on both the 2-week and 14-month DTT images. (C) DTT results showing the bilateral STTs in 2 normal subjects (51- and 57-year-old women).
DTI scanning was performed twice (at 2 weeks and 14 months after onset) by using a 6-channel head coil on a 1.5-T Philips Gyroscan Intera (Philips, Best, the Netherlands) with single-shot echo-planar imaging. For each of the 32 noncollinear diffusion sensitizing gradients, 67 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96, reconstructed to matrix = 192 × 192, field of view = 240 mm × 240 mm, TR = 10,398 milliseconds, TE = 72 milliseconds, parallel imaging reduction factor (SENSE factor) = 2, echo-planar imaging factor = 59, b = 1000 s/mm2, NEX = 1, and slice thickness = 2.5 mm. The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library was used for DTI data analysis. Eddy current correction was applied to correct for head motion effects and image distortion. FMRIB Diffusion Software with routines option (0.5-mm step lengths, 5000 streamline samples, curvature thresholds = 0.2) was used for fiber tracking.[9] For reconstruction of the STT, the seed region of interest (ROI) was placed on the posterolateral medulla on an axial slice, and the target ROI was placed on the primary somatosensory cortex on an axial slice.[3] Out of 5000 samples generated from each seed voxel, results for each contact were visualized with the threshold and weighting of tract probability at a minimum of one streamline through each voxel for analysis.
On DTT of the 2-week postonset DTI scans, the configuration of the STT was well-preserved in both hemispheres. However, in contrast to those 2-week postonset results, the 14-month postonset DTT results showed partial tearing and thinning in the left STT (Fig. 1B). Regardless, both the 2-week and 14-month postonset DTT showed that the left STT passed through the vicinity of the thalamic lesion.
3. Discussion
In this patient, we suggest that the delayed-onset CPSP could be ascribed to perilesional neural degeneration of the left STT. The reasons for that conclusion are as follows. The patient reported neuropathic pain in the right arm and leg at approximately 6 months after onset and the pain slowly intensified with the passage of time. Because an electromyographic assessment at 14 months after onset failed to show any abnormal signs in the left arm and leg, we ruled out peripheral neuropathy or radiculopathy. In contrast, the left STT on the 14-month postonset DTT showed partial tearing and thinning compared with a well-preserved STT on the 2-week postonset DTT, indicating progressive degeneration of the left STT. As a result, we have assumed that the left STT was injured by perilesional neural degeneration that had started at the thalamic level on the left side.
Stroke is reported to be accompanied by neural degeneration.[10,11] Numerous studies have reported on Wallerian degeneration, which is a process characterized by degeneration of the nerve axon and its myelin sheath distal to the stroke lesion, and which starts immediately after or during the acute stage of stroke.[10,11] However, few studies have been reported on perilesional neural degeneration in patients with stroke.[12] Jang and Seo[12] reported a patient who showed delayed degeneration of the corticospinal tract following spontaneous hemorrhage in the basal ganglia. Regarding the possible pathophysiologic mechanism of such perilesional neural degeneration in the left thalamus, previous studies have reported that injury of perilesional white matter might be associated with a chemical mechanism; for example, a blood clot can cause extensive damage to neural tissue by releasing potentially damaging substances, such as free iron, that may generate free radicals or induce inflammatory cytokine release.[12–15] Regarding the delayed-onset CPSP, Jang et al [2017] reported a patient who developed delayed-onset central pain due to degeneration of the STT resulting from degeneration of the isthmic transcallosal fibers following a traumatic corpus callosum hemorrhage.[16]
In conclusion, by using DTT, we were able to demonstrate STT degeneration in a patient who showed delayed-onset CPSP following thalamic hemorrhage. This study indicates the diagnostic importance of performing a DTT-based evaluation of the STT in patients exhibiting delayed-onset CPSP following intracerebral hemorrhage. However, because this is a single case report, these results have limitations; therefore, further studies involving larger number of subjects are warranted.
Author contributions
Methodology: JongHoon Kim, HanDo Lee.
Writing – original draft: HanDo Lee.
Writing – review & editing: SungHo Jang.
Footnotes
Abbreviations: CPSP = central poststroke pain, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, MR = magnetic resonance, ROI = region of interest, STT = spinothalamic tract.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A3A11043447).
The authors report no conflicts of interest to disclose.
References
- [1].Seghier ML, Lazeyras F, Vuilleumier P, et al. Functional magnetic resonance imaging and diffusion tensor imaging in a case of central poststroke pain. J Pain 2005;6:208–12. [DOI] [PubMed] [Google Scholar]
- [2].Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol 2009;8:857–68. [DOI] [PubMed] [Google Scholar]
- [3].Hong JH, Son SM, Jang SH. Identification of spinothalamic tract and its related thalamocortical fibers in human brain. Neurosci Lett 2010;468:102–5. [DOI] [PubMed] [Google Scholar]
- [4].Goto T, Saitoh Y, Hashimoto N, et al. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain 2008;140:509–18. [DOI] [PubMed] [Google Scholar]
- [5].Hong JH, Bai DS, Jeong JY, et al. Injury of the spino-thalamo-cortical pathway is necessary for central post-stroke pain. Eur Neurol 2010;64:163–8. [DOI] [PubMed] [Google Scholar]
- [6].Hong JH, Choi BY, Chang CH, et al. The prevalence of central poststroke pain according to the integrity of the spino-thalamo-cortical pathway. Eur Neurol 2012;67:12–7. [DOI] [PubMed] [Google Scholar]
- [7].Jang SH, Lee J, Yeo SS. Central post-stroke pain due to injury of the spinothalamic tract in patients with cerebral infarction: a diffusion tensor tractography imaging study. Neural Regen Res 2017;12:2021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the Visual Analogue Scale. Fam Pract Res J 1993;13:15–24. [PubMed] [Google Scholar]
- [9].Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? NeuroImage 2007;34:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu C, Zhu C, Zhang Y, et al. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. NeuroImage 2009;47:451–8. [DOI] [PubMed] [Google Scholar]
- [11].Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010;31:1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jang SH, Seo JP. Delayed leg weakness due to peri-lesional neural degeneration in a patient with intracerebral haemorrhage: case report. Acta Neurol Belg 2016;116:91–3. [DOI] [PubMed] [Google Scholar]
- [13].Chua CO, Chahboune H, Braun A, et al. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke 2009;40:3369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yeo SS, Choi BY, Chang CH, et al. Periventricular white matter injury by primary intraventricular hemorrhage: a diffusion tensor imaging study. Eur Neurol 2011;66:235–41. [DOI] [PubMed] [Google Scholar]
- [15].Yeo SS, Choi BY, Chang CH, et al. Evidence of corticospinal tract injury at midbrain in patients with subarachnoid hemorrhage. Stroke 2012;43:2239–41. [DOI] [PubMed] [Google Scholar]
- [16].Jang SH, Chang CH, Jung YJ, et al. Delayed-onset central pain due to degeneration of ischemic transcallosal fibers after corpus callosum hemorrhage. Am J Phys Med Rehabil 2017;96:E177–80. [DOI] [PubMed] [Google Scholar]


