Abstract
For contrast ultrasound imaging, the most efficient contrast agents comprise highly compressible gas-filled microbubbles. These micrometer-sized particles are typically filled with low-solubility perfluorocarbon gases, and coated with a thin shell, often a lipid monolayer. These particles circulate in the bloodstream for several minutes; they demonstrate good safety and are already in widespread clinical use as blood pool agents with very low dosage necessary (sub-mg per injection). As ultrasound is an ubiquitous medical imaging modality, with tens of millions of exams conducted annually, its use for molecular/targeted imaging of biomarkers of disease may enable wider implementation of personalized medicine applications, precision medicine, non-invasive quantification of biomarkers, targeted guidance of biopsy and therapy in real time. To achieve this capability, microbubbles are decorated with targeting ligands, possessing specific affinity towards vascular biomarkers of disease, such as tumor neovasculature or areas of inflammation, ischemia-reperfusion injury or ischemic memory. Once bound to the target, microbubbles can be selectively visualized to delineate disease location by ultrasound imaging. This review discusses the general design trends and approaches for such molecular ultrasound imaging agents, which are currently at the advanced stages of development, and are evolving towards widespread clinical trials.
Keywords: microbubbles, microbubble contrast agents, targeting, imaging, molecular imaging, targeted imaging
1. Introduction
In molecular imaging, techniques are developed for visualization, characterization, and measurement of biological processes at molecular or cellular level [1]. Molecular imaging probes were developed to determine the expression of specific molecular markers at different stages of diseases [2]. In comparison with other molecular imaging modalities, especially positron emission tomography (PET) and single-photon emission computed tomography (SPECT), ultrasound–based molecular imaging has many advantages: a very good safety profile (when compared with X-ray and Gd MRI contrast), lack of ionizing radiation, high spatial and temporal resolution, excellent contrast agent sensitivity, low cost, high portability and availability [3–6]. Medical diagnostic ultrasound involves use of compressional waves in the 1– 50 MHz range. The ultrasound waves are scattered and reflected by structures in the body as a function of differing acoustic impedances (the product of the speed of sound and tissue density) of insonified tissues. Different structures also scatter ultrasound differently as functions of the composition and geometry. Microbubbles are the most efficient ultrasound contrast agents. Gas-filled microbubbles (unlike surrounding biological tissue, e.g. blood) compress and expand readily in the ultrasound field giving rise to a strong point source-like scattered signals that contain harmonics of the transmitted signal: vibrational response of microbubbles is non-linear. The primarily linear response of tissue and nonlinear (harmonic) response of microbubbles gives rise to a family of tissue / microbubble signal separation schemes (see below, section 4). Initially, when various imaging modalities were assessed for their usability in targeted/molecular imaging, ultrasound was rated as incapable [1,7]. However, microbubble usefulness in this area has been proven in preclinical studies, and lately in clinical trials, so we can now discuss the successful strategies, as well as the alternatives to avoid. In this review it is impractical to assess all the available literature, so references are provided as examples of existing research trends.
In ultrasound targeted/molecular imaging, microbubbles that attach to receptors on the endothelium via a specific ligand-receptor bond indicate the presence of specific molecular markers on the vessel wall [3–5]. The outcome of this research effort looks quite promising, almost ripe for the large scale clinical trials, with initial clinical studies (e.g. NCT03009266, NCT02398266) already completed and reported [8,9].
2. Microbubble design: from physics to chemistry and biology
In order to formulate microbubbles useful for clinical ultrasound imaging (especially targeted/molecular imaging), one has to accommodate a set of conflicting requirements. First, contrast material has to be very stable on storage. At the same time, it has to be fully biocompatible and biodegradable, and, preferably, exit the body quickly and completely, to avoid any side effects for the patients. The injected dose has to be minimal, and detection sensitivity has to be high, so that the small fraction of the contrast agent that is accumulated in the target may be imaged successfully. Nonspecific accumulation in the non-target areas should be insignificant. Targeting ligands that decorate the surface of the contrast agent particles should assure firm adhesion on the target vessel wall, and retention in the presence of adjacent blood flow. Although it is not easy to address all these requirements simultaneously, the goal of practical clinical use now looks more achievable.
2.1. Microbubble parameters: particle size.
Gas microbubble-based ultrasound contrast agents were immediately (and incorrectly) suspected of causing embolic events upon intravenous administration [10]. To overcome this concern, the microbubble size must be tightly controlled, so that microbubbles are able to recirculate freely, just like red blood cells, through the capillaries. Therefore, we should impose an upper size limit of the microbubbles as ~3 – 5 micrometers. In a similar trend, clinical preparations of blood pool contrast agents, e.g., ~98% of the particles are specified to be under ten micrometers in size [11], so a small fraction of administered bubbles might deposit in the capillaries until they lose some gas from the core and deflate. The preferred lower limit of the bubble size derives from physics: Rayleigh scattering by a particle is proportional to the sixth power of its diameter. Therefore, as microbubble dimension reduces, the acoustic scattering diminishes precipitously even after accounting for the level of echogenicity arising from the low-mass, compressible, gas filled core. The stability of smaller bubbles may also be an issue: due to the higher surface-to-volume ratio, gas loss from these particles is much faster than from larger microbubbles. Nanobubble [12] and bubble-liposome [13] concepts had been developed over past decade, with the goal of developing nanoparticle contrast agents capable of extravasation through the leaky tumor endothelial lining. Recently, interesting results have been obtained [14], but clinical translation of these particles has not yet been approached. Therefore, we will focus this review on microbubble particles.
2.2. Microbubble shell parameters: stability.
The most stable gas-filled microbubbles with outstanding robustness and storage are made using a solid thick material (e.g. glass shell) [15]. They may vary from several to several dozen micrometers in size, and the shell may have micrometer thickness. These bubbles are stable in the aqueous media for years (bubbles of this kind in an aqueous dispersion float to the top of the media and remain unchanged there for several years). However, medical imaging use of these particles is highly unlikely, because they are not biodegradable and will not exit the body. Biocompatible biodegradable polymers are available to replace glass, for instance, polylactide/poly-lactide-co-glycolide (used in degradable surgical sutures). Polymer bubbles can be made by emulsion processes and include lyophilization or spray drying as the final step, to remove the internal core and create an internal void to be filled with gas. This formulation is very appropriate for the long-term storage stability: without lyophilization, in the aqueous medium, these polymers will hydrolyse and degrade. PLGA-based particles had been in preclinical development and clinical trials; for one of such blood pool agents, AI-700/Imagify, from Acusphere (Boston, MA) Phase 3 clinical trials were completed [16] but it had not been approved by regulatory authorities in US and application had been withdrawn in Europe. Another agent, made of a different polymer, cyanoacrylate, the base of a biocompatible surgical glue, Sonovist/SHU563A (Schering, Berlin), also quite stable on storage, had very reasonable circulation half-life in the bloodstream, but also did not make a transition to clinical use. Alternative versions of this polymer bubble are now under intense assessment in preclinical setting [17]. Recently, a hybrid approach has been proposed, a combination of silica and polymer in a particle formulation [18], but it is too early to tell whether it will progress towards clinical use.
A much simpler formulation, with the shell based on human albumin, had been in clinical development early, and is approved for clinical use; Optison (FS069) agent is made from perfluoropropane gas that is dispersed in aqueous medium containing human albumin [19]. The shell of this particle is “all-natural”, with guaranteed biocompatibility: albumin is present in human plasma at ~50 mg/mL, and is widely administered to patients in large doses. Albumin-based particles do not induce immune response [20].
An earlier version of this agent, Albunex, the first FDA-approved “micro-device” microbubble, contained air, not perfluorocarbon, as the gas core. The circulation time of these air-filled microbubbles was too low to be practically useful at that time, especially if the test subject was breathing oxygen [21,22]. While a rigid shell, made of glass or a thick polymer, is a good barrier for gas penetration, a thinner and relatively “loose” shell made of denatured albumin (about 12 – 15 nm thick) is not a good barrier to the transfer of nitrogen and its dissolution in the surrounding bloodstream (especially if blood is nitrogen- poor). This leads to the rapid microbubble collapse and contrast signal loss. Perfluorinated gases, such as perfluorocarbons, possess a much lower solubility in aqueous media (including blood) and, hence they are retained inside the microbubbles in the bloodstream longer than in the case of air cores. The resultant agents are more amenable to clinical application. Interestingly, both Optison and Albunex are provided as premade products, in the form of aqueous dispersion, stable for extended periods of time (years) during refrigerated storage. They retain their particle concentration and size distribution within specification upon storage in this type of formulation.
The lowest available shell thickness for continuous microbubble coating is provided by the use of a lipid monolayer. A monomolecular layer has thickness ~2 nm (half of a typical lipid membrane); hydrophilic heads of lipid molecules point towards the aqueous media, and hydrophobic fatty acid tails point towards gas phase of the bubble. There are several lipid-based formulations approved for clinical use, from Imagent/AFO150 (dimiristoyl phosphatidylcholine, with C14 fatty acid) [23] to MRX115/DMP115/Definity [11] (dipalmitoyl phosphatidylcholine, C16 fatty acid) to BR1/Sonovue/Lumason (distearoyl phosphatidylcholine, C18 fatty acid) [24] and NC100100/Sonazoid (hydrogenated phosphatidylserine, mostly C18 fatty acid [25]. Generally, longer chain lipids with a higher phase transition temperature confer improvement in long-term shell stability [26]. However, none of the above clinical contrast agents require long-term stability of bubbles: they are provided as precursors (which are stable on storage) and bubbles are manufactured in the clinical setting, minutes or hours prior to use. Also, longer-chain fatty acid lipids generally favor prolonged circulation time [27], but most of the described agents provide sufficient circulation time for clinical imaging, especially if an appropriate perfluorocarbon gas core is used. Currently, the most popular precursor strategy worldwide is based on a lyophilized dry powder of water-soluble compound (PEG or sugar) in which remnants of bubble shells are interspersed, in dry state, with the low-solubility perfluorinated gas headspace. After addition of water, the matrix water-soluble compound is dissolved and bubbles are immediately formed, to be used within several hours [24].
2.3. Microbubble acoustic response: influence of size and shell parameters.
The acoustic response of microbubbles is dependent on the particle size, shell design, and shape of the applied ultrasound waveform [28]. Diagnostic ultrasound usually employs frequencies in the range from 1 and 50 MHz. The higher frequencies assure finer spatial resolution (down to tens of micrometers) but shorter depth of tissue penetration (< 1 cm), making it useful for small animal studies, superficial examinations or intravascular catheter-based imaging. It had been suggested that resonance frequency of the micrometer-size gas bubbles is within MHz range, so there are some efforts to tailor the microbubble size to match the frequency used in imaging. However, Rayleigh scattering dependence, i.e., preference for the larger bubbles, may prove beneficial for the enhancement of acoustic backscatter on a per-particle basis [29]. Thin-shelled bubbles (made of albumin or lipid monolayers) compress and expand in the ultrasound field with only modest interference from the bubble coating [30], although compression-only behaviour is observed sometimes [31]. This is not the case for thicker-shelled polymer bubbles: it was found that in order to obtain significant acoustic backscatter signal, higher acoustic pressure must be applied, to “crack” the shell so that the gas microbubble could compress and expand [32]. Critical “cracking” acoustic pressure is dependent on the microbubble shell thickness [33], i.e., these microbubbles must be destroyed in order to be imaged.
2.4. Shell composition and biocompatibility of targeted microbubbles.
Obviously, the most biocompatible microbubbles are fabricated using human albumin as the shell material. Optison is quite safe: it is in constant clinical use without reports of a substantial frequency of serious adverse events (a post-marketing open-label clinical trial NCT00730964 did not report any serious adverse events during contrast echocardiography exams for >1000 patients). However, for the molecular imaging purposes, chemical modification of the shell with the targeting ligand (peptide, mimetic or an antibody fragment) is required. The covalently modified albumin might not be recognized by the body as its own, and anti-hapten antibodies might develop. Therefore, the use of lipids as the shell components appears more appropriate. Liposome formulations are widely used in clinical practice as intravascular therapeutic agents, with the large doses of lipids administered to patients. Ligand-lipid conjugates have been in research and preclinical development for liposome targeting since 1980s; later, a PEG spacer arm between the targeting ligand and receptor was added [34]. Admixing a small molecule targeting ligand-PEG-lipid component with the carrier shell lipids to form a monolayer shell is a trivial task [35,36]. It may be preferred to use tiered architecture of the coating, where a thick brush of PEG would minimize complement activation [37,38]. The latest example of this approach is the non-targeted BR38 [39] and targeted BR55 microbubble formulation [40]. Polymer bubbles based on PLGA [41] or cyanoacrylate [42] have also been used for the targeting ligand attachment; PEG-lipid has also been combined with polymers in a single bubble shell [43,44], but these agents are not in clinical trials at the time of this writing. Consequently, lipid-based microbubbles may continue to dominate the field.
3. Biomechanics of microbubble targeting: how to optimize.
The act of microbubble targeting can be described as a balance between adhesion (retention) and shear flow forces. A microbubble in the bloodstream touches the wall that has specific receptors, biomarkers of disease, and the specific targeting ligand on the surface of the bubble may attach to the receptor, if the ligand-receptor binding kinetics is favorable. Depending on the strength of the ligand-receptor bond, and the number of bonds formed, microbubbles may attach to the target surface (e.g. endothelial lining in the area of disease). If shear force that is applied on the bubble by the flow of blood is lower than the adhesion force, the bubble will stay adherent. Thus, a fraction of circulating bubbles that pass through the target tissue is retained with every passage, and microbubbles accumulate at the target area, so that ultrasound contrast imaging of the target tissue can be achieved.
3.1. Microbubble adhesion to the target: ligand-receptor pair interactions.
Adhesion efficacy and bond strength are obviously dependent on the number of ligand-receptor pairs that form when bubble comes in contact with the target. Microbubble adhesion level in the target tissue (assessed as the adherent contrast ultrasound signal) is directly related to the level of expression of the target receptor on endothelial cells in the target vasculature; this fact has been established quite early, for MAdCAM-1-targeted microbubbles [45].
Bubble adhesion strength [36] and resistance to shear flow [46] were measured initially using simple in vitro video microscopy, where biotinylated bubbles were put in contact with avidin-coated surfaces, and it was found that increase of ligand surface density on the bubble helps improve bubble retention on the target surface. Therefore, it might be beneficial to maximize surface density of the ligand on the bubble shell. For the microbubble contrast agent now in clinical trials (BR55) the amount of the peptide ligand has been reported: a heterodimeric peptide is present on the bubble shell at ~400,000 molecules per bubble [40]. The shell of a clinical bubble, Sonazoid [25], that is targeted to phagocytic cells, consists of phosphatidylserine, a natural biomarker of phagocytosis, which implies ~10 – 20 million targeting molecules per bubble.
3.2. Bubble adhesion and ligand-receptor type: fast vs slow interaction kinetics.
Target biomarker receptor interaction with the targeting ligand has to be a rapid process: microbubbles in the blood flow move relatively quickly (blood velocity in larger vessels can approach 1 m/s). Flow rate is distributed in the vessels as a blunted parabola [47], with the fastest flow in the center and slowest at the periphery, closest to vessel wall, but shear forces by the endothelium coat surface are significant. It had been noticed in the flow chamber setting, that microbubbles targeted to P-selectin via anti-P-selectin antibodies are targeted to the receptor-coated surface with reasonable efficiency at slower flows (up to ~1 dyn/cm2 wall shear stress). At faster flow, and consequent higher shear force, the adhesion of the microbubbles to the target is decreased, even though the flux of microbubbles by the target is increased [48]. If bubbles were allowed to adhere to the P-selectin-coated surface in static conditions first, they were retained on the target even despite much faster flow conditions (some bubbles were still present even at ~70 dyn/cm2, when receptor surface density was ~100 molecules/µm2). This result could be interpreted as the inability of these antibodies to have a chance to grab onto the receptor molecules in faster flow, i.e., kinetic constant of association of antibody and antigen was not high enough to ensure successful targeted adhesion in the high shear flow conditions. But once multiple antibody-antigen bonds form, microbubbles become much more resistant to flow shear. In nature, leukocytes use rapidly binding PSGL-1 molecule to attach to P- and E-selectin on vascular endothelium, specifically, the glycosulfopeptide on the distal terminus of PSGL-1, in high shear flow (in natural flow conditions in vivo, in certain vessels, wall shear stress may exceed 10 dyn/cm2). When these glycosulfopeptide ligand molecules were placed on the microbubbles, successful microbubble targeting in fast flow was achieved [49].
Even shorter carbohydrate fragments of PSGL-1, such as Sialyl Lewis X or Sialyl Lewis A, that are known to bind to P- and E-selectin rapidly and have proven to be useful in the high shear flow conditions for microbubble targeting [50]. When these molecules were clustered on a polymer chain and placed on the bubble shell, effective targeting in a parallel plate flow chamber was reported at wall shear stress of ~4.5 dyn/cm2, where anti-P-selectin antibody was completely inefficient [51]. An even more exciting result (40 dyn/cm2) was demonstrated for P-selectin and platelet targeting by Sialyl Lewis A polymer-coated bubbles [52]. This was especially intriguing given the fact that monomer Sialyl Lewis X or Sialyl Lewis A demonstrates rather low equilibrium Kd, in millimolar range, which is many orders of magnitude worse than a typical antigen-antibody interaction (nanomolar or better affinity). As the density of the carbohydrate ligand molecules on the bubble surface was high, cooperative multipoint interaction became possible, so a large number of rapidly forming bonds resulted in excellent adhesion and retention of microbubbles in high shear flow. Thus, the most beneficial strategy for microbubble targeting may be the use of high surface density of small molecule ligands that rapidly bind to the target receptor, even if their individual affinity is low, i.e., equilibrium Kd is high, but overall avidity between the bubble and target surface assures tight adhesion and retention on the target surface.
Additional strategy to consider may be the use of multi-targeting: combine ligands on the surface of each microbubble, for instance, placing on each microbubble the molecules of Sialyl Lewis X and anti-ICAM-1 antibody [50] or polymeric version of Sialyl Lewis X and anti-VCAM-1 antibody [53] at the same time, where a combination has improved microbubble targeting efficacy. Triple-targeted microbubbles have also been described, directed at once towards αvβ3, P-selectin, and VEGFR2 [54].
3.3. PEG spacer as a dynamic tether and barrier.
Decorating microbubbles with a hydrophilic polymer brush (e.g., PEG) is useful for a number of reasons. First of all, PEG-lipid (e.g., PEG stearate) is surface-active and may rapidly stabilize newly-formed gas-water interface, so that microbubble generation is efficient, with minimal conversion of bulk gas phase to macroscopic bubbles. A second reason relates to the enhancement of microbubble stability during storage: a dense brush of grafted water-soluble PEG polymers on top of the thin (e.g., a lipid monolayer) microbubble shell helps minimize the chance of direct bubble-to-bubble shell contact and slows down microbubble fusion process; thus some of the microbubble formulations in the aqueous media are stable for many months. Third reason is important from the standpoint of in vivo application: the use of a PEG brush minimizes nonspecific adhesion of the microbubbles to biological surfaces [37] and may reduce uptake by the phagocytic RES cells. Thus, it is beneficial to have a grafted brush of PEG on the surface of microbubbles. Some microbubble formulations that are currently in clinical use (Definity [55]) or in clinical trials (BR38 [39]) do carry such a brush. Therefore, it may be beneficial to also have the targeting ligand molecules anchored to the microbubble shell via a long spacer arm, so that the targeting ligand has a higher probability of reaching beyond the PEG brush and binding to the target receptor. Successful use of extended flexible PEG spacer arm for targeted adhesion has been known for liposomes [56] and lipid layers [57]. The concept of a tiered-brush architecture has been proposed and implemented [36], where a shorter and more dense PEG brush is used to ensure bubble stability, and longer-chain PEG is used for the attachment of the targeting ligand molecules.
There is yet another reason to have an extended flexible spacer arm for the ligand attachment: its presence improves targeting by providing rapid adaptive spatial positioning between the ligand and receptor molecule pairs. In a non-adaptive scenario, a ligand is firmly and rigidly attached to the surface of the bubble (a solid lipid, protein or polymer shell offers minimal spatial mobility). Likewise, receptors on the surface of vascular endothelium are embedded in the biomembrane and may be anchored in place by their attachment to the cytoskeleton matrix. Therefore, during the limited time of contact between the microbubble and target surfaces in flow, the probability of a productive ligand-receptor bond formation is minimized, especially the desirable formation of multiple bond-receptor pairs. However, if a ligand is tethered to a long spacer arm (preferably a flexible and extended one, such as PEG) the probability of a productive ligand-receptor binding event is improved, and microbubbles bind to the target surface more efficiently [58].
3.4. Shell parameters: extra surface, folds and shape.
It is well known that leukocytes have an extended surface area and microvilli to bind to activated endothelium on the vessel wall [59]. This feature ensures firm adhesion as compared with poorly deformable solid spheres, which would have much lower contact area with the target, and thus much lower number of ligand-receptor bond pairs would form. Spherical microbubbles, especially the ones made of the solid lipids with high melting point (such as DSPC, with Tc ~56 oC) are not easily deformable (the higher the phase transition temperature, the lower the deformability [60]). However, it is simple to generate extra shell surface area on a microbubble coating: transient (seconds) pressurization of the bubbles at ~1 atm results in a partial gas loss, with the lipid shell forming “wrinkles” on the outside of the bubble. Targeted bubbles with such extra shell demonstrate a significant improvement of their targeting capabilities [61], both in vitro and in vivo; microbubbles adherent on the surface assume non-spherical teardrop shape elonglated in the direction of the flow, which may help reduce the dislodging shear forces.
4. Ultrasound imaging for molecular probes
The goal of ultrasound molecular imaging is to selectively detect and enhance echo signals derived from the molecularly adherent microbubbles via specific ligand-receptor bindings, while suppressing signals derived from the background tissues, freely circulating microbubbles, and non-specifically adherent microbubbles (non-specific adhesion) [3–5]. Over several decades, multiple techniques were developed to reach this goal. Typically, a complete ultrasound molecular imaging protocol consists of three sections: differentiation of microbubbles from background tissue, differentiation of adherent from freely circulating microbubbles, and differentiation of molecularly adherent from non-specifically adherent microbubbles.
4.1. Differentiation of microbubbles from background tissue
Biological tissues (except lung and bone) predominantly consist of water, which has low compressibility. Consequently, there is only relatively weak scattering and reflection from impinging ultrasound wave propagation [62]. However, perfluorocarbon gas-filled microbubbles, possessing a very low density and much higher compressibility than tissue, generate ultrasonic backscattering many orders of magnitude larger than biological soft tissues [63]. Ultrasound medical systems have demonstrated ability to resolve single microbubble [64]. Consequently, ultrasound molecular imaging is one of the most sensitive molecular imaging modalities, with single-microbubble sensitivity reported [5].
4.1.1. Nonlinear signal detection for microbubbles
In traditional B-mode imaging with fundamental frequency, images of the summed backscatter from both the microbubbles and background tissue are generated, without any discrimination between the two signal sources. As mentioned above, microbubbles oscillate nonlinearly in the excitation acoustic field due to their high compressibility and elasticity of the shell [65]. Therefore, nonlinear scattering of ultrasound waves results in the generation of echo energy at harmonics of the transmitted center frequency. These harmonics include subharmonics [66,67], “second” harmonic (twice the fundamental frequency) [68], and super harmonics [69]. Based on the assumption that the nonlinear response of background tissue is substantially lower compared to that of microbubbles, harmonic imaging [70,71] was initially developed by implementing frequency domain filtering to isolate nonlinear frequency components (harmonics) from the microbubbles and suppress linear (fundamental) frequency components from the tissue (Figure 1A).
Figure 1.
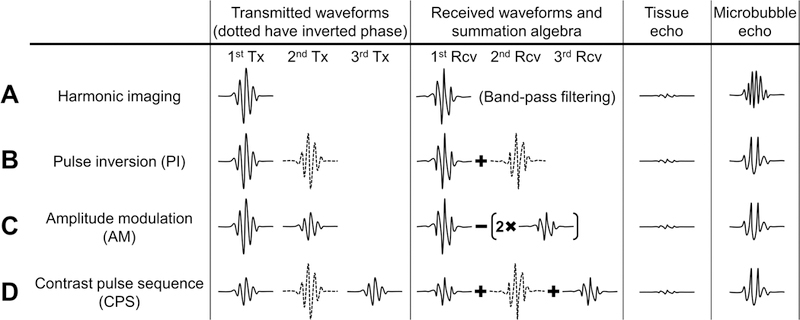
Nonlinear detection techniques of microbubbles. (A) Harmonic imaging. (B) Pulse inversion (PI). (C) Amplitude modulation (AM). Here is an example that the amplitude of the first transmitted pulse is twice the amplitude of the second transmitted pulse. (D) Contrast pulse sequence (CPS). Adapted from Caskey et al. [4] with permission, Copyright © 2011 Elsevier B.V.
In addition to single pulse harmonic imaging, most ultrasound contrast imaging has been based on multi-pulse nonlinear detection techniques [67]. Typically, two successive pulses are transmitted with application of modulation to either transmitted phase or amplitude. Received radio frequency (RF) echo signal records are stored in a temporary “buffer” for rapid processing. The echo records from linear responses of the background tissue are cancelled by coherent summation (in the case of pulse inversion [72]) or coherent scaled subtraction (in the case of amplitude modulation [67]), leaving only the residual signals, attributable to the nonlinear response of microbubbles. The most commonly used two-pulse nonlinear detection sequences are pulse inversion (PI) (Figure 1B) and amplitude modulation (AM) (also known as power modulation, PM) (Figure 1C) [67,72,73]. In pulse inversion, the second pulse is an inverted replica of the first pulse and transmitted after a suitable delay. In amplitude modulation, two consecutive pulses are transmitted with the different acoustic levels. Thereafter, the backscattered echoes are rescaled and summed to enhance the nonlinear response. The combination of pulse inversion and amplitude modulation results in the “Contrast Pulse Sequence” (CPS) which significantly enhances the contrast-to-tissue ratio [74,75] (Figure 1D). This is considered to be the most sensitive technique for microbubble detection [76,77]. Although the contrast-to-tissue ratio is significantly increased by using above nonlinear detection methods, some tissue echoes (e.g. vessel wall interface) may remain present, although reduced, resulting in false positives in the isolation the microbubble signals [78].
4.1.2. Ultrafast ultrasound imaging for microbubbles
In traditional line-by-line scanning with focused transmitted waves, excessive transmission energy may have negative impact on the survival of microbubbles, limiting their life time and thus echogenicity [77]. In the last decade, ultrafast ultrasound imaging was introduced for a dense time sampling of the entire imaging plane [79–81]. Typically, multiple plane waves [80,82] or diverging waves [83] with different steering angles are transmitted sequentially. Thanks to the coherent summation of the received backscattered echoes, contrast and signal-to-noise ratio can be regained [80,81]. Coherent plane wave compounding has demonstrated equivalent B-mode image quality as in traditional line-by-line focused approach with only a third of the insonifications [80]. Ultrafast imaging can also be used for ultrasound contrast agents. Microbubble tracking has been demonstrated by ultrafast imaging with high temporal resolution [84]. Recently, the combination of ultrafast plane wave imaging and amplitude modulation offered higher frame rate and greater contrast-to-background ratio compared to traditional focused approaches [77].
4.2. Differentiation of adherent from freely circulating microbubbles
4.2.1. Waiting period
The simplest approach to differentiate the signals arising from adherent microbubbles from those due to freely circulating microbubbles is based on employing a waiting period (e.g. 10 min) [40,85]. After the injection of microbubbles, ultrasound imaging is suspended to allow the clearance of freely circulating microbubbles from the blood stream. When the signal of freely circulating microbubbles is low, imaging is resumed to observe the signal attributed to adherent microbubbles. Consequently, this straightforward approach results in long procedure times (tens of minutes) and requires high stability of microbubbles. Additionally, it might not provide accurate quantitative assessment of molecular targets due to the effects of dislodged adherent microbubbles and residual freely circulating microbubbles.
4.2.2. Destruction-replenishment method
The most commonly used method to differentiate adherent microbubbles from freely circulating microbubbles is the destruction-replenishment method derived from parametric imaging of blood flow [86] (Figure 2). Briefly, a nonlinear contrast mode is used to detect microbubble signals. After injection of microbubbles, a few minutes are allowed for microbubbles to circulate and bind to the region of interest. Once the microbubble binding is sufficient, the pre-destruction intensity is obtained. Thereafter, high intensity destruction pulses are transmitted to burst all microbubbles within the field of view. Assuming the freely circulating microbubbles will replenish the region of interest, the post-destruction intensity is obtained after the transmission of destruction pulses. The adherent microbubble signal is identified by subtracting the post-destruction intensity (assumed to be residual freely circulating microbubbles) from the pre-destruction intensity (assumed to be adherent microbubbles and freely circulating microbubbles) (Figure 2C). This method provides more accurate and quantitative measurements of adherent microbubbles, and consumes less time than the aforementioned waiting period method. In addition, it is widely implemented in pre-clinical applications including the detection of angiogenesis [87,88], cancer [89,90], inflammation [91–93], and atherosclerosis [94,95]. However, this method lacks real-time capability due to the time-consuming post-processing for the pre- and post-destruction subtraction steps. Additionally, the high intensity destruction pulses are undesirable in clinical applications, to avoid the potential bio-effects caused by microbubble cavitation [96–98].
Figure 2.
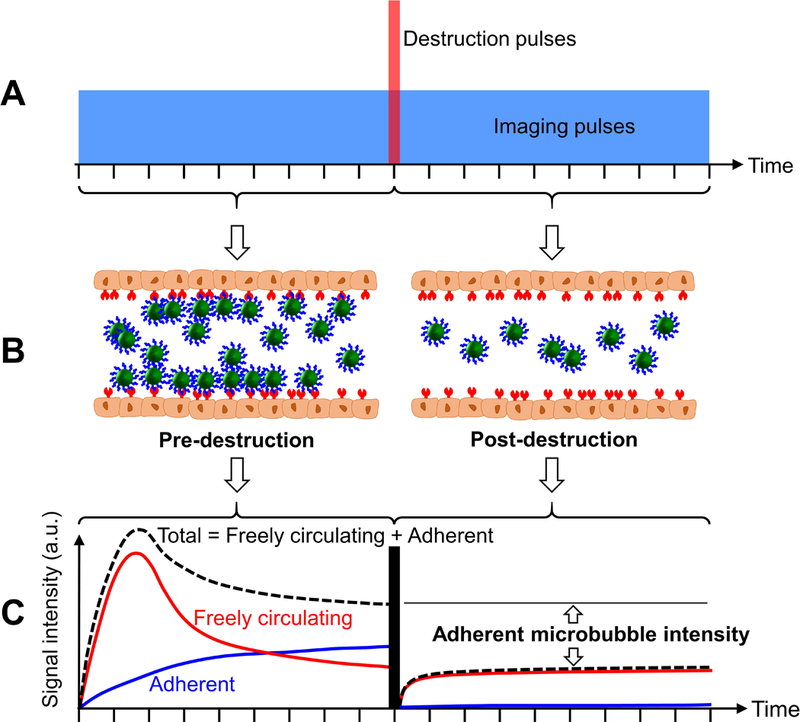
Schematic illustrations and algorithm for the destruction-replenishment method. (A) Pulse sequence of the method. The vertical (red) bar indicates the highintensity destruction pulses used to burst all the freely circulating and adherent microbubbles within the field of view. The horizontal (blue) box indicates the imaging pulses. The amplitudes of the destruction and imaging pulses are not to scale. (B) Graphical illustrations of microbubble dynamics in pre- and post-destruction stages. (C) The total acoustic signal intensity is the summation of freely circulating microbubble intensity and adherent microbubble intensity. The adherent microbubble intensity is achieved by subtracting the post-destruction intensity (assumed to be freely circulating microbubbles) from the pre-destruction intensity (assumed to be adherent microbubbles and freely circulating microbubbles). (C) is adapted from Steinl and Kaufmann [162], under Creative Commons Attribution License (2015).
4.2.3. Inter-frame signature-based methods
In order to further accelerate imaging procedure and eliminate the use of destruction pulses, another category of methods was developed to isolate adherent microbubbles based on the inter-frame signature of microbubbles. These methods are capable of performing real-time or near real-time imaging and clinically translatable signal quantification. In general, freely circulating microbubbles are fast-moving microbubbles and exhibit a more chaotic signature. Meanwhile, adherent microbubbles are slow-moving or stationary. Consequently, image signals of circulating microbubbles are transiently measurable while signals of adherent microbubbles are more stationary.
Slow-time frequency (frequency in frame-to-frame dimension) is one parameter used to quantify the inter-frame signature of microbubbles. Freely circulating microbubbles exhibit high slow-time frequencies while adherent microbubbles exhibit low slow-time frequencies. Low-pass slow-time filtering is designed to suppress high-frequency circulating microbubble signal while retain low-frequency adherent microbubble signal [99–102] (Figure 3). Similarly, slow-time averaging is designed to cancel out more random signals derived from circulating microbubbles while retain stationary signals from adherent microbubbles [103,104] (Figure 3). “Dwell time” is another threshold-determined parameter used to quantify the inter-frame signature of microbubbles. Freely circulating microbubbles exhibit short “dwell time” while adherent microbubbles exhibit longer “dwell time” (> 24 s). Consequently, adherent microbubbles could be isolated from circulating microbubbles based on “dwell time” threshold [105] (Figure 3). New methods including singular spectrum-based targeted molecular (SiSTM) imaging are introduced to differentiate adherent microbubbles from both freely circulating microbubbles and background tissue based on the inter-frame statistical properties of microbubbles [78,106]. Circulating microbubbles exhibit low echo correlation in slow-time dimension while adherent microbubbles exhibit high correlation. Appropriate band-pass filtering is performed to isolate signals from adherent microbubbles.
Figure 3.
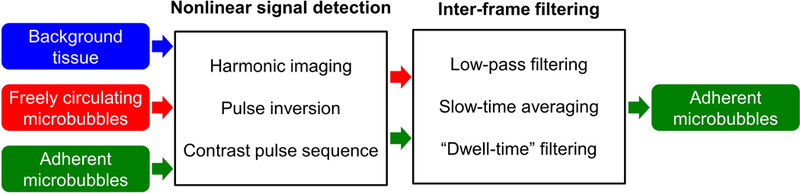
Examples of inter-frame filtering-based methods. Two-step filtering (nonlinear signal detectionþinter-frame filtering) is performed to isolate adherent microbubble signal. Harmonic imaging [99–102], pulse inversion [103,104], and contrast pulse sequence [105] were used in the nonlinear signal detection step. Slow-time low-pass filtering [99–102], slow-time averaging [103,104], and ‘dwell-time’ filtering [105] were used in the inter-frame filtering step.
4.3. Differentiation of molecularly adherent from non-specifically adherent microbubbles
All aforementioned ultrasound molecular imaging techniques are designed to isolate adherent microbubbles from freely circulating microbubbles and background tissue. They rely on an assumption that all adherent microbubbles are due to specific ligand-receptor bindings. However, adherent microbubbles typically comprise a combination of specifically and non-specifically adherent microbubbles. The non-specifically adherent microbubbles (non-specific adhesion) may result in overestimate of molecularly adherent microbubbles and even false positives in current methods. To solve this problem, typically a separate control microbubble (plain microbubbles without any conjugation or microbubbles conjugated to isotype control microbubbles) injection is performed to estimate the non-specific adhesion background using existing techniques [105]. The true molecularly adherent microbubble intensity is achieved by subtracting the adherent microbubble intensity of control microbubble injection (assumed to be non-specific adhesion) from that of targeted microbubble injection (assumed to be both molecularly adherent microbubbles and non-specific adhesion). Consequently, a long inter-injection waiting period (at least 20 min) is required for clearance of previously injected microbubbles between the two injections of microbubbles. Although many ultrasound molecular imaging techniques claim the near real-time or real-time imaging capability, the two extra steps (control microbubble injection and inter-injection waiting) can result in a complex imaging protocol and long imaging procedure time (30 – 60 min). The long and complex imaging protocols could potentially delay clinical adoption and increase procedure costs; however, at the preclinical investigational testing stage proper controls are necessary to elucidate fine mechanisms of microbubble targeting, and to ensure lack of nonspecific accumulation of bubbles in the target and non-target tissues.
4.4. Acoustic radiation force (ARF) aids molecular imaging signal detection.
In addition to dual-targeting microbubble design and new imaging algorithm development, imaging efficacy of molecularly adherent microbubbles may be enhanced by improving the microbubble attachment to molecular targets. Acoustic radiation force (ARF) is used to mechanically push microbubbles towards vascular endothelial cell wall, therefore, improving the ligand-receptor proximity [107–112]. Additionally, usage of ARF reduces the linear velocity of circulating microbubbles by pushing a larger fraction of them to the distal vessel wall [107]. In vitro studies have demonstrated over 100-fold increase of microbubble adhesion due to ARF usage [109,110]. Additionally, the image signal-to-noise ratio increased significantly due to application of ARF [111]. In vivo validations demonstrate that the application of ARF enhances the detection sensitivity and diagnostic utility of ultrasound molecular imaging [113,114].
While the efficacy of ARF in small vessel environments (e.g. tumor) has been demonstrated, studies have been performed in large vessel environments with application of ARF in vitro and ex vivo [78,99,103,104,115]. Due to the high flow rate and shear forces, ARF is essential in large vessel to push microbubbles towards distal vessel wall. Combinations of ARF and slow-time filtering technique resulted in real-time ultrasound molecular imaging in swine carotid artery, ex vivo [104]. The combination of ARF and singular value filtering resulted in an enhanced real-time ultrasound molecular imaging in large vessels [78]. Recently, a new technique based on modulated ARF is introduced to isolate molecularly adherent microbubbles in large blood vessel environments without the need of separate control microbubble injection [115–117] (Figure 4). A parameter referred as residual-to-saturation ratio (RSR) was extracted to quantify adherent microbubbles (Figure 4C). The modulated ARF imaging exhibited a reliable and rapid imaging protocol (3 min) in vivo [118], and the capability to quantify molecular marker concentration in vitro [116].
Figure 4.
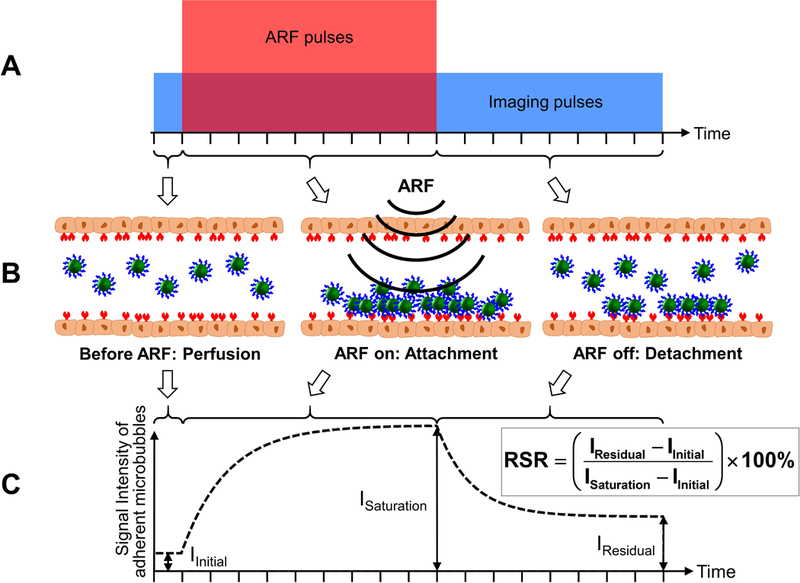
Schematic illustrations and algorithm for the modulated acoustic radiation force (ARF)-based method. (A) Pulse sequence of the method. The tall (red) box indicates the acoustic radiation force (ARF) pulses. The wide (blue) box indicates the imaging pulses. The amplitudes of the ARF and imaging pulses are not to scale. (B) Graphical illustrations of microbubble dynamics in the three stages: before ARF, ARF on, and ARF off. (C) Signal intensity of adherent microbubbles on the bottom vessel wall. IInitial represents the signal intensity of the baseline. ISaturation represents the signal intensity with saturated adherent microbubbles. IResidual represents the signal intensity of remaining adherent microbubbles after the cessation of ARF. Residual-to-saturation ratio (RSR) is calculated based on the equation above and is used to quantify the adherent microbubble concentration. Adapted from Wang et al. [115,116] with permission, ©Copyright, Elsevier B.V., 2015 (A); © Copyright, 2014, Institute of Physics and Engineering in Medicine (C).
5. Biomedical applications of ultrasound molecular imaging
Biomedical applications of ultrasound molecular imaging are focused on the non-invasive detection, quantification, and monitoring of disease-related receptors expressed on endothelium for the purpose of diagnosing disease and monitoring therapeutic efficacies. Three major categories of pre-clinical applications are molecular imaging of angiogenesis, inflammation, and thrombosis.
5.1. Molecular imaging of angiogenesis
For vascular endothelium involved in angiogenesis, over-expression of VEGF receptors (e.g. VEGFR2), integrins (e.g. αvβ3), and endoglin were identified as the molecular targets (Table 1). Other molecular targets include prostate-specific membrane antigen (PSMA) [119], secreted frizzled related protein-2 (SFRP2) [120], Thymocyte differentiating antigen 1 (Thy1) [121], and B7-H3 (CD276) [122]. Antibodies, peptides, and small molecules were successfully used to target those molecular markers. Dual, or triple, targeted microbubbles were also developed to increase binding efficacy [54,123–127]. Detection of angiogenesis has been achieved in murine tumor models including breast and pancreatic tumors [54,87–90,105,121,123,128–133]. Rat [40,134,135] and hen [136] were also used as tumor models for detection of angiogenesis. Studies have shown that VEGFR2-targeted microbubbles allow highly accurate detection of breast cancer in a murine model [89]. Additionally, microbubbles targeted to VEGFR2 and endoglin were used to monitor therapeutic effects of gemcitabine chemotherapy in pancreatic tumors [123]. The typical injection dose for the detection of angiogenesis was between 1×107 to 5×107 microbubbles. Recently, the minimum and diagnostic effective microbubble dose was decreased down to 1×106 microbubbles for a murine tumor model [137]. Potential clinical usefulness of targeted contrast ultrasound imaging of the vessel wall biomarkers manifested in angiogenesis is clear: imaging of malignant tumor-related neovasculature may be used to assess the location, size, and perhaps malignancy levels of tumor nodules. This information may also be quite useful for the intervention guidance, such as the needle biopsy of prostate or breast cancer, as well as guidance of tumor ablation procedures. In the more distant future, when therapeutic angiogenesis becomes clinical reality, assessment of neovasculature biomarkers could provide information on the treatment efficacy and augment tissue perfusion information.
Table 1:
Pre-clinical applications of ultrasound molecular imaging
| Application | Disease | Animal | Molecular target | Reference |
|---|---|---|---|---|
| Angiogenesis | Breast tumor | Mouse | αvβ3 | [128] |
| Mouse | VEGFR2 | [87,89] | ||
| Mouse | VEGFR2, αvβ3, P-selectin | [54] | ||
| Rat | VEGFR2 | [40] | ||
| Pancreatic tumor | Mouse | VEGFR2 | [90] | |
| Mouse | VEGFR2, VEGF, CD105 | [123] | ||
| Mouse | Thy1/CD90 | [121] | ||
| Rat | VEGFR2 | [134,135] | ||
| Glioma tumor | Rat | αvβ3 | [136] | |
| Ovarian tumor | Hen | αvβ3 | [157] | |
| Other tumor | Mouse | αvβ3 | [158,159] | |
| Mouse | VEGFR2 | [88,105,129–133] | ||
| Mouse | VEGFR2, αvβ3 | [124] | ||
| Mouse | VEGFR2, αvβ3, endoglin | [125,126] | ||
| Mouse | VEGFR2, αvβ3, P-selectin | [127] | ||
| Mouse | alpha(v)-integrins | [160] | ||
| Mouse | B7-H3/CD276 | [122] | ||
| Mouse | PSMA | [119] | ||
| Mouse | SFRP2 | [120] | ||
| Inflammation | Atherosclerosis | Mouse | VCAM-1 | [94,138] |
| Mouse | VCAM-1, P-selectin | [95] | ||
| Swine | VCAM-1, ICAM-1 | [139] | ||
| Primate | VCAM-1, P-selectin | [140] | ||
| Renal ischemia | Mouse | Leukocyte | [154] | |
| Myocardial ischemia | Mouse | P-selectin | [92,93] | |
| Mouse | P-selectin, E-selectin | [141] | ||
| Rat | E-selectin | [142] | ||
| Rat | P-selectin, E-selectin | [143] | ||
| Primate | P-selectin, E-selectin | [144] | ||
| Transplant rejection | Rat | ICAM-1 | [91] | |
| Bowel disease | Mouse | P-selectin | [145] | |
| Mouse | MAdCAM-1 | [45] | ||
| Mouse | P-selectin, E-selectin | [146] | ||
| Swine | P-selectin, E-selectin | [147] | ||
| Thrombosis | Acute thrombosis | Mouse | Glycoprotein IIb/IIIa | [151,161] |
| Rat | Glycoprotein IIb/IIIa | [150] | ||
5.2. Molecular imaging of Inflammation
For vascular endothelium undergoing inflammation, over-expression of selectins (e.g. P-selectin, E-selectin), vascular cell adhesion molecules-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) were identified as the molecular targets (Table 1). Inflammation response of atherosclerosis was detected using VCAM-1, ICAM-1, and P-selectin targeted microbubbles in a murine model [94,95,138], a swine model [139] and a primate model [140]. Myocardial ischemia was detected using P-selectin and/or E-selectin targeted microbubbles in a murine model [92,93,141], a rat model [142,143], and a primate model [144]. Additionally, inflammatory bowel disease was imaged using P-selectin and E-selectin targeted microbubbles in a murine model [145,146] and a swine model [147]. Dual-targeted microbubbles were utilized to further enhance targeting [95,139–141,143,144,146]. Potential clinical usefulness of acute and chronic inflammation cannot be underestimated, and expand well beyond traditional imaging of infection. As demonstrated in small and large animal models, inflammation of vasculature in Crohn’s disease or in the inflammatory bowel disease models can be monitored by targeted microbubbles directed towards MAdCAM −1 [45] or P- and E-selectin [145–147], respectively, avoiding the need for the frequent MRI or CT. Likewise, ischemic conditions, such as ischemia-reperfusion injury, may lead to the significant inflammatory response of vascular endothelium. Such a condition may manifest itself following myocardial infarction or in the acute kidney injury setting (where contrast ultrasound imaging of activated leukocytes on vascular endothelium with phosphatidylserine-carrying bubbles, or targeting P- or E-selectins directly, might potentially be able to detect this condition prior to the full scale injury development). Especially exciting are the efforts to detect molecular signatures of transient ischemic events hours after blood flow has restored, with so-called “ischemic memory” imaging agents. This might be especially useful for the emergency room and even in the ambulance scenarios, given the excellent portability of ultrasound imaging equipment.
5.3. Molecular imaging of thrombosis
Thrombotic complications, manifested as local deposition of activated platelets, fibrin, and tissue factor on vessel wall, are associated with the rupture or erosion of an atherosclerosis plaque [148]. It is highly desirable to rapidly and noninvasively detect and characterize acute thrombus via molecular imaging (Table 1). Thrombus formation has been imaged in animal models of disease with the molecular targets, including platelet adhesion molecules αIIbβ3 integrin, GPIbα, fibrin/fibrinogen, and tissue factor [149]. Molecular imaging of human thrombus was achieved in a rat model with microbubbles targeted to glycoprotein IIb/IIIa receptor [150]. Recently, molecular imaging of thrombosis in mouse model was demonstrated with glycoprotein IIb/IIIa-targeted microbubbles [151]. In this study, microbubbles were conjugated to the antibody via binding to a Ligand-Induced Binding Site (LIBS) [151]. Blood clot imaging might find clinical use in the assessment of stroke, monitoring of vulnerable plaque locations and status, thrombi that appear due to atrial fibrillation, as well as for the deep vein thrombosis assessment, although larger clots in the vessels should be clearly visible as negative contrast without molecularly targeted microbubbles. Uniqueness of molecular imaging might be with the ability to detect small thrombi and microthrombi that do not extend significantly from the vessel wall surface, or are located in the microvasculature.
6. Targeted microbubbles in clinical trials and in clinical usage
6.1. Microbubbles targeted by phosphatidylserine
Technically, there is one targeted bubble formulation already in widespread clinical usage, approved in Japan a decade ago: this perfluorobutane-containing microbubble is phosphatidylserine-based Sonazoid (formely NC100100), a microbubble that accumulates in the normal liver parenchyma via Kupffer cell uptake [152]. The lipid shell of this bubble consists of a single lipid, fully hydrogenated egg phosphatidylserine (hence egg allergy is listed as a contraindication). This lipid is an established marker of phagocytic uptake of cells and particles.
Sonazoid may be useful for periodic liver cancer monitoring in higher-risk population, in case when frequent checks by CT or MRI are not feasible. It may also be very useful as a tool for image-guided biopsies and targeted ablation procedures. In addition, phosphatidylserine-carrying bubbles will be taken up by the spleen macrophages (similar to the uptake of aged red blood cells [153]) and any other phagocytic cells that are exposed to blood. Such formulations have been in widespread preclinical animal studies since early this century [154], when they were also proposed as a tool for imaging of inflamed vasculature, via uptake by neutrophils that accumulate there. This agent may be useful for imaging of ischemia-reperfusion events e.g., in myocardium and kidney [155]. Recently, there was a clinical trial listed at clinicaltrials.gov (NCT03009266) that plans to investigate this contrast material as an ischemic memory agent in myocardial ischemia. There are two potential targeting mechanisms discussed, one being leukocyte uptake, and another, complement-mediated binding to endothelium [156]; longevity of ultrasound contrast signal in vivo may determine whether the former or latter mechanism is prevalent, because inside the cells microbubbles will deflate much slower.
6.2. VEGFR2-targeted microbubbles
BR55 microbubble is based on a perfluorobutane/nitrogen gas core, with a “solid” (DSPC) lipid monolayer shell, decorated with PEG brush and distally located heterodimeric peptide that possesses nanomolar affinity to VEGFR2.
This material has been investigated in a number of clinical trials, starting with an exploratory (Phase 0) trial in conjunction with radical prostathectomy, where ex-vivo immunohistology evaluation was compared with pre-surgery contrast ultrasound in 24 patients [9] (also listed as NCT01253213 clinical trial). The exploratory character of this study limited the amount of targeting peptide to < 0.1 mg per patient. This study was supplemented with additional clinical testing (including Phase 2) in prostate (listed as NCT02142608), as well as ovarian and breast cancer studies [8] and demonstrated successful targeting. As with Sonazoid, the usefulness of this contrast agent may extend beyond diagnostic imaging, to assist image-guided biopsy and ablation procedures.
7. Conclusion and future challenges.
Ultrasound possesses underappreciated qualities as a molecular imaging modality, although it is capable of single microbubble detection sensitivity. High signal specificity is challenging to achieve, due to the difficulties in separating the signal arising from a bound targeted microbubble from free flowing (non-adhered) microbubbles and tissue. A number of targeted imaging algorithms have proven successful; some operate in real-time, whereas earlier approaches involve “dwell” period. Targeted microbubble design (as a thin ligand-decorated shell encasing a perfluorocarbon gas core) has been discussed since two decades ago [35], and has finally progressed to the clinical trials stage, which look reasonably promising [8]. The pace of innovative design (microbubble formulation as well as ultrasound signal processing) remains vibrant, so further improvements may be anticipated. We may expect the appearance of generic blood pool microbubbles agents as microbubble formulation patents expire, so contrast ultrasound imaging becomes more accessible, as long as imaging equipment continues to provide and improve contrast-specific presets and pulse sequences. Based on this foundation, novel targeting ligands to the endothelial biomarkers of diseases, will drive diagnostic imaging innovation and patent exclusivity, to ensure interest in the investment into clinical translation, both in imaging, and in image-guided therapeutic interventions. Medical imaging is a highly competitive environment, where usage is often decided by the existence of already established protocols, which may be difficult to change, unless a clear clinical benefit is demonstrated. Another confounding factor is the lack of tradition of contrast agent administration during ultrasound exams (unlike in all other imaging modalities); this may be addressed if important clinical information will become available via contrast ultrasound approach. As ultrasound imaging equipment is the most widespread, portable (with hand-held units and USB probes plugged in laptops), inexpensive, and has an excellent safety profile, the use of ultrasound contrast will hopefully enable the widest worldwide use of molecular imaging of diseases, for diagnostic purposes and as a tool to aid the image-guided interventions.
Acknowledgements.
A.L. Klibanov is supported in part via NIH R01 EB023055, awarded by the National Institute Of Biomedical Imaging And Bioengineering of the National Institutes of Health. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References:
- [1].Weissleder R, Mahmood U. Molecular Imaging. Radiology 2001;219:316–333. [DOI] [PubMed] [Google Scholar]
- [2].Hong H, Yang Y, Liu B, et al. Imaging of Abdominal Aortic Aneurysm: the present and the future. Curr. Vasc. Pharmacol 2010;8:808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin. Radiol 2010;65:567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caskey CF, Hu X, Ferrara KW. Leveraging the power of ultrasound for therapeutic design and optimization. J. Control. Release 2011;156:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: Moving toward clinical translation. Eur. J. Radiol 2015;84:1685f–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gessner R, Dayton PA. Advances in molecular imaging with ultrasound. Mol. Imaging 2010;9:117–127. [PMC free article] [PubMed] [Google Scholar]
- [7].Wolf GL. Targeted Delivery of Imaging Agents: An Overview. In: Torchilin VP, editor. Handb. Target. Deliv. Imaging Agents Boca Raton: CRC Press; 1995. p. 3–22. [Google Scholar]
- [8].Willmann JK, Bonomo L, Carla Testa A, et al. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J. Clin. Oncol 2017;35:2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smeenge M, Tranquart F, Mannaerts CK, et al. First-in-Human Ultrasound Molecular Imaging With a VEGFR2-Specific Ultrasound Molecular Contrast Agent (BR55) in Prostate Cancer: A Safety and Feasibility Pilot Study. Invest. Radiol 2017;52:419–427. [DOI] [PubMed] [Google Scholar]
- [10].Oyama Y, Spencer M. Cardiopulmonary effects of intravenous gas embolism; with special reference to fate of intravascular gas bubbles. Jpn. Circ. J 1971;35:1541–1549. [DOI] [PubMed] [Google Scholar]
- [11].DEFINITY® (Perflutren Lipid Microsphere) Injectable Suspension. [Internet]. p. 1–18 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf.
- [12].Krupka TM, Solorio L, Wilson RE, et al. Formulation and characterization of echogenic lipid-Pluronic nanobubbles. Mol. Pharm 2010;7:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki R, Takizawa T, Negishi Y, et al. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control. Release 2007;117:130–136. [DOI] [PubMed] [Google Scholar]
- [14].Perera RH, Wu H, Peiris P, et al. Improving performance of nanoscale ultrasound contrast agents using N,N-diethylacrylamide stabilization. Nanomedicine 2017;13:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu C-H, Chen C, Irimia D, et al. Fast sorting of CD4+ T cells from whole blood using glass microbubbles. Technol. (Singap World Sci) 2015;3:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Senior R, Monaghan M, Main ML, et al. Detection of coronary artery disease with perfusion stress echocardiography using a novel ultrasound imaging agent: two Phase 3 international trials in comparison with radionuclide perfusion imaging. Eur. J. Echocardiogr 2009;10:26–35. [DOI] [PubMed] [Google Scholar]
- [17].Paefgen V, Doleschel D, Kiessling F. Evolution of contrast agents for ultrasound imaging and ultrasound-mediated drug delivery. Front. Pharmacol 2015;6:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tsao NH, Hall EAH. Enzyme-Degradable Hybrid Polymer/Silica Microbubbles as Ultrasound Contrast Agents. Langmuir 2016;32:6534–6543. [DOI] [PubMed] [Google Scholar]
- [19].Cohen JL, Cheirif J, Segar DS, et al. Improved left ventricular endocardial border delineation and opacification with OPTISON (FS069), a new echocardiographic contrast agent. Results of a phase III Multicenter Trial. J. Am. Coll. Cardiol 1998;32:746–752. [DOI] [PubMed] [Google Scholar]
- [20].Geny B, Bischoff P, Muan B, et al. Safety of a new transpulmonary echocontrast agent (Albunex) in repeated echocardiographic studies in patients. Clin. Cardiol 1997;20:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wible JH, Wojdyla JK, Bales GL, et al. Inhaled gases affect the ultrasound contrast produced by Albunex in anesthetized dogs. J. Am. Soc. Echocardiogr 1996;9:442–451. [DOI] [PubMed] [Google Scholar]
- [22].Geiser EA, Buss DD, Wible JHJ, et al. Evidence for a relation between inspired gas mixture and the left ventricular contrast achieved with Albunex in a canine model. Clin. Cardiol 1996;19:289–295. [DOI] [PubMed] [Google Scholar]
- [23].CENTER FOR DRUG EVALUATION AND RESEARCH APPROVAL PACKAGE FOR: APPLICATION NUMBER 21–191. [Internet]. p. 1–21 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-191_Imagent_Approv.pdf.
- [24].Lumason® (sulfur hexafluoride lipid-type A microspheres). [Internet] Available from: http://imaging.bracco.com/us-en/lumason.
- [25].Hvattum E, Uran S, Sandbæk AG, et al. Quantification of phosphatidylserine, phosphatidic acid and free fatty acids in an ultrasound contrast agent by normal-phase high-performance liquid chromatography with evaporative light scattering detection. J. Pharm. Biomed. Anal 2006;42:506–512. [DOI] [PubMed] [Google Scholar]
- [26].Pu G, Borden MA, Longo ML. Collapse and shedding transitions in binary lipid monolayers coating microbubbles. Langmuir 2006;22:2993–2999. [DOI] [PubMed] [Google Scholar]
- [27].Wu SY, Chen CC, Tung YS, et al. Effects of the microbubble shell physicochemical properties on ultrasound-mediated drug delivery to the brain. J. Control. Release 2015;212:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Jong N, Emmer M, van Wamel A, et al. Ultrasonic characterization of ultrasound contrast agents. Med. Biol. Eng. Comput 2009;47:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sirsi S, Feshitan J, Kwan J, et al. Effect of Microbubble Size on Fundamental Mode High Frequency Ultrasound Imaging in Mice. Ultrasound Med. Biol 2010;36:935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dayton PA, Morgan KE, Klibanov AL, et al. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999;46:220–232. [DOI] [PubMed] [Google Scholar]
- [31].de Jong N, Emmer M, Chin CT, et al. “Compression-only” behavior of phospholipid-coated contrast bubbles. Ultrasound Med. Biol 2007;33:653–656. [DOI] [PubMed] [Google Scholar]
- [32].Chitnis PV, Koppolu S, Mamou J, et al. Influence of Shell Properties on High-Frequency Ultrasound Imaging and Drug Delivery Using Polymer-Shelled Microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013;60:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chitnis PV, Lee P, Mamou J, et al. Rupture threshold characterization of polymer-shelled ultrasound contrast agents subjected to static overpressure. J. Appl. Phys 2011;109:84906–8490610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 2006;66:6732–6740. [DOI] [PubMed] [Google Scholar]
- [35].Klibanov AL, Hughes MS, Marsh JN, et al. Targeting of ultrasound contrast material. An in vitro feasibility study. Acta Radiol. Suppl 1997;412:113–120. [PubMed] [Google Scholar]
- [36].Kim DH, Klibanov AL, Needham D. The Influence of Tiered Layers of Surface-Grafted Poly(ethylene glycol) on Receptor−Ligand-Mediated Adhesion between Phospholipid Monolayer-Stabilized Microbubbles and Coated Glass Beads. Langmuir [Internet] 2000;16:2808–2817. Available from: 10.1021/la990749r. [DOI] [Google Scholar]
- [37].Fisher NG, Christiansen JP, Klibanov A, et al. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J. Am. Coll. Cardiol 2002;40:811–819. [DOI] [PubMed] [Google Scholar]
- [38].Chen CC, Borden MA. The role of poly(ethylene glycol) brush architecture in complement activation on targeted microbubble surfaces. Biomaterials 2011;32:6579–6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schneider M, Anantharam B, Arditi M, et al. BR38, a new ultrasound blood pool agent. Invest. Radiol 2011;46:486–494. [DOI] [PubMed] [Google Scholar]
- [40].Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest. Radiol 2010;45:89–95. [DOI] [PubMed] [Google Scholar]
- [41].Ottoboni S, Short RE, Kerby MB, et al. Characterization of the in vitro adherence behavior of ultrasound responsive double-shelled microspheres targeted to cellular adhesion molecules. Contrast Media Mol. Imaging 2006;1:279–290. [DOI] [PubMed] [Google Scholar]
- [42].Fokong S, Theek B, Wu Z, et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J. Control. Release 2012;163:75–81. [DOI] [PubMed] [Google Scholar]
- [43].Duncanson WJ, Figa MA, Hallock K, et al. Targeted binding of PLA microparticles with lipid-PEG-tethered ligands. Biomaterials 2007;28:4991–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Duncanson WJ, Oum K, Eisenbrey JR, et al. Targeted binding of PEG-lipid modified polymer ultrasound contrast agents with tiered surface architecture. Biotechnol. Bioeng 2010;106:501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bachmann C, Klibanov AL, Olson TS, et al. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 2006;130:8–16. [DOI] [PubMed] [Google Scholar]
- [46].Klibanov AL, Hughes MS, Villanueva FS, et al. Targeting and ultrasound imaging of microbubble-based contrast agents. Magn. Reson. Mater. Physics, Biol. Med 1999;8:177–184. [DOI] [PubMed] [Google Scholar]
- [47].Long DS, Smith ML, Pries AR, et al. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. Proc. Natl. Acad. Sci. U. S. A 2004;101:10060–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Takalkar AM, Klibanov AL, Rychak JJ, et al. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J. Control. Release 2004;96:473–482. [DOI] [PubMed] [Google Scholar]
- [49].Rychak JJ, Li B, Acton ST, et al. Selectin ligands promote ultrasound contrast agent adhesion under shear flow. Mol. Pharm 2006;3:516–524. [DOI] [PubMed] [Google Scholar]
- [50].Weller GER, Villanueva FS, Tom EM, et al. Targeted ultrasound contrast agents: in vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol. Bioeng 2005;92:780–788. [DOI] [PubMed] [Google Scholar]
- [51].Klibanov AL, Rychak JJ, Yang WC, et al. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol. Imaging 2006;1:259–266. [DOI] [PubMed] [Google Scholar]
- [52].Guenther F, Von Zur Muhlen C, Ferrante EA, et al. An ultrasound contrast agent targeted to P-selectin detects activated platelets at supra-arterial shear flow conditions. Invest. Radiol 2010;45:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ferrante EA, Pickard JE, Rychak J, et al. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J. Control. Release 2009;140:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Warram JM, Sorace AG, Saini R, et al. A triple-targeted ultrasound contrast agent provides improved localization to tumor vasculature. J. Ultrasound Med 2011;30:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tsutsui JM, Xie F, Cano M, et al. Detection of retained microbubbles in carotid arteries with real-time low mechanical index imaging in the setting of endothelial dysfunction. J. Am. Coll. Cardiol 2004;44:1036–1046. [DOI] [PubMed] [Google Scholar]
- [56].Lee RJ, Low PS. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim. Biophys. Acta 1995;1233:134–144. [DOI] [PubMed] [Google Scholar]
- [57].Jeppesen C, Jeppesen C 1, Wong JY et al. Impact of Polymer Tether Length on Multiple Ligand-Receptor Bond Formation. Science 2001;293:465–468. [DOI] [PubMed] [Google Scholar]
- [58].Ham AS, Klibanov AL, Lawrence MB. Action at a distance: lengthening adhesion bonds with poly(ethylene glycol) spacers enhances mechanically stressed affinity for improved vascular targeting of microparticles. Langmuir 2009;25:10038–10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Park EYH, Smith MJ, Stropp ES, et al. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters. Biophys. J 2002;82:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim DH, Costello MJ, Duncan PB, et al. Mechanical Properties and Microstructure of Polycrystalline Phospholipid Monolayer Shells: Novel Solid Microparticles. Langmuir [Internet] 2003;19:8455–8466. Available from: 10.1021/la034779c. [DOI] [Google Scholar]
- [61].Rychak JJ, Lindner JR, Ley K, et al. Deformable gas-filled microbubbles targeted to P-selectin. J. Control. Release 2006;114:288–299. [DOI] [PubMed] [Google Scholar]
- [62].Klibanov AL. Ultrasound molecular imaging with targeted microbubble contrast agents. J. Nucl. Cardiol 2007;14:876–884. [DOI] [PubMed] [Google Scholar]
- [63].De Jong N, Hoff L, Skotland T, et al. Absorption and scatter of encapsulated gas filled microspheres: theoretical considerations and some measurements. Ultrasonics 1992;30:95–103. [DOI] [PubMed] [Google Scholar]
- [64].Klibanov AL, Rasche PT, Hughes MS, et al. Detection of individual microbubbles of ultrasound contrast agents: imaging of free-floating and targeted bubbles. Invest. Radiol 2004;39:187–195. [DOI] [PubMed] [Google Scholar]
- [65].Leighton TG. The Acoustic Bubble. J. Acoust. Soc. Am 1994.
- [66].Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonics 2000;38:93–98. [DOI] [PubMed] [Google Scholar]
- [67].de Jong N, Frinking PJ., Bouakaz A, et al. Detection procedures of ultrasound contrast agents. Ultrasonics 2000;38:87–92. [DOI] [PubMed] [Google Scholar]
- [68].de Jong N, Cornet R, Lancée CT. Higher harmonics of vibrating gas-filled microspheres. Part two: measurements. Ultrasonics 1994;32:455–459. [Google Scholar]
- [69].Bouakaz A, Frigstad S, Ten Cate FJ, et al. Super harmonic imaging: A new imaging technique for improved contrast detection. Ultrasound Med. Biol 2002;28:59–68. [DOI] [PubMed] [Google Scholar]
- [70].Burns PN, Powers JE, Simpson DH, et al. Harmonic power mode Doppler using microbubble contrast agents: an improved method for small vessel flow imaging. Proc. IEEE Ultrason. Symp 1994;3:1547–1550. [Google Scholar]
- [71].Chang PH, Shung KK, Wu SJ, et al. Second harmonic imaging and harmonic Doppler measurements with Albunex. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995;42:1020–1027. [Google Scholar]
- [72].Shen C-C, Chou Y-H, Li P-C. Pulse inversion techniques in ultrasonic nonlinear imaging. J. Med. Ultrasound 2005;13:3–17. [Google Scholar]
- [73].Simpson DH, Chin CT, Burns PN. Pulse inversion Doppler: a new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999;46:372–382. [DOI] [PubMed] [Google Scholar]
- [74].Phillips PJ. Contrast pulse sequences (CPS): imaging nonlinear microbubbles. IEEE Int. Ultrason. Symp 2001;1739–1745.
- [75].Phillips P, Gardner E. Contrast-agent detection and quantification. Eur. Radiol 2004;14:4–10. [PubMed] [Google Scholar]
- [76].Eckersley RJ, Tang M-X, Chetty K, et al. Microbubble contrast agent detection using binary coded pulses. Ultrasound Med. Biol 2007;33:1787–1795. [DOI] [PubMed] [Google Scholar]
- [77].Viti J, Vos H, De Jong N, et al. Detection of Contrast Agents: Plane Wave vs Focused Transmission. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015;3010:1–1. [DOI] [PubMed] [Google Scholar]
- [78].Mauldin FW, Dhanaliwala AH, Patil AV, et al. Real-time targeted molecular imaging using singular value spectra properties to isolate the adherent microbubble signal. Phys. Med. Biol 2012;57:5275–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jensen JA, Holm O, Jensen LJ, et al. Ultrasound research scanner for real-time synthetic aperture data acquisition. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005;52:881–891. [DOI] [PubMed] [Google Scholar]
- [80].Montaldo G, Tanter M, Bercoff J, et al. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009;56:489–506. [DOI] [PubMed] [Google Scholar]
- [81].Tanter M, Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014;61:102–119. [DOI] [PubMed] [Google Scholar]
- [82].Tiran E, Deffieux T, Correia M, et al. Multiplane wave imaging increases signal-to-noise ratio in ultrafast ultrasound imaging. Phys. Med. Biol 2015;60:8549–8566. [DOI] [PubMed] [Google Scholar]
- [83].Papadacci C, Pernot M, Couade M, et al. High-contrast ultrafast imaging of the heart. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014;61:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Couture O, Bannouf S, Montaldo G, et al. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol 2009;35:1908–1916. [DOI] [PubMed] [Google Scholar]
- [85].Lindner JR, Song J, Christiansen J, et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001;104:2107–2112. [DOI] [PubMed] [Google Scholar]
- [86].Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473–483. [DOI] [PubMed] [Google Scholar]
- [87].Lee DJ, Lyshchik A, Huamani J. Relationship between retention of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted ultrasonographic contrast agent and the level of VEGFR2 expression in an in vivo breast cancer model. J. Ultrasound Med 2008;2:855–866. [DOI] [PubMed] [Google Scholar]
- [88].Willmann JK, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology 2008;246:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bachawal SV, Jensen KC, Lutz AM, et al. Earlier detection of breast cancer with ultrasound molecular imaging in a transgenic mouse model. Cancer Res 2013;73:1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pysz MA, Machtaler SB, Seeley ES, et al. Vascular endothelial growth factor receptor type 2-targeted contrast-enhanced US of pancreatic cancer neovasculature in a genetically engineered mouse model: potential for earlier detection. Radiology 2015;274:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation 2003;108:218–224. [DOI] [PubMed] [Google Scholar]
- [92].Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation 2007;115:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kaufmann BA, Lewis C, Xie A, et al. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur. Heart J 2007;28:2011–2017. [DOI] [PubMed] [Google Scholar]
- [94].Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 2007;116:276–284. [DOI] [PubMed] [Google Scholar]
- [95].Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler. Thromb. Vasc. Biol 2010;30:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Miller DL, Dou C, Wiggins RC. Frequency Dependence of Kidney Injury Induced by Contrast-Aided Diagnostic Ultrasound in Rats. Ultrasound Med. Biol 2008;34:1678–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wang DS, Panje C, Pysz MA, et al. Cationic versus Neutral Microbubbles for Ultrasound-mediated Gene Delivery in Cancer. Radiology 2012;264:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Panje CM, Wang DS, Willmann JK. Ultrasound and microbubble-mediated gene delivery in cancer: progress and perspectives. Invest. Radiol 2013;48:755–769. [DOI] [PubMed] [Google Scholar]
- [99].Zhao S, Kruse DE, Ferrara KW, et al. Selective imaging of adherent targeted ultrasound contrast agents. Phys. Med. Biol 2007;52:2055–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Needles A, Couture O, Foster FS. A method for differentiating targeted microbubbles in real time using subharmonic micro-ultrasound and interframe filtering. Ultrasound Med. Biol 2009;35:1564–1573. [DOI] [PubMed] [Google Scholar]
- [101].Hu X, Zheng H, Kruse DE, et al. A sensitive TLRH targeted imaging technique for ultrasonic molecular imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010;57:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hu X, Caskey CF, Mahakian LM, et al. In vivo validation and 3D visualization of broadband ultrasound molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2013;3:336–349. [PMC free article] [PubMed] [Google Scholar]
- [103].Patil AV, Rychak JJ, Allen JS, et al. Dual frequency method for simultaneous translation and real-time imaging of ultrasound contrast agents within large blood vessels. Ultrasound Med. Biol 2009;35:2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Patil AV, Rychak JJ, Klibanov AL, et al. Real-time technique for improving molecular imaging and guiding drug delivery in large blood vessels: in vitro and ex vivo results. Mol. Imaging 2011;10:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Pysz MA, Guracar I, Tian L, et al. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant. Imaging Med. Surg 2012;2:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wang S, Mauldin FW Jr, Hossack JA. Decorrelation-based adherent microbubble identification as a faster alternative to singular spectrum-based targeted molecular (SiSTM) imaging of large blood vessels. IEEE Int. Ultrason. Symp 2013;1829–1832.
- [107].Dayton P, Klibanov A, Brandenburger G, et al. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med. Biol 1999;25:1195–1201. [DOI] [PubMed] [Google Scholar]
- [108].Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J. Acoust. Soc. Am 2002;112:2183–2192. [DOI] [PubMed] [Google Scholar]
- [109].Rychak JJ, Klibanov AL, Hossack JA. Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles: in vitro verification. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005;52:421–433. [DOI] [PubMed] [Google Scholar]
- [110].Rychak JJ, Klibanov AL, Ley KF, et al. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound Med. Biol 2007;33:1132–1139. [DOI] [PubMed] [Google Scholar]
- [111].Zhao S, Borden M, Bloch SH, et al. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol. Imaging 2004;3:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Borden MA, Sarantos MR, Stieger SM, et al. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol. Imaging 2006;5:139–147. [PMC free article] [PubMed] [Google Scholar]
- [113].Gessner RC, Streeter JE, Kothadia R, et al. An in vivo validation of the application of acoustic radiation force to enhance the diagnostic utility of molecular imaging using 3-d ultrasound. Ultrasound Med. Biol 2012;38:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Borden MA, Streeter JE, Sirsi SR, et al. In vivo demonstration of cancer molecular imaging with ultrasound radiation force and buried-ligand microbubbles. Mol. Imaging 2013;12:1–8. [PMC free article] [PubMed] [Google Scholar]
- [115].Wang S, Hossack JA, Klibanov AL, et al. Binding dynamics of targeted microbubbles in response to modulated acoustic radiation force. Phys. Med. Biol 2014;59:465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wang S, Mauldin FW, Klibanov AL, et al. Ultrasound-based measurement of molecular marker concentration in large blood vessels: a feasibility study. Ultrasound Med. Biol 2015;41:222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wang S, Wang CY, Unnikrishnan S, et al. Optical verification of microbubble response to acoustic radiation force in large vessels with in vivo results. Invest. Radiol 2015;50:772–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wang S, Unnikrishnan S, Herbst EB, et al. Ultrasound molecular imaging with modulated acoustic radiation force-based beam sequence in mouse abdominal aorta : a feasibility study. IEEE Int. Ultrason. Symp 2015; Access# 15601588
- [119].Wang L, Li L, Guo Y, et al. Construction and in vitro/in vivo targeting of PSMA-targeted nanoscale microbubbles in prostate cancer. Prostate 2013;73:1147–1158. [DOI] [PubMed] [Google Scholar]
- [120].Tsuruta JK, Klauber-DeMore N, Streeter J, et al. Ultrasound molecular imaging of secreted frizzled related protein-2 expression in murine angiosarcoma. PLoS One 2014;9:e86642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Foygel K, Wang H, MacHtaler S, et al. Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology 2013;145:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lutz AM, Bachawal SV, Drescher CW, et al. Ultrasound molecular imaging in a human CD276 expression-modulated murine ovarian cancer model. Clin. Cancer Res 2014;20:1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Korpanty G, Carbon JG, Grayburn PA, et al. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res 2007;13:323–330. [DOI] [PubMed] [Google Scholar]
- [124].Willmann JK, Lutz AM, Paulmurugan R, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008;248:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Deshpande N, Ren Y, Foygel K, et al. Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 2011;258:804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Leguerney I, Scoazec J-Y, Gadot N, et al. Molecular ultrasound imaging using contrast agents targeting endoglin, vascular endothelial growth factor receptor 2 and integrin. Ultrasound Med. Biol 2015;41:197–207. [DOI] [PubMed] [Google Scholar]
- [127].Sorace AG, Saini R, Mahoney M, et al. Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J. Ultrasound Med 2012;31:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Anderson CR, Hu X, Zhang H, et al. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Invest. Radiol 2011;46:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Anderson CR, Rychak JJ, Backer M, et al. scVEGF microbubble ultrasound contrast agents: a novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest. Radiol 2010;45:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bzyl J, Lederle W, Rix A, et al. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur. Radiol 2011;21:1988–1995. [DOI] [PubMed] [Google Scholar]
- [131].Wang H, Kaneko OF, Tian L, et al. Three-dimensional ultrasound molecular imaging of angiogenesis in colon cancer using a clinical matrix array ultrasound transducer. Invest. Radiol 2015;50:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sugimoto K, Moriyasu F, Negishi Y, et al. Quantification in molecular ultrasound imaging: a comparative study in mice between healthy liver and a human hepatocellular carcinoma xenograft. J. Ultrasound Med 2012;31:1909–1916. [DOI] [PubMed] [Google Scholar]
- [133].Wei S, Fu N, Sun Y, et al. Targeted contrast-enhanced ultrasound imaging of angiogenesis in an orthotopic mouse tumor model of renal carcinoma. Ultrasound Med. Biol 2014;40:1250–1259. [DOI] [PubMed] [Google Scholar]
- [134].Tardy I, Pochon S, Theraulaz M, et al. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Invest. Radiol 2010;45:573–578. [DOI] [PubMed] [Google Scholar]
- [135].Frinking PJA, Tardy I, Theraulaz M, et al. Effects of acoustic radiation force on the binding efficiency of BR55, a VEGFR2-specific ultrasound contrast agent. Ultrasound Med. Biol 2012;38:1460–1469. [DOI] [PubMed] [Google Scholar]
- [136].Ellegala DB, Leong-Poi H, Carpenter JE, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003;108:336–341. [DOI] [PubMed] [Google Scholar]
- [137].Spivak I, Rix A, Schmitz G, et al. Low-dose molecular ultrasound imaging with E-selectin-targeted PBCA microbubbles. Mol. Imaging Biol 2015;18:180–190. [DOI] [PubMed] [Google Scholar]
- [138].Khanicheh E, Qi Y, Xie A, et al. Molecular imaging reveals rapid reduction of endothelial activation in early atherosclerosis with apocynin independent of antioxidative properties. Arterioscler. Thromb. Vasc. Biol 2013;33:2187–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Hamilton AJ, Huang S-L, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J. Am. Coll. Cardiol 2004;43:453–460. [DOI] [PubMed] [Google Scholar]
- [140].Chadderdon SM, Belcik JT, Bader L, et al. Proinflammatory endothelial activation detected by molecular imaging in obese nonhuman primates coincides with onset of insulin resistance and progressively increases with duration of insulin resistance. Circulation 2014;129:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Davidson BP, Kaufmann B a, Belcik JT, et al. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J. Am. Coll. Cardiol 2012;60:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Leng X, Wang J, Carson A, et al. Ultrasound detection of myocardial ischemic memory using an e-selectin targeting Peptide amenable to human application. Mol. Imaging 2014;16:1–9. [PMC free article] [PubMed] [Google Scholar]
- [143].Hyvelin J-M, Tardy I, Bettinger T, et al. Ultrasound molecular imaging of transient acute myocardial ischemia with a clinically translatable P- and E-selectin targeted contrast agent: correlation with the expression of selectins. Invest. Radiol 2014;49:224–235. [DOI] [PubMed] [Google Scholar]
- [144].Davidson BP, Chadderdon SM, Belcik JT, et al. Ischemic memory imaging in nonhuman primates with echocardiographic molecular imaging of selectin expression. J. Am. Soc. Echocardiogr 2014;27:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Deshpande N, Lutz AM, Ren Y, et al. Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology 2011;262:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Wang H, Machtaler S, Bettinger T, et al. Molecular imaging of inflammation in inflammatory bowel disease with a clinically translatable dual-selectin-targeted US contrast agent: comparison with FDG PET/CT in a mouse model. Radiology 2013;267:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wang H, Felt SA, Machtaler S, et al. Quantitative Assessment of Inflammation in a Porcine Acute Terminal Ileitis Model: US with a Molecularly Targeted Contrast Agent. Radiology 2015;276:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat. Rev. Drug Discov 2004;3:913–925. [DOI] [PubMed] [Google Scholar]
- [149].Lindner JR. Molecular imaging of thrombus: technology in evolution. Circulation 2012;125:3057–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 2007;38:1508–1514. [DOI] [PubMed] [Google Scholar]
- [151].Wang X, Hagemeyer CE, Hohmann JD, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation 2012;125:3117–3126. [DOI] [PubMed] [Google Scholar]
- [152].Forsberg F, Liu JB, Merton DA, et al. Gray scale second harmonic imaging of acoustic emission signals improves detection of liver tumors in rabbits. J. Ultrasound Med 2000;19:557–563. [DOI] [PubMed] [Google Scholar]
- [153].Allen TM, Williamson P, Schlegel RA. Phosphatidylserine as a determinant of reticuloendothelial recognition of liposome models of the erythrocyte surface. Proc. Natl. Acad. Sci. U. S. A 1988;85:8067–8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 2000;102:2745–2750. [DOI] [PubMed] [Google Scholar]
- [155].Christiansen JP, Leong-Poi H, Klibanov AL, et al. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation 2002;105:1764–1767. [DOI] [PubMed] [Google Scholar]
- [156].Mott B, Packwood W, Xie A, et al. Echocardiographic Ischemic Memory Imaging Through Complement-Mediated Vascular Adhesion of Phosphatidylserine-Containing Microbubbles. JACC Cardiovasc. Imaging 2016;9:937–946. [DOI] [PubMed] [Google Scholar]
- [157].Barua A, Yellapa A, Bahr JM, et al. Enhancement of ovarian tumor detection with alphavbeta3 integrin-targeted ultrasound molecular imaging agent in laying hens: a preclinical model of spontaneous ovarian cancer. Int. J. Gynecol. Cancer 2014;24:19–28. [DOI] [PubMed] [Google Scholar]
- [158].Willmann JK, Kimura RH, Deshpande N, et al. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J. Nucl. Med 2010;51:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Jun HY, Park SH, Kim HS, et al. Long residence time of ultrasound microbubbles targeted to integrin in murine tumor model. Acad. Radiol 2010;17:54–60. [DOI] [PubMed] [Google Scholar]
- [160].Leong-Poi H, Christiansen J, Klibanov AL, et al. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation 2003;107:455–460. [DOI] [PubMed] [Google Scholar]
- [161].Schumann PA, Christiansen JP, Quigley RM, et al. Targeted-microbubble binding selectively to GPIIb IIIa receptors of platelet thrombi. Invest. Radiol 2002;37:587–593. [DOI] [PubMed] [Google Scholar]
- [162].Steinl D, Kaufmann B. Ultrasound Imaging for Risk Assessment in Atherosclerosis. Int. J. Mol. Sci 2015;16:9749–9769. [DOI] [PMC free article] [PubMed] [Google Scholar]


