Abstract
Purpose
Given the diverse and aggressive nature of soft tissue sarcomas (STSs), a need exists for more-precise therapy. Patient-derived orthotopic xenografts (PDOXs) provide a unique platform for personalized treatment. Thus, identification of patient and treatment factors that predict PDOX establishment is important. This study assessed the feasibility of incorporating PDOXs into the clinical setting and identifying factors associated with PDOX establishment.
Patients and Methods
From May 2015 to May 2016, 107 patients with biopsy-proven or potential STS were enrolled. Tumor samples were obtained intraoperatively and orthotopically implanted into nude mice in the corresponding anatomic location. PDOXs were considered established after engraftment and serial passage. Factors associated with establishment were analyzed by logistic regression and time to establishment by time-to-event analysis.
Results
Only high-grade tumors established (32 of 72 [44.4%]). The establishment rate (ER) varied by neoadjuvant therapy and treatment response, with the highest ER among untreated high-grade tumors (26 of 42 [61.9%]). Tumors exposed to radiation preoperatively did not establish (zero of 11 [0%]), and tumors exposed to neoadjuvant chemotherapy had a lower ER(31.9%) than untreated tumors. Only STSs with minimal pathologic response to neoadjuvant treatment (≤ 30%) established a PDOX (six of 18 [33.3%]). Median establishment time was 54 days, which varied by neoadjuvant therapy but was not statistically significant (P = .180).
Conclusion
To our knowledge, in the largest STS PDOX study to date, we demonstrate a 62% ER among untreated high-grade tumors with a median establishment time of 54 days. Neoadjuvant therapy, particularly radiation, and pathologic response to treatment were associated with a reduced rate of PDOX establishment.
INTRODUCTION
Over the past decade, there has been an evolution toward a personalized approach to the treatment of cancer in which individual patient and tumor characteristics are used to guide therapy. Recent research in sarcoma has focused on characterizing the histologic subtypes and genetic mutations that drive tumorigenesis to inform such targeted treatments. Although this work has elucidated the vast histologic and genetic diversity of sarcomas, limited progress has been made in the development of effective targeted therapies that have been successfully translated into the clinical setting.
Although approximately one third of sarcomas harbor specific translocations or mutations that may be amenable to targeted therapies, two thirds show a complex karyotype with multiple rearrangements, duplications, or deletions.1 This genetic variation has hindered the development of effective targeted therapies and has led researchers to direct interventions at specific pathologic sub-types. Even among these studies, the use of various combinations of chemotherapeutic agents has yielded only modest improvements in both response rates and disease-free survival. In general, regardless of the combination, soft tissue sarcomas (STSs) have a relatively low response rate to systemic therapy (20% to 30%). Although responders tend to have significantly improved outcomes, nonresponders endure the toxic adverse effects of treatment without survival benefit.2,3
Collectively, the rarity, histologic diversity, high risk of metastasis, and poor response rates to systemic therapy highlight the rationale and immediate need for more personalized sarcoma therapy. Patient-derived xenografts (PDXs), which are tumor and patient specific, provide an ideal platform for personalized care in sarcoma. PDXs maintain genetic similarity with and mimic the therapeutic responses of a patient’s tumor.4 Within STSs, multiple subcutaneous PDX models have been developed and have shown promise for replicating the tumor histology, clinical chemo-sensitivity, growth kinetics, and local disease progression of a patient’s tumor.5–8
Patient-derived orthotopic xenograft (PDOX) models in which the patient-derived tissue is implanted into the corresponding anatomic location by a technique called surgical orthotopic implantation (SOI) have also been shown to produce patterns of invasion and metastatic spread that have not been demonstrated in the subcutaneous PDX models.8–15 As well, PDOX models have been shown to faithfully reproduce the histology of the human tumor16,17 and to have unique clinical applicability. In particular, PDOX models have been shown to produce tumor response and resistance that mirror that of the patient.18 Smith et al19 demonstrated that xenograftability of liposarcomas correlate directly with patient outcomes. By building upon the results of these previous studies, PDOX models seem to present the best opportunity to test multiple potentially active systemic agents (chemotherapy, targeted therapy, etc) in a preclinical model, which shields patients from the potential toxicity of inactive drugs and identifies effective therapies that improve outcomes. Therefore, given the overall promise of PDOX in sarcomas, we assessed the feasibility of generating individual PDOX models in the clinical setting and determined factors associated with successful development of xeno-grafts among patients with STSs.
PATIENTS AND METHODS
This study was reviewed and approved by the University of California, Los Angeles, institutional review board. From May 2015 to May 2016, patients who underwent resection of biopsy-proven or potential STS were offered enrollment in this trial. All patients elected to enroll and were consented preoperatively.
Mice
Athymic nu/nu female nude mice (AntiCancer, San Diego, CA) 4 to 6 weeks old were used to xenograft human tissues. All animal studies were conducted with an AntiCancer institutional animal care and use committee protocol specifically approved for this study and in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under assurance number A3873–1.8,18 All surgical experiments were done under anesthesia and with analgesia to minimize suffering. Anesthesia was administered by subcutaneous injection of a 0.02-mL solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate. During surgery, animal response was monitored to ensure adequate depth of anesthesia. Animals were observed daily and humanely killed by CO2 inhalation when they met the humane end point criteria: severe tumor burden (>20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion, or body temperature drop. All animals were fed an autoclaved laboratory rodent diet and housed in a barrier facility on a high-efficiency particulate arrestance–filtered rack under standard conditions of 12-hour light/dark cycles. These procedures are similar to the standard of care at our facility and have been previously documented.8,18
Tumor Acquisition and Xenograft Establishment
Intraoperatively at the time of tumor resection, a tissue sample was obtained by the operating surgeon (F.C.E.) and transported to AntiCancer-PDOX on ice for immediate SOI in nude mice. Each tumor sample was divided into 5-mm fragments. Tumor fragments were then implanted both subcutaneously and orthotopically into nude mice using a well-validated protocol.8,15,18,20–24 SOI procedures were then followed for orthotopic models specific to each tumor site.8,11,15,18,20–27 For extremity tumors, 10-mm skin incisions were made in the corresponding anatomic site, and then a single tumor fragment was placed orthotopically between muscle layers. For intra-abdominal or retroperitoneal tumors, a 10-mm median or lateral abdominal incision was made to place a tumor fragment orthotopically. Wounds were closed with 6–0 nylon suture (Ethilon; Ethicon, Bridgewater, NJ).
Nude mice were monitored for tumor growth for up to 6 months after implantation. Growth monitoring was carried out by caliper measurement. PDOX that had no growth by 6 months were considered failures. Once a PDOX had reached 500 mm3, it was considered engrafted. Engrafted tumors were then serially passaged. Successfully passaged PDOXs were considered established. In every PDOX, the histopathology of the established tumors was compared with the original patient’s tumor and verified for concordance by a specialized sarcoma pathologist (S.M.D.); this method for histopathologic validation has been previously published by our group.8,15,18,20,23,24,26,28 Established PDOXs were maintained for future study of potential systemic agents.
Statistical Methods
For all patients enrolled in the trial, demographics and clinical data were prospectively collected, and xenograft data (time to establishment) were obtained in collaboration with AntiCancer. Histo-logic subtypes were determined for all tumors by a specialized sarcoma pathologist. Subtypes with > 10 tumors were included as separate covariates; all other subtypes were classified as other. Tumors were classified by presentation as either primary or metastatic/recurrent and by location as extremity or abdominal/retroperitoneal. Neoadjuvant treatment information was collected for all patients. Only therapy that occurred in the neoadjuvant setting before tumor excision was included in this analysis because the tumor sample used for xeno-graft implantation was only exposed to therapy that occurred before resection. Radiation therapy was only considered to occur during the neoadjuvant period if it had occurred within 10 weeks before tumor excision. Tumors exposed to neoadjuvant therapy were graded by their pathologic response on the basis of the percent response indicated by a specialized sarcoma pathologist.
Given that xenografts were established only from high-grade tumors, factors associated with establishment and time to establishment were analyzed among the high-grade cohort. The rate of establishment, which indicated that the tumor was successfully engrafted and serially passaged, was measured in days from the time of original engraftment to the time adequate growth was achieved from serial passage. Factors associated with establishment (also referred to as xenograft-ability) were analyzed by univariable logistic regression. Factors associated with establishment time were analyzed with survival functions using log-rank tests for equality and nonparametric methods for distribution.
RESULTS
Study Population
All 107 patients approached for study enrollment elected to enroll. The clinicopathologic characteristics of these patients are listed in Appendix Table A1 (online only). Similar numbers of male and female patients were enrolled (48% female). Median patient age was 61 years (range, 16 to 91 years), and median tumor size was 7.6 cm (range, 0.9 to 38 cm). Tumor location was evenly distributed (42% extremity, 58% abdominal/retroperitoneal). The majority of tumors were high grade (67%), and the most common subtypes were liposarcoma (25%) and leiomyosarcoma (14%). Tumors spanned 28 histologic subtypes (Appendix Table A2, online only). Given that only high-grade tumors were established, the high-grade subpopulation is the focus of the remaining analysis.
High-Grade Cohort
PDOX development was attempted for 72 patients with high-grade STSs. Patient and tumor characteristics for the high-grade cohort are listed in Table 1. For patients who underwent neoadjuvant therapy, the median time between completion of neoadjuvant therapy and tumor resection was 35 days (range, 9 to 74 days).
Table 1.
Patient and Tumor Characteristics for All High-Grade Tumors and by Xenograft Success
| High Grade Only (n = 72) | Successful Xenografts Only (n = 32) | Unsuccessful Xenografts Only (n = 40) | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Sex | ||||||
| Female | 34 | 47.2 | 16 | 50.0 | 18 | 45.0 |
| Male | 38 | 52.8 | 16 | 50.0 | 22 | 55.0 |
| Grade | ||||||
| Low | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| High | 72 | 100.0 | 32 | 100.0 | 40 | 100.0 |
| Presentation | ||||||
| Primary | 44 | 61.1 | 20 | 62.5 | 24 | 60.0 |
| Recurrent or metastatic | 28 | 38.9 | 12 | 37.5 | 16 | 40.0 |
| Location | ||||||
| Abdomen/retroperitoneum | 41 | 56.9 | 15 | 46.9 | 26 | 65.0 |
| Extremity | 31 | 43.1 | 17 | 53.1 | 14 | 35.0 |
| Size, cm | ||||||
| < 10 | 48 | 66.7 | 22 | 68.8 | 26 | 65.0 |
| > 10 | 24 | 33.3 | 10 | 31.3 | 14 | 35.0 |
| Subtype | ||||||
| Leiomyosarcoma | 15 | 20.8 | 8 | 25.0 | 7 | 17.5 |
| Liposarcoma | 13 | 18.1 | 8 | 25.0 | 5 | 12.5 |
| NOS/spindle cell/UPS | 15 | 20.8 | 7 | 21.9 | 8 | 20.0 |
| Other | 29 | 40.3 | 9 | 28.1 | 20 | 50.0 |
| Neoadjuvant CT (any) | ||||||
| Yes | 24 | 33.3 | 6 | 18.8 | 18 | 45.0 |
| No | 48 | 66.7 | 26 | 81.3 | 22 | 55.0 |
| Neoadjuvant XRT (any) | ||||||
| Yes | 11 | 15.3 | 0 | 0.0 | 11 | 27.5 |
| No | 61 | 84.7 | 32 | 100.0 | 29 | 72.5 |
| Neoadjuvant therapy | ||||||
| None | 42 | 58.3 | 26 | 81.3 | 16 | 40.0 |
| CT alone | 19 | 26.4 | 6 | 18.8 | 13 | 32.5 |
| XRT alone | 6 | 8.3 | 0 | 0.0 | 6 | 15.0 |
| CT + XRT | 5 | 6.9 | 0 | 0.0 | 5 | 12.5 |
| Xenograftability | ||||||
| Success | 32 | 44.4 | 32 | 100.0 | 0 | 0.0 |
| Failure | 40 | 55.6 | 0 | 0.0 | 40 | 100.0 |
| Median age, years (range) | 60.5 (16–91) | 64.5 (16–91) | 57 (0–89) | |||
| Median size, cm (range) | 7.25 (0.9–35.5) | 7.25 (1.5–23.8) | 7.15 (0.9–35.5) | |||
| Median time to establish, days (range) | 53.5 (9–184) | 53.5 (9–184) | — | |||
Abbreviations: CT, chemotherapy; NOS, not otherwise specified; UPS, undifferentiated pleomorphic sarcoma; XRT, radiation therapy.
Factors Associated With Establishment of Sarcoma PDOX Model
No low-grade STSs were established as PDOXs (zero of 32). Among high-grade STSs, 32 PDOXs (44.4%) were established. Establishment was similar by sex, presentation, and location but varied by neoadjuvant therapy (Table 2). None of the STSs exposed to radiation within 10 weeks before tumor excision established (zero of 11), which resulted in a 0% establishment rate (odds ratio [OR], 0.00 compared with no preoperative radiation; P = .001). Univariable analysis demonstrated that the likelihood of establishment was reduced with any neoadjuvant chemotherapy (OR, 0.282; P = .022) and increased without neoadjuvant therapy (OR, 6.50; P = .001). In a subgroup analysis, the time between completing neoadjuvant chemotherapy and surgical resection did not demonstrate an effect on the likelihood of xenograft establishment. No difference in the likelihood of PDOX establishment was found by sex, age, presentation (primary, recurrent, or metastatic), location, subtype, or size.
Table 2.
Factors Associated With Patient-Derived Orthotopic Xenograft Establishment Among High-Grade Tumors
| Establishment Rate Among High-Grade Tumors (n = 72) | Univariable Logistic Regression (n = 72) | |||
|---|---|---|---|---|
| % (No.) | P | OR | P | |
| Sex | ||||
| Female | 42.1 (16 of 38) | .673 | 1.222 | .673 |
| Male | 47.1 (16 of 34) | Reference | ||
| Grade | ||||
| Low | 0.0 (0) | |||
| High | 44.4(32 of 72) | |||
| Presentation | ||||
| Primary | 45.5 (20 of 44) | .829 | 1.111 | .829 |
| Recurrent or metastatic | 42.9(12 of 28) | Reference | ||
| Location | Reference | |||
| Abdomen/retroperitoneum | 36.6(15 of 41) | .123 | ||
| Extremity | 54.8 (17 of 31) | 2.105 | .125 | |
| Size, cm | ||||
| < 10 | 45.8 (22 of 48) | .737 | ||
| > 10 | 41.7 (10 of 24) | |||
| Subtype | ||||
| Leiomyosarcoma | 53.3 (8 of 15) | .436 | 2.540 | .155 |
| Liposarcoma | 61.5(8 of 13) | .171 | 3.555 | .069 |
| NOS/spindle cell/UPS | 46.7(7 of 15) | .846 | 1.944 | .310 |
| Other | 31.0 (9 of 29) | .06 | Reference | |
| Neoadjuvant CT (any) | ||||
| Yes | 25.0 (6 of 24) | .457 | 0.282 | .022* |
| No | 54.2 (26 of 48) | Reference | ||
| Neoadjuvant XRT (any) | ||||
| Yes | 0(0 of 11) | .001* | 0.000 | .001* |
| No | 52.5 (32 of 61) | Reference | ||
| Neoadjuvant therapy (any) | ||||
| None | 61.9 (26 of 42) | .009 | 6.500 | .001* |
| CT alone | 31.6 (6 of 19) | .652 | 0.479 | .192 |
| XRT alone | 0.0(0 of 6) | .041* | 0.000 | .023 |
| CT + XRT | 0 (0 of 5) | .055 | 0.000 | .039 |
| Age, years | 1.027 | .075 | ||
| Size, cm | 1.01 | .801 | ||
Abbreviations: CT, chemotherapy; NOS, not otherwise specified; OR, odds ratio; UPS, undifferentiated pleomorphic sarcoma; XRT, radiation therapy.
Significant to P < .05.
Establishment rates varied by subtype (Appendix Table A2). Among subtypes with at least three high-grade tumors, PDOX success rates were highest among liposarcoma (66.7%), myofibrosarcoma (60.0%), and leiomyosarcoma(53.3%). Only one patient with osteosarcoma and one with rhabdomyosarcoma were enrolled, and both tissue samples led to a successful PDOX.
Among high-grade primary STSs, 45.5% (20 of44) established. Establishment rates were higher among primary extremity STSs, tumors > 10 cm, liposarcomas, and tumors that had been exposed to less preoperative therapy (no neoadjuvant chemotherapy, no neoadjuvant radiation, or no treatment). Variations in establishment rate for primary and recurrent tumors are listed in Table 3. Among recurrent or metastatic high-grade tumors, 42.9% (12 of 28) were established as PDOXs. Establishment rates for recurrent or metastatic tumors were similar by location and size but were higher among leiomyosarcomas, liposarcomas, and not otherwise specified (NOS)/spindle cell/undifferentiated pleomorphic sarcoma (UPS) sub-type compared with others and among tumors exposed to less preoperative therapy. On the basis of establishment rates alone, neoadjuvant treatment seems to have a consistent effect on primary and metastatic/recurrent STSs (Table 3).
Table 3.
Primary Versus Recurrent/Metastatic Tumors: Factors Associated With Patient-Derived Orthotopic Xenograft Establishment
| All High-Grade Primary Tumors (n = 44) | High-Grade Primary Establishment Rates (n = 20) | All High-Grade Metastatic/Recurrent Tumors (n = 28) | High-Grade Metastatic/Recurrent Establishment Rates (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Sex | ||||||||
| Female | 20 | 45.5 | 8 | 40.0 | 14 | 50.0 | 8 | 57.1 |
| Male | 24 | 54.5 | 12 | 50.0 | 14 | 50.0 | 4 | 28.6 |
| Location | ||||||||
| Abdomen/retroperitoneum | 23 | 52.3 | 7 | 30.4 | 18 | 64.3 | 8 | 44.4 |
| Extremity | 21 | 47.7 | 13 | 61.9 | 10 | 35.7 | 4 | 40.0 |
| Size, cm | ||||||||
| < 10 | 24 | 54.5 | 8 | 33.3 | 24 | 85.7 | 10 | 41.7 |
| > 10 | 20 | 45.5 | 12 | 60.0 | 4 | 14.3 | 2 | 50.0 |
| Subtype | ||||||||
| Leiomyosarcoma | 11 | 25.0 | 4 | 36.4 | 4 | 14.3 | 4 | 100.0 |
| Liposarcoma | 7 | 15.9 | 4 | 57.1 | 6 | 21.4 | 4 | 66.7 |
| NOS/spindle cell/UPS | 11 | 25.0 | 5 | 45.5 | 4 | 14.3 | 2 | 50.0 |
| Other | 15 | 34.1 | 7 | 46.7 | 14 | 50.0 | 2 | 14.3 |
| Neoadjuvant CT (any) | ||||||||
| Yes | 12 | 27.3 | 2 | 16.7 | 12 | 42.9 | 4 | 33.3 |
| No | 32 | 72.7 | 18 | 56.3 | 16 | 57.1 | 8 | 50.0 |
| Neoadjuvant XRT (any) | ||||||||
| Yes | 13 | 29.5 | 0 | 0.0 | 7 | 25.0 | 0 | 0.0 |
| No | 31 | 70.5 | 20 | 64.5 | 21 | 75.0 | 12 | 57.1 |
| Neoadjuvant therapy | ||||||||
| None | 27 | 61.4 | 18 | 66.7 | 15 | 53.6 | 8 | 53.3 |
| CT alone | 7 | 15.8 | 2 | 28.6 | 12 | 42.9 | 4 | 33.3 |
| XRT alone | 5 | 11.4 | 0 | 0.0 | 1 | 3.6 | 0 | 0.0 |
| CT + XRT | 5 | 11.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Xenograftability | ||||||||
| Success | 20 | 45.5 | 20 | 100.0 | 12 | 42.9 | 12 | 100.0 |
| Failure | 24 | 54.5 | 0 | 0.0 | 16 | 57.1 | 0 | 0.0 |
| Median age, years (range) | 61.5 (26–91) | 66 (30–91) | 59.5 (16–84) | 62.5 (16–84) | ||||
| Median size, cm (range) | 9.1 (2.3–35.5) | 9.1 (3.5–23.8) | 4.65 (0.9–17.5) | 6.1 (1.5–14.6) | ||||
| Median time to establish, days (range) | 64 (14–184) | 64 (14–184) | 46.5 (9–119) | 46.5 (9–119) | ||||
Abbreviations: CT, chemotherapy; NOS, not otherwise specified; UPS, undifferentiated pleomorphic sarcoma; XRT, radiation therapy.
Among untreated STSs, 61.9% (26 of 42) were established. Within this subgroup was a particularly high establishment rate (85.7%) among NOS/spindle cell/UPS tumors at 85.7%. In addition, higher establishment rates were associated with primary tumors, extremity tumors, and tumors that were > 10 cm. Given the small numbers, these differences did not reach statistical significance on univariable analysis. All comparisons are listed in Table 4.
Table 4.
Untreated High-Grade Tumors: Factors Associated With Establishment
| High-Grade Untreated Tumors (n = 42) | High-Grade Untreated Establishment-Rate (n = 26) | Xenograft Establishment | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | OR | P | |
| Sex | ||||||
| Female | 21 | 50.0 | 12 | 57.1 | 0.667 | 0.526 |
| Male | 21 | 50.0 | 14 | 66.7 | ||
| Presentation | ||||||
| Primary | 27 | 64.3 | 18 | 66.7 | ||
| Recurrent or metastatic | 15 | 35.7 | 8 | 53.3 | ||
| Location | ||||||
| Abdomen/retroperitoneum | 22 | 52.4 | 12 | 54.5 | ||
| Extremity | 20 | 47.6 | 14 | 70.0 | 1.944 | 0.306 |
| Size, cm | ||||||
| < 10 | 30 | 71.4 | 17 | 56.7 | ||
| > 10 | 12 | 28.6 | 9 | 75.0 | 2.294 | 0.276 |
| Subtype | ||||||
| Leiomyosarcoma | 12 | 28.6 | 7 | 58.3 | ||
| Liposarcoma | 9 | 21.4 | 7 | 77.8 | ||
| NOS/spindle cell/UPS | 7 | 16.7 | 6 | 85.7 | ||
| Other | 14 | 33.3 | 6 | 42.9 | ||
| Xenograftability | ||||||
| Success | 26 | 61.9 | 26 | 100.0 | ||
| Failure | 16 | 38.1 | 0 | 0.0 | ||
| Median age, years (range) | 63 (16–91) | 66 (16–91) | 1.020 | 0.290 | ||
| Median size, cm (range) | 6.9 (0.9–23.8) | 8.25 (1.5–23.8) | 1.143 | 0.084 | ||
| Median time to establish, days (range) | 61.5 (9–184) | 61.5 (9–184) | ||||
Abbreviations: NOS, not otherwise specified; OR, odds ratio; UPS, undifferentiated pleomorphic sarcoma.
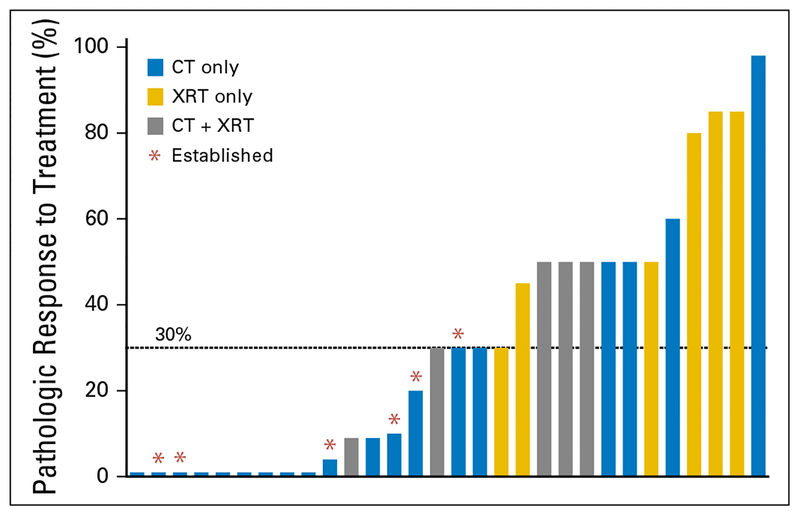
Among treated tumors, pathologic response varied from 0% to 98%. The only tumors that established xenografts were those with minimal (≤30%) pathologic response (Fig 1). Within the subset of tumors with minimal pathologic response, the establishment rate was 33.3% (six of18). Tumors with > 30% pathologic response did not establish. Three factors were identified in this study that were associated with a 0% establishment rate: low grade, preoperative radiation, and pathologic response to treatment > 30%.
Fig 1.
Pathologic response is associated with establishment rate. Each bar indicates an individual tumor’s degree of pathologic response to treatment. Those tumors that were established as a patient-derived orthotopic xenograft are indicated with an asterisk. Only tumors with ≤ 30% pathologic response (minimal) were able to establish a patient-derived orthotopic xenograft. CT, chemotherapy; XRT, radiation therapy.
Factors Associated With Time to Establishment
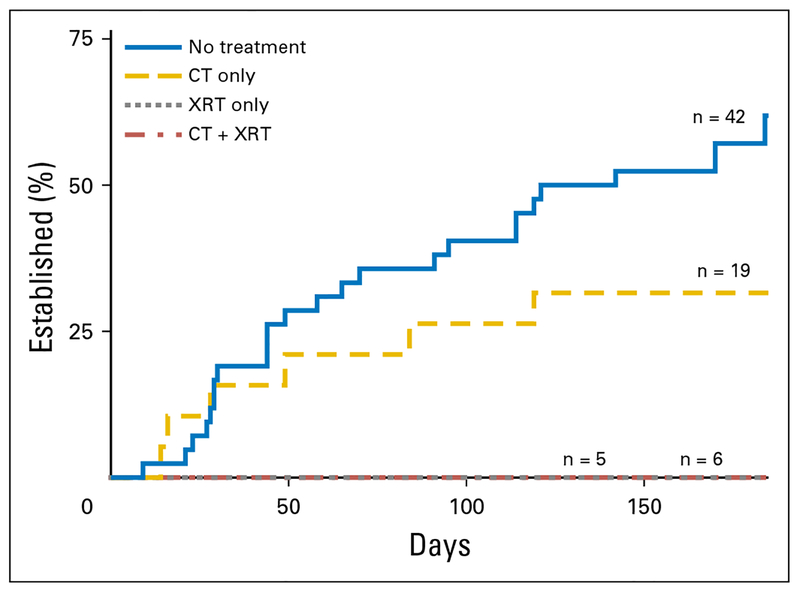
Among patients with an established PDOX (n = 32), the time to establishment varied between 9 and 184 days (median, 53.5 days). Compared with untreated patients, no difference in time to establishment was observed for patients who received neoadjuvant therapy (P = .180; Fig 2). Specifically, given that none of the grafts exposed to radiation established, no difference in time to establishment was found when untreated grafts were compared with those exposed to neoadjuvant chemotherapy alone. No difference in time to establishment was seen by tumor size (P = .321), location (P=.158), presentation (P=.234), or degree of pathologic response (P = .114). Time to establishment by subtype is listed in Appendix Table A2.
Fig 2.
Time to establishment varied by exposure to neoadjuvant therapy. Median time to establishment was 62 days and 39 days for the no-treatment and neoadjuvant chemotherapy (CT) groups, respectively (P = .180). None of the tumors exposed to radiation therapy (XRT; CT + XRT or XRT alone) established a patient-derived orthotopic xenograft.
DISCUSSION
PDXs are a promising approach to personalized medicine in the treatment of STSs. Given the rarity and histologic diversity of these tumors as well as the variable responses by subtype to chemotherapy, the ability to replicate a patient’s individual tumor in a mouse model provides an important opportunity for developing and personalizing therapy without placing patients at risk for treatment-related complications of ineffective therapy. Although previous studies of xenograft models in sarcoma have been done, few were directly connected to the clinical environment. The Champions Oncology trial, which developed 29 subcutaneous PDX models, indicated that growth rates varied and documented an establishment rate of 76% but did not indicate which patient or tumor factors predicted establishment.29 To appropriately select patients for xenograft studies, we first sought to understand which tumors are most likely to grow as xenografts.
In the current study, we prospectively enrolled all patients who underwent resection of an STS at a single institution over a 1-year period. The analysis demonstrates that the ability to develop xenografts highly depends on both tumor and patient treatment characteristics. Specifically, low-grade tumors universally failed to grow, whereas high-grade tumors had an overall establishment rate of 44.4% (32 of 72), which demonstrates that patients with low-grade tumors are unlikely to benefit from attempts at xenograft development. Furthermore, the study indicates that patients who receive no treatment before tumor resection are most likely to benefit, such that 61.9% of those patients’ tumors were successfully xenografted. In particular, when compared with STSs exposed to neoadjuvant therapy, untreated STSs were more than six times more likely to successfully establish a PDOX, which suggests that the optimal time for xenograft development is likely before the initiation of therapeutic interventions.
Among patients who underwent neoadjuvant therapy, radiation had the greatest negative impact on the likelihood of attaining a successful xenograft. The xenograft establishment rate for patients previously treated with radiation alone before resection was 0% (zero of six), compared with 32% for chemotherapy alone (six of 19). Given the difficulty in establishing a PDOX after radiation therapy, it may be best to recommend that patients who undergo radiation therapy, who may ultimately benefit from PDOX establishment, undergo tumor biopsy for xenograft establishment before proceeding with neoadjuvant radiation.
Pathologic response to treatment was also significantly associated with PDOX establishment. Specifically, patient tumors with a demonstrable treatment response (> 30% on pathologic review of the resected tumor) did not establish a PDOX. This finding suggests that patients whose tumors are able to be established as an orthotopic xeno-graft may similarly benefit the most from pre-clinical identification of active systemic agents on the platform that these models provide.
Among successfully established xenografts was a wide range in the time required to establish a graft (9 to 184 days). Although the Champions Oncology study suggested that time to graft establishment was correlated with tumor factors,29 the current study did not demonstrate this correlation. Specifically, we found no correlation between the time to establishment and previous treatment-, subtype-, or patient-related factors. The median time to establishment of a xenograft among those that established was 53 days, which indicates that xenografts can be developed over a short period. In the current model, serial drug testing can be done after establishment. We acknowledge that this amount of time required for xenograft establishment and serial drug testing may take longer than the patient’s recovery from surgery; therefore, at present we believe that the results of xenograft testing should be used to supplement care and potentially define second-line therapies rather than replace standard treatments.
The current study is limited by the sample size and diverse study population. Although statistical analyses of the overall group were feasible, smaller subgroup analyses were limited, and therefore, conclusions about individual subtypes or treatment factors could not be made with a high degree of certainty.
As we move forward with the use of PDXs as a tool for personalizing sarcoma therapy, we must be cognizant of the patient population with the greatest potential to benefit: patients with high-grade tumors that have not been exposed to neoadjuvant radiation therapy or those without a significant treatment response. These results also indicate that the optimal time for xenograft establishment is likely before the initiation of treatment or at a minimum, before radiation therapy. Given the benefit of neoadjuvant therapy, particularly neoadjuvant radiation therapy, we hypothesize that the optimal mechanism for attaining a tissue sample, without interrupting patient therapy, is through a pretreatment core needle biopsy, which we plan to study in the future.
In conclusion, this study provides substantial evidence that sarcoma PDOXs are feasible within the clinical setting and when used in the appropriate patient population, hold promise as a method for personalizing therapy in this diverse disease.
Acknowledgments
Support
Supported by the Robert Wood Johnson Clinical Scholars Program and the West Los Angeles Veteran Affairs Health Services Research and Development Center for Innovation (T.A.R.) and National Cancer Institute Grant No. CA213649 (R.M.H.).
APPENDIX
Table A1.
Patient and Tumor Characteristics (high grade and low grade)
| All Xenografts (n = 107) | ||
|---|---|---|
| No. | % | |
| Sex | ||
| Female | 51 | 47.7 |
| Male | 56 | 52.3 |
| Grade | ||
| Low | 35 | 32.7 |
| High | 72 | 67.3 |
| Presentation | ||
| Primary | 71 | 66.4 |
| Recurrent or metastatic | 36 | 33.6 |
| Location | ||
| Trunk | 62 | 57.9 |
| Extremity | 45 | 42.1 |
| Size, cm | ||
| < 10 | 67 | 62.6 |
| > 10 | 40 | 37.4 |
| Subtype | ||
| Leiomyosarcoma | 15 | 14.0 |
| Liposarcoma | 27 | 25.2 |
| NOS/spindle cell/UPS | 16 | 15.0 |
| Other | 49 | 45.8 |
| Neoadjuvant CT (any) | ||
| Yes | 31 | 29.0 |
| No | 76 | 71.0 |
| Neoadjuvant XRT (any) | ||
| Yes | 26 | 24.3 |
| No | 81 | 75.7 |
| Neoadjuvant therapy | ||
| None | 67 | 62.6 |
| CT alone | 23 | 21.5 |
| XRT alone | 9 | 8.4 |
| CT + XRT | 8 | 7.5 |
| Xenograftability | ||
| Success | 32 | 29.9 |
| Failure | 75 | 70.1 |
| Median age, years (range) | 61 (16–91) | |
| Median size, cm (range) | 7.6 (0.9–38) | |
| Median time to establish, days (range) | 49 (9–184) | |
Abbreviations: CT, chemotherapy; NOS, not otherwise specified; UPS, undifferentiated pleomorphic sarcoma; XRT, radiation therapy.
Table A2.
Histologic Subtypes
| High Grade | Establishment Rate Among High Grade | Time to Establishment (days) | |||||
|---|---|---|---|---|---|---|---|
| No. | No. | % High Grade | No. | % (success/high grade) | Median | Range | |
| Angiomyoplipoma | 1 | 0 | 0.0 | ||||
| Angiosarcoma | 1 | 1 | 100.0 | 0 | 0.0 | ||
| Chondrosarcoma | 1 | 0 | 0.0 | ||||
| Chordoma | 1 | 0 | 0.0 | ||||
| Dermatofibrosarcoma protuberans | 1 | 1 | 100.0 | 0 | 0.0 | ||
| Desmoid tumor | 1 | 0 | 0.0 | ||||
| Desmoplastic small round cell tumor | 2 | 100.0 | 0 | 0.0 | |||
| Embryonal sarcoma | 1 | 1 | 100.0 | 0 | 0.0 | ||
| Epithelioid sarcoma | 3 | 3 | 100.0 | 0 | 0.0 | ||
| Ewing sarcoma, extraosseous | 3 | 3 | 100.0 | 1 | 33.3 | 49 | 49 |
| Fibromyxoid sarcoma | 2 | 1 | 50.0 | 0 | 0.0 | ||
| Follicular dendritic cell sarcoma | 2 | 2 | 100.0 | 1 | 50.0 | 23 | 23 |
| GI stromal tumor | 7 | 4 | 57.1 | 1 | 25.0 | 84 | 84 |
| Intramuscular angioma | 1 | 0 | 0.0 | ||||
| Leiomyoma | 3 | 0 | 0.0 | ||||
| Leiomyosarcoma | 15 | 15 | 100.0 | 8 | 53.3 | 86 | 29–170 |
| Liposarcoma | 27 | 12 | 44.4 | 8 | 66.7 | 80 | 9–184 |
| Malignant peripheral nerve sheath tumor | 1 | 0 | 0.0 | ||||
| Myxofibrosarcoma | 5 | 5 | 100.0 | 3 | 60.0 | 70 | 14–121 |
| Myxoma | 3 | 0 | 0.0 | ||||
| NOS/spindle cell/UPS | 16 | 15 | 93.8 | 7 | 46.7 | 30 | 16–142 |
| Osteosarcoma, extraosseous | 1 | 1 | 100.0 | 1 | 100.0 | 27 | 27 |
| Phyllodes tumor | 1 | 1 | 100.0 | 0 | 0.0 | ||
| Radiation-associated sarcoma | 1 | 1 | 100.0 | 0 | 0.0 | ||
| Rhabdomyosarcoma | 1 | 1 | 100.0 | 1 | 100.0 | 44 | 44 |
| Schwannoma | 1 | 0 | 0.0 | ||||
| Solitary fibrous tumor | 3 | 0 | 0.0 | ||||
| Synovial sarcoma | 2 | 2 | 100.0 | 1 | 50.0 | 91 | 91 |
Abbreviations: NOS, not otherwise specified; UPS, undifferentiated pleomorphic sarcoma.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
Tara A. Russell
No relationship to disclose
Mark A. Eckardt
No relationship to disclose
Takashi Murakami
No relationship to disclose
Irmina A. Elliott
No relationship to disclose
Kei Kawaguchi
No relationship to disclose
Tasuku Kiyuna
No relationship to disclose
Kentaro Igarashi
No relationship to disclose
Yungfeng Li
No relationship to disclose
Joseph G. Crompton
No relationship to disclose
Danielle S. Graham
Stock and Other Ownership Interests: DaVita (I)
Sarah M. Dry
No relationship to disclose
Nicholas Bernthal
Honoraria: Daiichi Sankyo
Consulting or Advisory Role: Onkos Surgical, Bonesupport
Research Funding: Onkos Surgical
Jane Yanagawa
No relationship to disclose
Anusha Kalbasi
No relationship to disclose
Noah Federman
Stock and Other Ownership Interests: Genmab, Kite Pharma
Consulting or Advisory Role: Zelda Therapeutics
Bartosz Chmielowski
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Merck, Genentech, Roche, Eisai, Immunocore
Speakers’ Bureau: Genentech, Roche, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Genentech, Roche, Bristol-Myers Squibb, Janssen Pharmaceuticals, Merck
Arun S. Singh
Consulting or Advisory Role: Eli Lilly, Daiichi Sankyo
Speakers’ Bureau: Eli Lilly, Eisai
Travel, Accommodations, Expenses: Eli Lilly, Daiichi Sankyo, Eisai
Stock and Other Ownership Interests: AntiCancer-PDOX
Robert M. Hoffman
Stock and Other Ownership Interests: AntiCancer-PDOX
Fritz C. Eilber
Stock and Other Ownership Interests: AntiCancer-PDOX
REFERENCES
- 1.Sampson VB, Kamara DF, Kolb EA: Xenograft and genetically engineered mouse model systems of osteosarcoma and Ewing’s sarcoma: Tumor models for cancer drug discovery. Expert Opin Drug Discov 8:1181–1189, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donahue TR, Kattan MW, Nelson SD, et al. : Evaluation of neoadjuvant therapy and histopathologic response in primary, high-grade retroperitoneal sarcomas using the sarcoma nomogram. Cancer 116:3883–3891, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Eilber FC, Brennan MF, Eilber FR, et al. : Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer 101:2270–2275, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Houghton JA, Houghton PJ, Green AA: Chemotherapy of childhood rhabdomyosarcomas growing as xenografts in immune-deprived mice. Cancer Res 42:535–539, 1982 [PubMed] [Google Scholar]
- 5.Castellsagué J, Gel B, Rodríguez JF, et al. : Comprehensive establishment and characterization of orthoxenograft mouse models of malignant peripheral nerve sheath tumors for personalized medicine. EMBO Mol Med 7:608–627, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper JE, Cantor EL, Ehlen MS, et al. : A patient-derived xenograft model of parameningeal embryonal rhabdomyosarcoma for preclinical studies. Sarcoma 2015:826124, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monsma DJ, Cherba DM, Richardson PJ, et al. : Using a rhabdomyosarcoma patient-derived xenograft to examine precision medicine approaches and model acquired resistance. Pediatr Blood Cancer 61:1570–1577, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Hiroshima Y, Zhang Y, Zhang N, et al. : Patient-derived orthotopic xenograft (PDOX) nude mouse model of soft-tissue sarcoma more closely mimics the patient behavior in contrast to the subcutaneous ectopic model. Anticancer Res 35:697–701, 2015 [PubMed] [Google Scholar]
- 9.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A 89:5645–5649,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa T, Kubota T, Watanabe M, et al. : Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int J Cancer 53:608–612, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Hoffman RM: Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 15:451–452, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Fidler IJ: Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes Memorial Award Lecture. Cancer Res 50:6130–6138, 1990 [PubMed] [Google Scholar]
- 13.Garber K. Realistic rodents? Debate grows over new mouse models of cancer. J Natl Cancer Inst 98:1176–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RM: Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: A bridge to the clinic. Invest New Drugs 17:343–359, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Igarashi K, Kawaguchi K, Kiyuna T, et al. : Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle 16:91–94, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aparicio S, Hidalgo M, Kung AL: Examining the utility of patient-derived xenograft mouse models. Nature Research 15:311–316, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Byrne AT, Alférez DG, Amant F, et al. : Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 17:254–268, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Singh AS, Kiyuna T, et al. : Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 7:47556–47564, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KB, Tran LM, Tam BM, et al. : Novel dedifferentiated liposarcoma xenograft models reveal PTEN down-regulation as a malignant signature and response to PI3K pathway inhibition. Am J Pathol 182:1400–1411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyuna T, Murakami T, Tome Y, et al. : High efficacy of tumor-targeting Salmonella typhimurium A1-R on a doxorubicin- and dactolisib-resistant follicular dendritic-cell sarcoma in a patient-derived orthotopic xenograft PDOX nude mouse model. Oncotarget 7:33046–33054, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyuna T, Murakami T, Tome Y, et al. : Labeling the stroma of a patient-derived orthotopic xenograft (PDOX) mouse model of undifferentiated pleomorphic soft-tissue sarcoma with red fluorescent protein for rapid non-invasive imaging for drug screening. J Cell Biochem 118:361–365, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Zhao M, Hiroshima Y, et al. : Efficacy of tumor-targeting salmonella A1-R on a melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. PLoS One 11:e0160882, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawaguchi K, Igarashi K, Murakami T, et al. : Tumor-targeting Salmonella typhimurium A1-R combined with temozolomide regresses malignant melanoma with a BRAF-V600E mutation in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 7:85929–85936, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawaguchi K, Murakami T, Chmielowski B, et al. : Vemurafenib-resistant BRAF-V600E-mutated melanoma is regressed by MEK-targeting drug trametinib, but not cobimetinib in a patient-derived orthotopic xenograft (PDOX) mouse model. Oncotarget 7:71737–71743, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami T, DeLong J, Eilber FC, et al. : Tumor-targeting Salmonella typhimurium A1-R in combination with doxorubicin eradicate soft tissue sarcoma in a patient-derived orthotopic xenograft (PDOX) model. Oncotarget 7:12783–12790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami T, Igarashi K, Kawaguchi K, et al. : Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget 8:8035–8042, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi K, Igarashi K, Murakami T, et al. : Tumor-targeting Salmonella typhimurium A1-R sensitizes melanoma with a BRAF-V600E mutation to vemurafenib in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem 118:2314–2319, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Igarashi K, Kawaguchi K, Murakami T, et al. : Intra-arterial administration of tumor-targeting Salmonella typhimurium A1-R regresses a cisplatin-resistant relapsed osteosarcoma in a patient-derived orthotopic xenograft (PDOX) mouse model. Cell Cycle 16:1164–1170, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stebbing J, Paz K, Schwartz GK, et al. : Patient-derived xenografts for individualized care in advanced sarcoma. Cancer 120:2006–2015, 2014. [Erratum: Cancer 120:3588, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]