Abstract
Affecting 60 million patients, glaucoma is the second leading cause of blindness worldwide. Despite the availability of multiple medical and surgical treatments with effective intraocular pressure lowering, many patients still progress to become visually handicapped from glaucoma due to therapeutic failure. There is therefore a great need for novel therapies to improve the standard of care, and Rho kinase (ROCK) inhibitors represent a promising new class of drugs for treatment of glaucoma. ROCK inhibitors act by increasing facility of fluid outflow from the eye, thereby reducing intraocular pressure. ROCK inhibitors also have a vasodilatory effect on conjunctival vessels, which can lead to eye redness, a less than desirable cosmetic side effect for patients that would use this medication. Although there is promising data to support the clinical potential of this class of drug, the occurrence of conjunctival hyperemia remains a potential deterrent for use by patients. Studies are underway to assess alternative dosing strategies, delivery methods and prodrug formulations that may circumvent this unwanted side effect. This review provides an up-to-date account of the basic scientific data, as well as nonclinical and clinical studies to support use of ROCK inhibitors for treatment of glaucoma.
Keywords: glaucoma, intraocular pressure, Rho kinase, ROCK hyperemia, trabecular meshwork
Glaucoma is the second leading cause of blindness worldwide. In spite of availability of medical and surgical treatment, many patients with glaucoma currently continue to lose vision. Hence, there is a substantial need for novel mechanistic strategies to complement existing therapies. Rho-associated protein kinase (or Rho kinase [ROCK]) inhibitors represent a promising new class of molecules with the potential to treat glaucoma and address this need. This review summarizes the available basic science, nonclinical, and clinical evidence to support the use of ROCK inhibitors for treatment of glaucoma.
Introduction
Glaucoma is the second leading cause of blindness worldwide [1]. It is characterized by elevated intraocular pressure (IOP), optic nerve damage and visual field loss. Despite multiple medical and therapeutic treatment modalities that can effectively lower IOP, many of the 60 million patients with the disease become visually impaired and/or blind from therapeutic failure. A 2003 report estimates the total costs of glaucoma in the UK alone to be as high as UK£38 billion [2]. Clearly, there is an urgent need for improved management strategies.
Glaucoma can be classified into two major types – open-angle glaucoma (OAG) and angle-closure glaucoma (ACG) – with the former representing 74% of cases worldwide [1]. Both OAG and ACG involve an imbalance between aqueous humor production and outflow, leading to an increase in IOP, optic nerve damage with nerve fiber layer loss, and subsequent visual field loss. In simplistic terms, OAG involves abnormalities in the outflow pathway while ACG involves impaired access to that pathway. The remainder of this review will focus on OAG, and although the mechanisms of disease differ, some of the medical approaches discussed for OAG are also relevant to ACG.
In the normal eye, aqueous humor forms in ciliary processes of the ciliary body, passes through the pupil to the anterior chamber, and exits through the trabecular meshwork (TM). From the TM, aqueous humor exits through one of two routes. The trabecular route, often called the conventional outflow pathway, accounts for 40–96% of outflow in the human eye, depending on the age of the patient, and involves aqueous transport through the TM, into Schlemm’s canal (SC), and out into the episcleral and conjunctival venous system [3]. Cellular properties of the TM are critical for conventional outflow. High levels of RhoA in TM cells induce a contractile morphology, increased actin stress fibers, increased focal cell–cell adhesions, increased levels of phosphorylated myosin light chain (MLC), and increased extracellular matrix protein production. These changes lead to decreased aqueous humor drainage due to cellular and morphological changes in the TM cells [4]. The ciliary muscle (CM) also plays an important role in this route. Contraction of the CM leads to increased TM pore size and subsequent increased aqueous drainage. In contrast, the second or uveoscleral route accounts for 4–60% of outflow in the human eye and involves passage of aqueous humor between CM fibers and into the suprachoroidal space. The prostaglandin class of IOP-lowering agents is well recognized to work via this mechanism [5,6]. From there, aqueous humor drains into the venous system of the ciliary body, choroid and sclera [3,7,8].
In general, current medical, laser and surgical therapies for glaucoma act by either increasing aqueous outflow or decreasing aqueous production. As of 2001, the most commonly prescribed medications were β-blockers (74%), carbonic anhydrase inhibitors (10%) and latanoprost (7%) [9]. More recently, prosta glandin analogues, such as latanoprost, travaprost, bimatoprost and tafluprost, have become first-line agents for medical therapy and now carry the majority share of prescriptions. Nonetheless, a substantial proportion of glaucoma patients fail to respond adequately to medical therapy, with failure rates for the most common drugs ranging from 13% (latanoprost) to 45% (sympathomimetics) [9]. Although laser or surgical therapies serve as alternatives for patients who fail medical therapy, laser and surgical procedures are often only temporizing. In fact, postsurgical and post-laser patients frequently require additional medical therapy [10]. The same holds when laser or surgery is used as a first-line treatment [11–13]. Therefore, patients who have failed laser or surgical treatment or who are already on maximal medical therapy with existing medications are in great need of novel glaucoma therapies that work through unique mechanisms.
Glaucoma is a worldwide health problem, and many populations most burdened by this disease live in resource-poor settings that lack access to medical technology and trained medical workers. In such areas, medical therapies are more likely to impact the burden of disease than laser or surgical interventions. Hence, a substantial opportunity exists for a new glaucoma medication to address both individual and global health needs.
Of the many drug classes emerging to treat glaucoma, ROCK inhibitors appear to be one of the most promising. This review will provide an overview of the role of ROCK inhibitors as potential therapies for glaucoma, including a discussion of the basic biology of the Rho GTPase pathway, a review of preclinical studies of ROCK inhibitors, and review of ongoing clinical-stage ROCK inhibitor programs. In particular, it will include critical aspects of preclinical and clinical data for ROCK inhibitors, beyond that of other recent reviews of ocular antihypertensive agents [14,15].
Basic biology of the Rho GTPase pathway
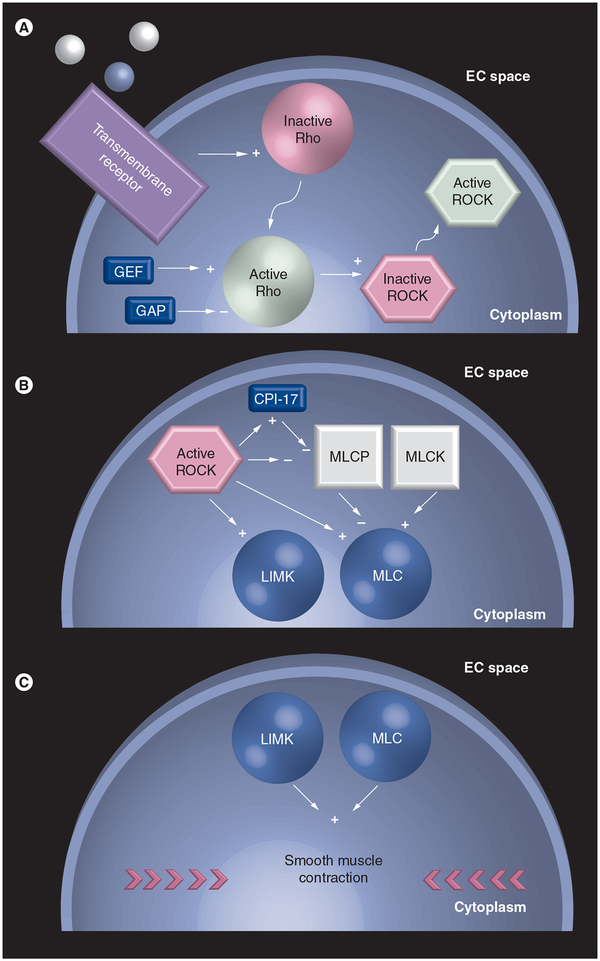
The Rho GTPase signal transduction pathway consists of a complex series of interactions between extracellular ligands, transmembrane receptors, intracellular enzymes and cytoskeletal effector proteins. Activation of the Rho GTPase pathway in the eye causes cellular and molecular changes within cells in the TM that results in contraction of smooth muscle-like cells and diminished aqueous humor drainage. Multiple studies support this molecular understanding of conventional outflow. In particular, in vitro studies using human and porcine TM cells infected with adenovirus containing a dominant-negative Rho-binding domain of ROCK have shown that ROCK inhibition induces cell rounding, and cell–cell detachment, and decreases actin stress fibers and focal adhesion staining [16]. In these studies, in addition to alterations consistent with TM relaxation and morphological changes, ROCK inhibition increased outflow in human anterior segments. Increased outflow has been shown to decrease IOP, making Rho GTPase signaling a promising target for glaucoma therapy [17–19]. A detailed illustration of the Rho GTPase pathway is provided in Figure 1.
Figure 1. Rho GTPase signaling pathway.
Ligand binds transmembrane receptor, leading to Rho activation and translocation from the plasma membrane to the cytosol. (A) In the cytosol, Rho activates ROCK, which partially translocates to the plasma membrane. GEF and GAP provide additional regulation, activating and inhibiting Rho, respectively. ROCK activates LIMK and phosphorylates MLC. (B) The latter involves ROCK inhibition of MLCP, which dephosphorylates MLC. (C) Activation of LIMK and phosphorylation of MLC lead to smooth muscle contraction. Straight arrows with a plus indicate activation, straight arrows with a minus indicate inhibition, and bent arrows indicate translocation.
EC: Extracellular; GAP: GTPase-activating protein; GEF: Guanine nucleotide exchange factor; LIMK: LIM kinase; MLC: Myosin light chain; MLCK: Myosin light chain kinase; MLCP: Myosin light chain phosphatase; ROCK: Rho kinase.
The Rho family of proteins (RhoA, RhoB and RhoC) belong to the Ras superfamily of GTPases. Rho GTPases receive inputs from one of four transmembrane receptor classes, including G-protein-coupled receptors, receptor tyrosine kinases, cytokine receptors and adhesion receptors, which collectively respond to signals from growth factors, cytokines and extracellular matrix proteins [20–23].
ROCKs are intracellular serine/threonine kinases located downstream of Rho in the signal transduction pathway (Figure 1), and as such they represent an important target in glaucoma therapy. Structurally, ROCKs consist of three domains: an N-terminal kinase domain, a C-terminal pleckstrin homology domain, and a coiled-coil domain. These domains interact with Rho–GTP to regulate ROCK activity. When present, Rho–GTP binds the coiled-coil domain and enhances ROCK activity. When absent, N-terminal kinase activity diminishes through an autoinhibitory intramolecular fold of the pleckstrin homology domain [24,25]. As with Rho, ROCK localization is important. ROCKs are primarily distributed in the cytoplasm; however, upon activation by Rho, ROCKs partially translocate to the membrane [26].
There are two known ROCK isoforms: ROCK-I and ROCK-II. Although both ROCK-I and ROCK-II are expressed in the CM and TM of humans and monkeys, the TM exhibits greater expression levels of both kinases [27]. These differences are consistent with the proposed mechanism of action of ROCK inhibitors, whereby inhibition of ROCKs cause increased aqueous outflow, leading to a decrease in IOP [28,29]. Mechanistically, ROCK activation induces contraction of smooth muscle-like cells in the eye; thus, protein levels predict that ROCK inhibition would lead to greater relaxation of the TM than of the CM. Since CM contraction increases outflow while TM contraction reduces it, greater relaxation of the TM would cause increased outflow [27,30,31]. Additional factors, such as local concentration of ROCK inhibitor and differences in activation/expression of other Rho GTPase pathway signaling molecules, may also contribute to IOP reduction.
Besides Rho GTPases and ROCKs, other downstream targets in this pathway are likely to play an important role in IOP regulation. For example, ROCKs regulate MLC phosphatase (MLCP), MLC, LIM domain kinase (LIMK) and CPI-17 [24]. Upon activation, ROCKs phosphorylate and inhibit MLCP, causing an increase in myosin regulatory light chain phosphorylation at serine 19. This facilitates myosin–actin binding and contraction of smooth muscle-like cells. In contrast to MLC kinase (MLCK) activity, ROCK-mediated regulatory light chain phosphorylation does not depend on intracellular calcium concentrations [32,33]. ROCK can also directly phosphorylate MLC; however, the physiologic importance of this direct phosphorylation is unclear. When calcium is absent and MLCK is off, MLC remains predominantly unphosphorylated [34].
The ROCK/LIMK/cofilin pathway is also important. ROCK activates LIMKs by phosphorylating threonine 508 on LIMK1 or threonine 505 on LIMK2 [35,36]. Activation results in downstream phosphorylation and inhibition of cofilin, a protein that normally stimulates actin disassembly [37,38]. Hence, LIMK activation causes actin filament stabilization. In contrast, inhibition of LIMK in the TM promotes actin depolymerization, which leads to tissue relaxation, increased outflow and decreased IOP [39,40].
Another effector in the Rho GTPase pathway is CPI-17. Studies have shown that ROCKs induce actin–myosin cross-bridging by phosphorylating threonine 38 on CPI-17 which, in turn, inhibits MLCP, leading to increased MLC phosphorylation. A Rho GTPase-independent pathway involving PKC can also induce threonine 38 phosphorylation on CPI-17, leading to smooth muscle contraction [41].
Preclinical studies
ROCK inhibitors
In the early 2000s, researchers began investigating the role of ROCK inhibition in increased aqueous outflow [28,29,42–44]. Since then, numerous ROCK inhibitors (Y-27632, Y-39983, HA-1077, H-1152P, 2,3-diaminopyrazines and benzothiophenes) have emerged from preclinical glaucoma studies and some have entered clinical trials (Table 1) [18,19,28,43,45–47]. This success has, in part, led to the identification of inhibitors of other components of the Rho GTPase pathway (ML-7, GGTI-DU40, statins and pyrrolopryimidines) that may also contribute to antiglaucoma strategies (Table 2) [40,48–50]. Beyond their utility in regulating aqueous flow, some inhibitors of the Rho GTPase pathway also reduce scar formation and inflammation following filtration surgery, suggesting that these inhibitors could play a dual role in glaucoma management.
Table 1.
Overview of preclinical experiments and results for Rho kinase inhibitors.
| ROCK inhibitors | Results |
|---|---|
| Y-27632 | ↓ IOP |
| ↑ Conventional outflow | |
| ↑ Pupil dilation | |
| ↓ Contraction of CM and TM cells | |
| ↑ Changes in TM and SC cell morphology | |
| ↑ SC cell monolayer permeability | |
| ↓ MLC phosphorylation in TM and SC cells | |
| ↑ Separation between inner wall and juxtacanalicular connective tissue corresponding to increased outflow | |
| ↓ Scar after surgery | |
| ↓ Scar in cell model | |
| HA-1077 AT877 Fasudil |
↓ IOP |
| ↑ Conventional outflow | |
| ↓ Actin bundles and focal adhesions in TM cells | |
| ↓ CM contraction | |
| ↓ Scar in cell model | |
| H-1152P | ↓ IOP |
| ↑ Conventional outflow | |
| ↑ Cell separation in TM cells | |
| ↓ Actin bundles and focal adhesions in TM cells | |
| ↓ MLC phosphorylation in TM cells | |
| ↓ Scar in cell model | |
| 2,3-diaminopyrazine | ↓ IOP |
| RKI983 SNJ-1656 Y-39983 |
↓ IOP |
| ↑ Conventional outflow | |
| ↓ TM contraction | |
| Benzothiophenes | ↓ IOP |
CM: Ciliary muscle; IOP: Intraocular pressure; MLC: Myosin light chain; ROCK: Rho kinase; SC: Schlemm’s canal; TM: Trabecular meshwork.
Table 2.
Overview of preclinical experiments and results for non-Rho kinase inhibitors that interact with the Rho GTPase pathway.
| Non-ROCK inhibitors (target of inhibitor) | Results |
|---|---|
| ML-7 (myosin light chain kinase) | ↓ TM contraction |
| ↓ MLC phosphorylation in TM cells | |
| ↓ Scar in cell model | |
| GGTI-DU40 (geranylgeranyl-transferase-1) | ↑ Outflow |
| ↑ Cell separation and cell rounding in TM cells | |
| ↓ Actin bundles and focal adhesions in TM cells | |
| ↓ MLC phosphorylation in TM cells | |
| ↓ Membrane localization of isoprenylated small GTPases and Gβγ in TM cells | |
| Lovastatin, compactin (HMG-CoA reductase) | ↑ Outflow |
| ↑ Cell rounding and actin depolymerization in CB and TM cells | |
| ↓ Focal adhesions in CB and TM cells | |
| ↓ MLC phosphorylation in TM and CB cells | |
| ↓ Membrane-bound Rho GTPase in TM cells | |
| ↓ Scar in cell model | |
| Pyrrolopyrimidines (LIM kinase-2) | ↓ IOP |
| ↑ Outflow | |
| H-7 (serine/threonine kinases) | ↓ IOP |
| ↑ Outflow | |
| ↑ Alterations in cell shape, actin cytoskeleton and focal adhesion complexes in TM cells | |
| ↑ Changes in morphology and contractility in SC and peri-SC cells |
CB: Ciliary body; IOP: Intraocular pressure; MLC: Myosin light chain; ROCK: Rho kinase; SC: Schlemm’s canal; TM: Trabecular meshwork.
Y-27632 is one of the most widely studied ROCK inhibitors. Researchers have examined its effects on IOP, outflow facility, contractility and cell shape in numerous systems. Administration of Y-27632 has been observed to reduce IOP in ocular normotensive rabbits and mice, and increase outflow facility in normotensive monkeys and rabbits, bovine eyes and enucleated porcine eyes [28,29,44,51–53]. These changes are associated with alterations in TM and CM contractility. Exposure to Y-27632 inhibits carbachol and endothelin-1-induced bovine TM contraction as well as contraction of human TM cells in a gel contraction assay [48,54,55]. Y-27632 also inhibits carbachol-induced contraction of bovine and rabbit CM [43,44].
Cellular changes accompany changes in IOP, outflow, and contraction induced by Y-27632. In human TM and SC cells, Y-27632 exposure induces alterations in cell shape, reductions in actin stress fibers, focal cell–cell adhesions, and phosphotyrosine staining; altered cell adhesiveness to fibronectin and collagen type I; and increased SC cell permeability [29,43,55,56]. Furthermore, in bovine eyes, increased separation between the inner wall endothelium of SC and the underlying juxtacanalicular connective tissue correlates with increased outflow facility [53]. Together, these results from multiple animals and cell-based systems suggest that Y-27632 reduces IOP and increases outflow through structural and functional changes in TM and/or CM cells.
HA-1077 (AT877; fasudil, Asahi Kasei Pharma Corp., Japan) is a systemically administered ROCK inhibitor that is currently approved and indicated for the treatment of cerebral vasospasm following subarachnoid hemorrhage [57]. In addition to its benefit within the CNS, researchers have examined its potential role in glaucoma management. In 2001, Honjo and colleagues investigated its role in modulating aqueous outflow. They found that topical, intracameral or intravitreal administration of HA-1077 to ocular normotensive rabbits decreased IOP and increased outflow facility, while administration to human TM cells disrupted actin bundles and focal adhesion formation. Additionally, the authors reported that HA-1077 reduced carbachol-induced contraction of isolated bovine CM strips [43]. In 2009, Fukunaga et al. recapitulated these results in a rabbit model of ocular hypertension, generated by water loading. Although topical delivery of 1, 2 and 3 mM HA-1077 decreased IOP relative to controls, a few cases of conjunctival injection were observed in 3 mM-treated eyes [17].
H-1152P is a methyl derivative of HA-1077 and is more potent and specific for ROCK than Y-27632 [33,58]. Topical administration to ocular normotensive rabbits, monkeys and rats reduces IOP, as does administration to ocular hypertensive rabbits [18,59,60]. H-1152P additionally increases outflow facility in enucleated porcine eyes [33]. In conjunction with changing hydrodynamics, cellular changes also occur. Specifically, H-1152P exposure to TM decreases MLC phosphorylation in human and porcine cells, and increases cell separation, reduces actin stress fibers and decreases focal adhesions in human cells [33,60]. In rat eyes, cellular changes include an expansion of intracellular space and loss of extracellular material in the juxtacanalicular region [60]. Aside from the clear benefit for IOP reduction, the main side effect of H-1152P is conjunctival congestion [18].
Recently, Henderson et al. developed a 2,3-diaminopyrazine derivative (2,3-DPD) based on a screen of a kinase library. Topical administration of 300- and 600-μg doses of 2,3-DPD to ocular hypertensive monkeys reduced IOP by 33 and 37%, respectively [45]. At moderate doses, this reduction compared well with values seen with Y-39983 and H-1152P, while at higher doses, the reduction was sustained for longer than that of H-1152P. A total of 6 h following administration, both H-1152P and a 300-μg dose of 2,3-DPD yielded a 25% reduction in IOP, versus a reduction of 30–35% with administration of a 600-μg dose of 2,3-DPD. Of note, 2,3-DPD exhibited high ROCK activity and selectivity in vitro with minimal side effects in vivo; that is, rabbits receiving 2,3-DPD displayed only mild hyperemia [45].
Y-39983 (Mitsubishi Pharma Corp., Japan; also called SNJ-1656, Senju Pharmaceutical Co., Japan; RKI983, Novartis, Switzerland) is a ROCK inhibitor derived from Y-27632. Topical administration of Y-39983 has been observed to increase conventional outflow in ocular normotensive rabbits and reduce IOP in normotensive rabbits, monkeys and mice, as well as reduce IOP in dexamethasone-induced ocular hypertensive mice [46,51,61]. Y-39983 also causes cellular and biochemical changes. Exposure of monkey TM to Y-39983 inhibits carbachol-induced contraction, while delivery to CM causes minimal relaxation [27]. In biochemical assays, Y-39983 displays greater selectivity for ROCK than Y-27632. Like other ROCK inhibitors, Y-39983 causes mild local side effects such as conjunctival hyperemia [46]. A 26–28-day administration of Y-39983 four-times daily was observed to produce sporadic punctuate subconjunctival hemorrhage in rabbits and monkeys during the administration period. However, decreasing this regimen to two- or three-times daily was shown to eliminate this side effect [46].
In 2010, Davis and colleagues described a novel benzothiophene-containing ROCK inhibitor, which they discovered through an ultrahigh-throughput enzymatic activity screen and structure–activity relationship studies [47]. Using a cynomolgus monkey model of laser-induced ocular hypertension, they reported that topical administration of their compound caused a reduction in IOP 1 h after application that lasted 6 h. Furthermore, they compared their inhibitor to Y-39983 and observed comparable in vivo efficacy and in vitro activity. The authors did not, however, evaluate the presence or absence of side effects as reported for Y-39983 [47].
Other inhibitors of the Rho GTPase pathway
Results from studies examining the effects of Rho inhibition on aqueous outflow have in part motivated researchers to examine other aspects of Rho GTPase signaling as possible targets for glaucoma therapy. Examples include ML-7, GGTI-DU40, HMG-CoA reductase inhibitors (statins), pyrrolopryimidines and H-7 [40,48–50,62]. ML-7 is a relatively specific, although somewhat weak, inhibitor of smooth muscle MLCK. Scientists have hypothesized that ML-7 administration reduces TM contractility and increases aqueous outflow [63]. Examining this possibility, Tian et al. found that ML-7 does indeed increase outflow facility in cynomolgus monkeys, while Rosenthal et al. reported that ML-7 administration causes bovine TM relaxation ex vivo [48,64]. These results, however, do not tell the whole story – Tian et al. also noted that mydriasis accompanied increased aqueous outflow [64]. Such effects on pupillary dilation would not be desirable for a clinical drug candidate.
In contrast to ML-7, which targets a kinase downstream of ROCK, other inhibitors are known to target upstream kinases. For example, GGTI-DU40 and statins both disrupt synthesis of Rho’s isoprenyl tail, thereby perturbing Rho’s ability to localize to the plasma membrane. GGTI-DU40 is a geranylgeranyltransferase-1 inhibitor, which Peterson et al. described in 2006 [65]. It has a Ki of 0.8 nM and is highly selective for geranylgeranyltransferase-1 in vitro and in live cells [63]. In 2008, Rao et al. evaluated this molecule in the context of glaucoma. When exposed to porcine TM cells, GGTI-DU40 induced cell separation and cell rounding along with reductions in actin stress fibers, focal adhesions, cell–cell junctions and MLC phosphorylation. In addition, GGTI-DU40 increased outflow facility when exposed to organ-cultured porcine eye anterior segments [49].
Statins, which also inhibit isoprenyl tail synthesis, are commonly used to treat hyperlipidemia; however, they may also be beneficial in glaucoma management [66–69]. Some of the strongest evidence for this comes from a study describing the interactions between statins and Rho GTPase signaling. In their 2005 report, Song et al. exposed porcine TM cells, CB cells and organ-cultured eye anterior segments to lovastatin and compactin. These inhibitors were observed to alter cell shape and cytoskeletal organization, decrease MLC phosphorylation and increase outflow [50]. This led the authors to hypothesize that HMG-CoA reductase inhibition interferes with Rho GTPase activity by disrupting isoprenyl tail synthesis and subsequently Rho GTPase localization to the plasma membrane. In support of this idea, Song et al. conducted further experiments demonstrating that statin exposure does indeed disrupt localization and that coadministration of geranylgeranyl pyrophosphate normalizes cell shape and cytoskeletal organization [50]. Although this evidence is compelling, case–control studies examining associations between glaucoma progression and statin use have been less conclusive, indicating a need for further research [70,71]. In 2009, Harrison et al. described a novel pyrrolopyrimidine class of LIMK2 inhibitors, which they identified through an inhibition screen of cofilin phosphorylation in vitro and in porcine TM cells. In their analyses, Harrison et al. examined the efficacy of their compounds in both a dexamethasone-induced ocular hypertension mouse model and in a pig eye perfusion assay. Administration of their pyrrolopyrimidine derivatives reduced IOP and increased outflow facility, respectively [40].
H-7 is a broad serine/threonine kinase inhibitor that interferes with Rho GTPase signaling and many other pathways. Evidence suggests that H-7 administration induces cellular changes in components of the conventional outflow pathway [72–75]. For example, H-7 has been shown to induce alterations in cell shape, actin cytoskeleton, and associated focal adhesion complexes in human TM cells [72]. In studies performed on monkeys, H-7 was observed to induce inner wall protrusion, inner wall cell relaxation and expansion of intercellular spaces in the juxtacanalicular meshwork [73,74], as well as increase outflow and reduce IOP [62,73–76]. Although these effects on outflow and IOP reduction appear promising, H-7 lacks kinase specificity, inhibiting PKC (6.0 μm), PKA (3.0 μm), PKG (5.8 μm), MLCK (97 μm) [77] and ROCK (200–500 μm) [78] at micromolar concentrations, making H-7 a less than ideal drug candidate.
Decreased scar formation after glaucoma surgery
In addition to their ability to decrease IOP, inhibitors of the Rho GTPase pathway, including Y-27632, HA-1077, H-1152, ML-7 and lovastastin, may reduce scar formation following glaucoma filtration surgery. Many individuals suffering from progressive and/or inadequately controlled glaucoma undergo filtration surgery due to failure of alternative treatments to adequately and effectively lower IOP; however, scar formation following surgery is not uncommon and very often leads to surgical failure. Current treatments do exist to reduce scar formation; however, these are often associated with less than desirable side effects [79]. Hence, there is a need for better antiproliferatives. This need has motivated scientists to investigate Rho GTPase signaling in the context of scar formation and inflammation. In two separate studies, Meyerter-Vehn and colleagues reported that Y-27632, HA-1077, H-1152, ML-7 and lovastatin prevent TGF-β-induced human tenon fibroblast contraction and myofibroblast transdifferentiation, processes implicated in scar formation [79,80]. In another study using a rabbit model of glaucoma filtration surgery, Honjo et al. reported a reduction in collagen deposition and scar formation in the sclerostomy area of rabbits following Y-27632 administration [81]. Together, these cellular and animal studies suggest that inhibitors of Rho GTPase signaling could improve outcomes of glaucoma filtration surgery separate from their role in decreasing IOP, thus increasing their benefit to the glaucoma patient.
Additional benefits of ROCK inhibitors for glaucoma
In addition to targeting the conventional outflow pathway to reduce IOP, ROCK inhibitors alter ocular biology in numerous other ways that are postulated to be beneficial in glaucoma management. Notably, ROCK inhibitors can improve several factors important for neuroprotection: ocular blood flow, ganglion cell survival and axonal regeneration. Several mechanisms have been proposed to explain this neuroprotection. In a study by Lingor et al., ROCK inhibition was shown to interfere with myelin-derived inhibitors of axonal regeneration [82], thereby promoting axonal growth in rats. In another study in mice, ROCK inhibition diminished reactive gliosis, a known factor in neuronal loss [83]. In a third study, ROCK inhibition prevented leukocyte infiltration, improved blood flow and prevented endothelial disarrangement, which led to loss of retinal ganglion cells during the reperfusion phase of transient retinal ischemia in rats [84]. Other potential mechanisms of neuroprotection have been discussed elsewhere but are beyond the scope of this review [23,85].
As discussed above, evidence from animal studies suggests that ROCK inhibitors possess neuroprotective properties by virtue of their effects on ocular blood flow, ganglion cell survival and axonal regeneration. What remains unknown is whether such properties confer a clinical benefit and can prevent progression of disease in patients with glaucoma. Further clinical studies are required to assess the neuroprotective benefit of ROCK inhibitors independent of their effects on IOP.
Clinical studies
As discussed above, glaucoma is the second leading cause of blindness worldwide and represents a significant economic burden globally for populations affected by this disease. Despite receiving a combination of medical therapies, laser treatment and surgery, many patients continue to lose vision from therapeutic failure and inadequate IOP control. ROCK inhibitors represent a class of antiglaucomatous drugs with a novel mechanism of action and strong potential to augment available treatment regimens through use as combination or monotherapy. Currently, multiple ROCK inhibitors are in varying stages of clinical development (Table 3). The statuses of these clinical programs are discussed herein.
Table 3.
Overview of ROCK inhibitors in clinical development.†
| ROCK inhibitors | Company | Stage of development |
|---|---|---|
| AR-12286 | Aerie Pharmaceuticals | Phase II, current |
| K-115 | Kowa Company, Ltd. | Phase III, anticipated |
| INS117548 | Merck | Stalled after completion of Phase I |
| RKI1983/SNJ-1656/Y-39983 | Novartis | Stalled after completion of Phase II |
| ATS907 | Altheos, Inc. | Phase I/II, pending in 2011 |
| DE-104 | Santen Pharmaceuticals | Discontinued after completion of Phase I/II |
Based on on preclinical data, Rho kinase inhibitors decrease intraocular pressure by increasing conventional outflow.
AR-12286
AR-12286 (Aerie Pharmaceuticals) is a ROCK inhibitor currently in clinical development for treatment of elevated IOP in glaucoma. In September 2009, a double-masked, randomized, placebo-controlled dose–response study assessing the safety and ocular hypotensive effect of AR-12286 (0.05, 0.1 and 0.25%) was completed in patients with ocular hypertension (ClinicalTrials. gov identifier: NCT00902200 [101]). Administration of 0.25% solution twice-daily reduced IOP by 28%, or −6.8 mmHg, the greatest reduction reported in this study, but patients in this group exhibited trace to moderate conjunctival hyperemia lasting up to 4 h. In patients who received the 0.25% solution once-daily at bedtime, significant IOP reduction was attained, with hyperemia observed in only a minority of patients (10%) [86].
In August 2010, a comparative study with latanoprost was completed in patients with elevated IOP (ClinicalTrials.gov identifier: NCT01060579 [101]). The authors concluded that 0.5% AR-12286 at bedtime provided better control of diurnal IOP than 0.25% AR-12286 twice-daily. However, AR-12286 was found to be less efficacious than latanaprost in lowering IOP by a mean IOP difference of 0.9 mmHg. Adverse effects associated with 0.5% AR-12286 included stinging upon instillation and conjunctival hyperemia that typically resolved during sleep [87].
A third study was completed in normal volunteers in December 2010 to compare two formulations of 0.5% AR-12286 and assess systemic drug exposure (ClinicalTrials.gov identifier: NCT01250197 [101]). In this study, 8 days of daily administration of topical 0.5% AR-12286 resulted in systemic concentrations greater than 1 ng/ml in five out of 18 subjects. Overall, administration of 0.5% AR-12286 was observed to reduce IOP by 18–41%. Trace to moderate conjunctival hyperemia was observed with AR-12286 and resolved within 8 h of dosing [88]. Future studies are planned to evaluate the 24-h ocular hypotensive efficacy of 0.5% AR-12286 in patients with OAG or ocular hypertension (ClinicalTrials.gov identifier: NCT01330979 [101]) and to evaluate the added benefit of AR-12286 in patients already taking latanoprost (ClinicalTrials. gov identifier: NCT01302249 [101]).
K-115
K-115 (Kowa Company, Ltd.) is a ROCK inhibitor in clinical development for treatment of glaucoma and ocular hypertension. In a study conducted in 2009, 28 patients with primary OAG or ocular hypertension were treated with 0.2% K-115, 0.4% K-115 or placebo twice-daily for 1 day (CTI-090708) [89]. IOP was recorded at 11 time points, and a maximum reduction in IOP was observed 2 h after administration. The 0.4% K-115 solution exhibited the greatest reductions, while mild, transient, conjunctival hyperemia was noted in all patients [89].
In a second study of K-115, 210 patients with primary OAG or ocular hypertension were treated with one of three doses of K-115 (0.1, 0.2 or 0.4%) or placebo twice-daily for a duration of 8 weeks (CTI-101015) [90]. Like the first study, the greatest reductions in IOP were seen 2 h after administration. The largest effect was seen in the 0.4% dose group. Mild, transient, conjunctival hyperemia was present in 65.3% of patients given 0.4% K-115, and a conjunctival hemorrhage was observed in one patient who received 0.1% solution.
Results of the above studies were presented at the 2011 ARVO meeting [89,90], and the sponsor reportedly plans to initiate Phase III trials in the near future [102].
INS117548
In June 2009, Inspire Pharmaceuticals (Merck) completed a Phase I clinical trial with its ROCK inhibitor INS117548 for treatment of IOP in ocular hypertension and OAG (ClinicalTrials. gov identifier: NCT00767793 [101]). According to Inspire’s 2010 Annual Report (Form 10-K), in this Phase I study, INS117548 exhibited mild IOP-lowering effects with dose-related tolerability issues, specifically ocular discomfort such as stinging and burning. Since that time, no further development has been reported to date.
RKI983/SNJ-1656/Y-39983
RKI983/SNJ-1656/Y-39983 (Novartis/Senju Pharmaceuticals/Mitsubishi Pharma Corp.) is a ROCK inhibitor in development for treatment of elevated IOP. Tanihara et al. reported results from a Phase I clinical trial in which healthy volunteers received varying doses of SNJ-1656 delivered once over a 24-h period (0.003, 0.01, 0.03, 0.05 or 0.1%, or placebo) [19]. A dose-dependent decrease in IOP was observed with SNJ-1656 administration, and IOP returned to baseline within 24 h. In a separate arm of this study, patients received multiple doses of SNJ-1656 over 7 days (0.05% once-daily, 0.1% once-daily, 0.05% twice-daily or 0.1% twice-daily). Twice-daily administration was associated with a greater reduction in IOP than once-daily administration, leading the authors to recommend further investigation of twice-daily dosing [19]. No systemic adverse effects were reported; however, some subjects demonstrated trace to mild hyperemia. These ocular effects were most evident with administration of higher concentrations. For example, in the single-dose arm of the study, six out of six eyes receiving 0.1% solution and five out of six eyes receiving 0.05% solution exhibited ocular hyperemia [19]. Administration of 0.1% SNJ-1656 also induced blurry vision in one subject and photophobia in another [19]. Fewer adverse effects were reported with lower concentrations of SNJ-1656 and all hyperemia resolved within 12 h [19].
In a related study, patients with primary OAG or ocular hyper-tension received twice-daily RKI983 (0.01, 0.03 or 0.05%), latanoprost 0.005% once-daily in the evening, or placebo (ClinicalTrials.gov identifier: NCT00515424 [101]). In this study, a dose-dependent reduction in IOP was observed for RKI983, but the magnitude of IOP reduction was less than latanoprost [103]. Treatment with RKI983 also resulted in dose-dependent conjunctival hyperemia and other ocular adverse effects that were more prominent than those associated with latanoprost [103].
In a third clinical study, twice-daily treatment with RKI983 (0.05 or 0.10%) was compared with once-daily latanoprost 0.005% for a duration of 1 month (ClinicalTrials.gov identifier: NCT00846989 [101]). Although both concentrations of RKI983 led to a reduction in average IOP compared with baseline, latanoprost 0.005% yielded greater reductions in IOP [104]. Of note, more than 40% of patients receiving RKI983 exhibited adverse effects (predominantly conjunctival hyperemia) versus 31.9% of patients receiving latanoprost [104]. Since completion of this study in April 2009, no further development has been reported for RKI983.
ATS907
ATS907 (Altheos, Inc.) is a topical ROCK inhibitor that is being developed for lowering IOP in OAG and ocular hypertension [105]. Phase IIb clinical trials are planned to initiate in the fourth quarter of 2011 [92]. The developers of ATS907 believe that it has an advantage over competing ROCK inhibitors due its unique pharmacologic properties [106], namely its high cellular permeability and ability to convert to a more potent metabolite, ATS907 M1, after entry into the anterior chamber [93]. It is postulated that this prodrug-like strategy could potentially achieve targeted clinical potency while reducing adverse effects such as conjunctival hyperemia. Although clinical data have not yet been reported for ATS907, preclinical studies examining the efficacy, safety, tolerability and pharmaceutical profile of ATS907 seem promising.
DE-104
DE-104 (Santen Pharmaceuticals/Ube Industries) was discontinued in 2010 after it failed to meet primary end points in Phase I/II trials [14].
Expert commentary
Glaucoma is a sight-threatening condition that affects millions of people worldwide. Our understanding of the pathophysiology of glaucoma is incomplete, yet evidence suggests that IOP reduction and effective control is critical for preventing optic nerve damage and continued visual loss [93]. Current methods for reduction of IOP include topical medical therapies, laser procedures and surgery. Despite the availability of these various treatment modalities, many patients continue to lose vision while undergoing treatment, creating a need for additional therapeutic modalities to complement the existing mechanisms for treating glaucoma.
ROCK inhibitors comprise a promising new class of potential drugs for the treatment of glaucoma. Basic scientific studies of the Rho GTPase pathway in cells have provided insight into the mechanisms by which ROCK inhibitors relax smooth muscle, while in vivo and ex vivo studies have provided further evidence that ROCK inhibitors can enhance facility of outflow through the TM and reduce IOP in various models of normal tension and ocular hypertension. Although clinical studies to date suggest that ROCK inhibitors have the potential to lower IOP in patients with glaucoma and ocular hypertension, further clinical studies are needed to determine whether the IOP-lowering potential is sufficient for use as a primary glaucoma therapy versus an adjunct to existing treatments. The fact that ROCK inhibitors are effective through a novel pathway (conventional TM outflow) makes them a very attractive adjunct therapy to currently approved pharmacotherapies.
While the efficacy profile of ROCK inhibitors is promising, concerns have risen over the potential for ocular side effects, namely conjunctival hyperemia. Even if hyperemia does not affect vision and is due to the direct vasodilatory effect, ocular redness is a potential cosmetic issue that may affect patient satisfaction. Strategies exist for circumventing ROCK inhibitor-related hyper-emia. For example, it may be possible to achieve sufficient efficacy with once-daily dosing at bedtime, in which case the hyperemia would be expected to resolve during sleep [87]. An alternative strategy involves use of a prodrug that is activated after entering the anterior chamber of the eye [92]. This prodrug-like approach has the potential to increase the therapeutic index of the ROCK inhibitor and avoid the conjunctival hyperemia. Although these strategies are promising, further clinical studies are necessary to assess the safety/tolerability profile of these therapeutic candidates. The results of these clinical studies will likely be an important determinant of the utility of ROCK inhibitors in the clinic and in reducing the global burden of glaucoma.
Five-year view
ROCK inhibitors have been under development for glaucoma for many years, and some programs have been discontinued owing to lack of therapeutic benefit and/or undesirable effects such as conjunctival hyperemia. Yet other ROCK inhibitors remain in development for glaucoma, the hope being to somehow optimize the therapeutic index and achieve minimal side effects without sacrificing therapeutic efficacy. In fact, Phase I and II studies are underway to determine the optimal timing of doses and delivery strategy for ROCK inhibitors. Results of these studies will be telling as to whether this class of medications can gain regulatory approval and commercial appeal for the treatment of glaucoma.
Key issues.
Glaucoma is the second leading cause of blindness, affecting 60 million patients worldwide.
Despite availability of multiple medical and surgical treatments, many patients continue to lose functional vision from therapeutic failure.
There is a need for novel therapies to improve upon the standard of care for glaucoma treatment.
Rho kinase (ROCK) inhibitors represent a promising new class of drugs for treatment of glaucoma.
ROCK inhibitors decrease intraocular pressure, which is beneficial for glaucoma, by relaxing the trabecular meshwork cells of the eye, a component of the conventional outflow pathway that is critical for drainage of ocular fluid.
ROCK inhibitors can have a vasodilatory effect that leads to eye redness, an undesirable cosmetic side effect.
Clinical studies are underway to further assess the efficacy and safety of this new class of potential glaucoma medications.
Clinical studies should be implemented to further evaluate the potential benefit that this class offers to glaucoma patients, even beyond intraocular pressure lowering.
Footnotes
Financial & competing interests disclosure
B Wirostko discloses that she is a retained consultant and has equity in Altheos, and she also consults for Merck and iVeena. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol 90(3), 262–267 (2006).•• Describes an estimation of the worldwide prevalence of angle closure and open-angle glaucoma in 2010 and 2020.
- 2.The Costs of Blindness: An Analysis of the Costs of Visual Impairment and Blindness in the United Kingdom. Report prepared for The Guide Dogs for the Blind Association (2003).
- 3.Fautsch MP, Johnson DH. Aqueous humor outflow: what do we know? Where will it lead us? Invest. Ophthalmol. Vis. Sci 47(10), 4181–4187 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol 295(5), C1057–C1070 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziai N, Dolan JW, Kacere RD, Brubaker RF. The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Arch. Ophthalmol 111(10), 1351–1358 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Toris CB, Camras CB, Yablonski ME. Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology 100(9), 1297–1304 (1993). [DOI] [PubMed] [Google Scholar]
- 7.Salmon JF. Glaucoma In: Vaughan & Asbury’s General Ophthalmology. McGraw-Hill Medical, London, UK: (2008). [Google Scholar]
- 8.Weinreb RN. Uveoscleral outflow: the other outflow pathway. J. Glaucoma 9(5), 343–345 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Althin R, Sforzolini BS, Dhawan R. Persistency and treatment failure in newly diagnosed open angle glaucoma patients in the United Kingdom. Br. J. Ophthalmol 88(11), 1391–1394 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis BA, Ianchulev T, Schofield JK, Minckler DS. Selective laser trabeculoplasty as a replacement for medical therapy in open-angle glaucoma. Am. J. Ophthalmol 140(3), 524–525 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst. Rev 2, CD004399 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Nagar M, Ogunyomade A, O’Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br. J. Ophthalmol 89(11), 1413–1417 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur. J. Ophthalmol 16(1), 100–104 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin. Ophthalmol 5, 667–677 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert Opin. Emerg. Drugs 16(1), 137–161 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Rao PV, Deng P, Maddala R, Epstein DL, Li CY, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol. Vis 11, 288–297 (2005). [PubMed] [Google Scholar]
- 17.Fukunaga T, Ikesugi K, Nishio M et al. The effect of the Rho-associated protein kinase inhibitor, HA-1077, in the rabbit ocular hypertension model induced by water loading. Curr. Eye Res 34(1), 42–47 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Nishio M, Fukunaga T, Sugimoto M et al. The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes. Curr. Eye Res 34(4), 282–286 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol 126(3), 309–315 (2008).•• Describes a Phase I clinical trial of SNJ-1656 for use in glaucoma. This is the first clinical trial of a Rho kinase inhibitor for glaucoma that has been published in a peer reviewed journal.
- 20.Kjoller L, Hall A. Signaling to Rho GTPases. Exp. Cell Res 253(1), 166–179 (1999).• Describes upstream signaling in the Rho GTPase pathway.
- 21.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol 150(3), 567–580 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol 42, 283–323 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs 21(3), 167–177 (2007).• Describes experimental evidence for the use of Rho GTPase and Rho kinase inhibitors for the treatment of glaucoma.
- 24.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67(9), 545–554 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Amano M, Yamamoto T et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15(9), 2208–2216 (1996).•• Describes one of the first characterizations of Rho kinase.
- 26.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem 270(49), 29051–29054 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Nakajima E, Nakajima T, Minagawa Y, Shearer TR, Azuma M. Contribution of ROCK in contraction of trabecular meshwork: proposed mechanism for regulating aqueous outflow in monkey and human eyes. J. Pharm. Sci 94(4), 701–708 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Honjo M, Tanihara H, Inatani M et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci 42(1), 137–144 (2001).• Describes one of the first in vivo evaluations of the effects of a Rho kinase inhibitor on intraocular pressure and outflow facility.
- 29.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci 42(5), 1029–1037 (2001). [PubMed] [Google Scholar]
- 30.Wiederholt M Direct involvement of trabecular meshwork in the regulation of aqueous humor outflow. Curr. Opin. Ophthalmol 9(2), 46–49 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Wiederholt M, Groth J, Strauss O. Role of protein tyrosine kinase on regulation of trabecular meshwork and ciliary muscle contractility. Invest. Ophthalmol. Vis. Sci 39(6), 1012–1020 (1998). [PubMed] [Google Scholar]
- 32.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol 522(Pt 2), 177–185 (2000).• Describes Rho GTPase signaling.
- 33.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp. Eye Res 80(2), 197–206 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol 4(6), 446–456 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J. Biol. Chem 276(1), 670–676 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem 275(5), 3577–3582 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Lappalainen P, Drubin DG. Cofilin promotes rapid actin filament turnover in vivo. Nature 388(6637), 78–82 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Arber S, Barbayannis FA, Hanser H et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393(6687), 805–809 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J. Mol. Med 80(10), 629–638 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Harrison BA, Whitlock NA, Voronkov MV et al. Novel class of LIM-kinase 2 inhibitors for the treatment of ocular hypertension and associated glaucoma. J. Med. Chem 52(21), 6515–6518 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Koyama M, Ito M, Feng J et al. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett 475(3), 197–200 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Kaufman PL, Gabelt B, Tian B, Liu X. Advances in glaucoma diagnosis and therapy for the next millennium: new drugs for trabecular and uveoscleral outflow. Semin. Ophthalmol 14(3), 130–143 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Honjo M, Inatani M, Kido N et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch. Ophthalmol 119(8), 1171–1178 (2001).• Describes one of the first in vivo evaluations of the effects of a Rho kinase inhibitor on intraocular pressure and outflow facility.
- 44.Waki M, Yoshida Y, Oka T, Azuma M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr. Eye Res 22(6), 470–474 (2001).• Describes one of the first in vivo evaluations of the effects of a Rho kinase inhibitor on intraocular pressure.
- 45.Henderson AJ, Hadden M, Guo C et al. 2,3-diaminopyrazines as Rho kinase inhibitors. Bioorg. Med. Chem. Lett 20(3), 1137–1140 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Tokushige H, Inatani M, Nemoto S et al. Effects of topical administration of y-39983, a selective Rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest. Ophthalmol. Vis. Sci 48(7), 3216–3222 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Davis RL, Kahraman M, Prins TJ et al. Benzothiophene containing Rho kinase inhibitors: efficacy in an animal model of glaucoma. Bioorg. Med. Chem. Lett 20(11), 3361–3366 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal R, Choritz L, Schlott S et al. Effects of ML-7 and Y-27632 on carbacholand endothelin-1-induced contraction of bovine trabecular meshwork. Exp. Eye Res 80(6), 837–845 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Rao PV, Peterson YK, Inoue T, Casey PJ. Effects of pharmacologic inhibition of protein geranylgeranyltransferase type I on aqueous humor outflow through the trabecular meshwork. Invest. Ophthalmol. Vis. Sci 49(6), 2464–2471 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest. Ophthalmol. Vis. Sci 46(7), 2424–2432 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Whitlock NA, Harrison B, Mixon T et al. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J. Ocul. Pharmacol. Ther 25(3), 187–194 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res 80(2), 215–225 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp. Eye Res 86(2), 271–281 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renieri G, Choritz L, Rosenthal R, Meissner S, Pfeiffer N, Thieme H. Effects of endothelin-1 on calcium-independent contraction of bovine trabecular meshwork. Graefes Arch. Clin. Exp. Ophthalmol 246(8), 1107–1115 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Koga T, Awai M, Tsutsui J, Yue BY, Tanihara H. Rho-associated protein kinase inhibitor, Y-27632, induces alterations in adhesion, contraction and motility in cultured human trabecular meshwork cells. Exp. Eye Res 82(3), 362–370 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci 48(5), 2105–2114 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Shibuya M, Suzuki Y, Sugita K et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J. Neurosurg 76(4), 571–577 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Sasaki Y, Suzuki M, Hidaka H. The novel and specific Rho-kinase inhibitor (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline)sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol. Ther 93(2–3), 225–232 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Yu W, Cao G, Qiu J et al. Evaluation of monkey intraocular pressure by rebound tonometer. Mol. Vis 15, 2196–2201 (2009). [PMC free article] [PubMed] [Google Scholar]
- 60.Yu M, Chen X, Wang N et al. H-1152 effects on intraocular pressure and trabecular meshwork morphology of rat eyes. J. Ocul. Pharmacol. Ther 24(4), 373–379 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitlock NA, Mcknight B, Corcoran KN, Rodriguez LA, Rice DS. Increased intraocular pressure in mice treated with dexamethasone. Invest. Ophthalmol. Vis. Sci 51(12), 6496–6503 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Tian B, Kaufman PL, Volberg T, Gabelt BT, Geiger B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch. Ophthalmol 116(5), 633–643 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Bain J, Mclauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J 371(Pt 1), 199–204 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tian B, Brumback LC, Kaufman PL. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey eye. Exp. Eye Res 71(6), 551–566 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Peterson YK, Kelly P, Weinbaum CA, Casey PJ. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J. Biol. Chem 281(18), 12445–12450 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Baigent C, Keech A, Kearney PM et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366(9493), 1267–1278 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Larosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 282(24), 2340–2346 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 326(7404), 1423 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation 101(2), 207–213 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Mcgwin G Jr, Mcneal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch. Ophthalmol 122(6), 822–826 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Owen CG, Carey IM, Shah S et al. Hypotensive medication, statins, and the risk of glaucoma. Invest. Ophthalmol. Vis. Sci 51(7), 3524–3530 (2010). [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Cai S, Glasser A et al. Effect of H-7 on cultured human trabecular meshwork cells. Mol. Vis 7, 145–153 (2001). [PubMed] [Google Scholar]
- 73.Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp. Eye Res 78(1), 137–150 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Sabanay I, Gabelt BT, Tian B, Kaufman PL, Geiger B. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Arch. Ophthalmol 118(7), 955–962 (2000). [PubMed] [Google Scholar]
- 75.Tian B, Gabelt BT, Peterson JA, Kiland JA, Kaufman PL. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Invest. Ophthalmol. Vis. Sci 40(1), 239–242 (1999). [PubMed] [Google Scholar]
- 76.Tian B, Wang RF, Podos SM, Kaufman PL. Effects of topical H-7 on outflow facility, intraocular pressure, and corneal thickness in monkeys. Arch. Ophthalmol 122(8), 1171–1177 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Hidaka H, Inagaki M, Kawamoto S, Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23(21), 5036–5041 (1984). [DOI] [PubMed] [Google Scholar]
- 78.Domnina LV, Ivanova OY, Pletjushkina OY et al. Marginal blebbing during the early stages of TNF-induced apoptosis indicates alteration in actomyosin contractility. Cell Biol. Int 28(6), 471–475 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Meyer-ter-Vehn T, Sieprath S, Katzenberger B, Gebhardt S, Grehn F, Schlunck G. Contractility as a prerequisite for TGF- beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest. Ophthalmol. Vis. Sci 47(11), 4895–4904 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Meyer-ter-Vehn T, Katzenberger B, Han H, Grehn F, Schlunck G. Lovastatin inhibits TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest. Ophthalmol. Vis. Sci 49(9), 3955–3960 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Honjo M, Tanihara H, Kameda T, Kawaji T, Yoshimura N, Araie M. Potential role of Rho-associated protein kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest. Ophthalmol. Vis. Sci 48(12), 5549–5557 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Lingor P, Tonges L, Pieper N et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 131(Pt 1), 250–263 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Tura A, Schuettauf F, Monnier PP, Bartz-Schmidt KU, Henke-Fahle S. Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Invest. Ophthalmol. Vis. Sci 50(1), 452–461 (2009). [DOI] [PubMed] [Google Scholar]
- 84.Hirata A, Inatani M, Inomata Y et al. Y-27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia. Graefes Arch. Clin. Exp. Ophthalmol 246(1), 51–59 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Sugiyama T, Shibata M, Kajiura S et al. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Invest. Ophthalmol. Vis. Sci 52(1), 64–69 (2011).• Describes the effect of a Rho kinase inhibitor on optic nerve head blood flow in vivo.
- 86.Williams RD, Novack GD, Van Haarlem T, Kopczynski C; AR-12286 Phase 2A study group. Ocular hypotensive effect of the rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol 152(5), 834–841.e1 (2011). [DOI] [PubMed] [Google Scholar]
- 87.Serle JB, Novack GD, Van Haarlem TJ, Kopczynski C, Ar-12286 Phase 2b Study Group. A 28-day active-controlled, Phase 2b study assessing the safety and ocular hypotensive efficacy of AR-12286 in patients with elevated intraocular pressure. ARVO Meeting Abstracts 52(6), 217 (2011). [Google Scholar]
- 88.Kopczynski C, Novack GD, Swearingen D, Van Haarlem TJ. Ocular hypotensive efficacy, safety, and systemic absorption of AR-12286 in normal volunteers. ARVO Meeting Abstracts 52(6), 6689 (2011). [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto T, Abe H, Kuwayama Y, Tanihara H, Araie M. Efficacy and safety of the Rho kinase inhibitor, K-115, over 24 hours in patients with primary open-angle glaucoma and ocular hypertension. ARVO Meeting Abstracts 52(6), 216 (2011). [Google Scholar]
- 90.Tanihara H, Abe H, Kuwayama Y, Yamamoto T, Araie M. Ocular hypotensive dose-response efficacy and safety of the Rho kinase inhibitor, K-115, in patients with primary open-angle glaucoma and ocular hypertension. ARVO Meeting Abstracts 52(6), 220 (2011). [Google Scholar]
- 91.Wortman M Rock-ing glaucoma prevention Altheos, Inc, CA, USA, START-UP, 27–28 (2010). [Google Scholar]
- 92.Kengatharan M, Wirostko BM, Umeno H, Hsu HH. Pharmaceutical profile Of a novel Rho kinase (ROCK) inhibitor ATS907 for reduction of IOP in glaucoma. ARVO Meeting Abstracts 52(6), 3106 (2011). [Google Scholar]
- 93.Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R. Intraocular pressure control and long-term visual field loss in the collaborative Initial Glaucoma Treatment Study. Ophthalmology 118(9), 1766–1773 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.ClinicalTrials.gov. http://clinicaltrials.gov
- 102.Novartis Clinical Trial Results Database. CRKI983A2101: A multicenter, randomized, placebo-controlled, latanoprost-controlled, parallel group study to assess the tolerability, safety and efficacy of RKI1983 (0.01%, 0.03%, and 0.05%) ophthalmic solution given twice a day over one week in patients with primary open angle glaucoma or ocular hypertension (2007). www.novctrd.com
- 103.Novartis Clinical Trial Results Database. CRKI983A2201: A 4-week multi-center, single-masked, randomized, latanoprost-controlled, parallel group study to assess the efficacy, tolerability and safety of RKI983 (0.05% and 0.10%) ophthalmic solution given twice a day versus once daily latnoprost 0.005%, in patients with primary open angle glaucoma or ocular hypertension (2009). www.novctrd.com
- 104.Kowa Announces the Efficacy and Safety of a Novel Rho Kinase Inhibitor (K-115) in the Treatment of Glaucoma (2011). www.kowa.co.jp/eng/news/press11050902.pdf
- 105.Altheos Completes $20 Million Series A Financing to Develop Next-Generation Glaucoma Treatment (2010). www.altheos.net/5-April-2010.html
- 106.Bouchie A Altheos mines its Rho-lodex. BioCentury, The Bernstein Report On BioBusiness (2010). www.biocentury.com