Abstract
Objectives:
Delays in the diagnosis and detection of bipolar disorder can lead to adverse consequences, including improper treatment and increased suicide risk. The Mood Spectrum Self-Report Measure (MOODS-SR) was designed to capture the full spectrum of lifetime mood symptomology with factor scores for depression and mania symptom constellations. The utility of the MOODS-SR as a tool to investigate homogenous subgroups was examined, with particular focus on a possible bipolar risk subgroup. Moreover, potential patterns of differences in MOODS-SR subtypes were probed using cognitive vulnerabilities, neuropsychological functioning, and ventral striatum connectivity.
Methods:
K-mean cluster analysis based upon factor scores of MOODS-SR was used to determine homogenous subgroupings within a healthy and remitted depressed young adult sample (N=86). Between-group comparisons (based upon cluster sub-groupings) were conducted on measures of cognitive vulnerabilities, neuropsychological functioning, and ventral striatum rs-fMRI connectivity.
Results:
Three groups of participants were identified: one with minimal symptomology, one with moderate primarily depressive symptomology, and one with more severe manic and depressive symptomology. Differences in impulsivity, neuroticism, conscientiousness, facial perception accuracy, and rs-fMRI connectivity exist between moderate and severe groups.
Conclusions:
Within a sample of people with and without depression histories, a severe subgroup was identified with potentially increased risk of developing bipolar disorder through use of the MOODS-SR. This small subgroup had higher levels of lifetime depression and mania symptoms. Additionally, differences in traits, affective processing, and connectivity exist between those with a more prototypic unipolar subgrouping and those with potential risk for developing bipolar disorder.
Keywords: bipolar disorder, depression, phenotype, risk factors, neuropsychology, resting state
Introduction
Major depressive disorder (MDD) is diagnosed as a separate illness from bipolar disorder (BD), yet the behavioral, clinical, performance, genetic, and neuroimaging differences between these groups have been limited to modest. Indeed, both MDD and BD share major depressive episodes, one reason why it is not surprising that there are overlapping traits in the literature.1–4 Furthermore, any measurement that yields a single score for MDD likely misrepresents the heterogeneity of the disorder,5 and does not necessarily rule out bipolar spectrum symptoms. Furthermore, early in the course of the illness, a substantial minority of those presenting with MDD will later be diagnosed with BD.6,7
It is well understood that BD is often missed in screening batteries for those presenting with mood spectrum pathology and initial evaluations for major depressive disorder.8 For example, BD is often incorrectly diagnosed and not treated until 10–20 years after onset, usually about 14 years.9 This underdiagnosis of hypomania and mania often leads to poorer prognosis due to delay in start of treatment, greater disruptions in the life course, adverse life events related to untreated manias, and increased risk for suicide.8,10 Difficulties in the diagnosis of BD can stem from many sources. Lack of subjective distress during hypomanic and manic episodes, and ‘normalization’ of some risky behaviors associated with mania during young adulthood (e.g., sleep deprivation, substance abuse, sexual experimentation)11,12 can contribute to under- or delayed diagnosis. Therefore, mania is a substantial, poorly perceived risk in the late adolescent and young adult period for those with a history of MDD. Faster and more accurate diagnosis is a current area of need for this age period.
A number of different methods have been employed to increase specificity in assessment tools for MDD and BD (e.g., defining more homogeneous groups by psychomotor disturbance levels or anxiety).13,14 These methods have demonstrated some success – for example, depressed individuals with lower reward responsiveness and higher anhedonia are more likely to have difficulty pursuing simple rewards7 and those with melancholic subtype depression tend to have more psychomotor slowing and set-shifting difficulty.15 More recently, large studies using RDoC approaches with dimensional strategies have been able to define subcategories: of mood disorders; one through use of Go/No-Go responding and another using event-related potentials.16,17
A combined dimensional and lifespan approach to mood disorders may aid in more accurate diagnoses by collecting more detailed information about prior episodes and periods of relative wellness. For example, spectrum models take into account that depression symptoms can be elevated repeatedly at various points over the lifespan or even that certain symptoms never occur at all, an idea not accounted for in binary categories of current or recent diagnosis.18 Moreover, some symptom patterns may not always be present at the point of assessment, yet may be pathognomonic. For example, severe anhedonia may only be present in one of many prior episodes and would not be specified within a current or most recent past diagnosis. Assessing the full spectrum of symptoms across the lifespan may aid in early detection and diagnosis. One way to measure the full spectrum of symptoms involved in mood disorders is to assess factors of depressive and manic symptoms over the lifetime, including prodromal and subthreshold behavioral manifestations, such as through the Mood Spectrum Self-Report Measure (MOODS-SR).19 Utilizing exploratory factor analysis, Cassano and colleagues18 identified six factors related to lifetime depression symptoms, including depressive mood (and anhedonia), psychomotor retardation, suicidality, and neurovegetative symptoms. In a separate report with patients diagnosed with BD, classical exploratory factor analysis revealed factors related to mania, including psychomotor activation, creativity (e.g., artistic creativity and sensitivity), mixed instability (e.g., sexual promiscuity, alcohol-related mood change and irritability, and changing jobs, residencies, friends and hobbies), inflated self-esteem, and wastefulness or recklessness (e.g., spending more money than one can afford, risk-taking behavior).8
Different lifetime subtypes of mood pathology might have different biomarkers, cognitive biases, affective traits, and personality traits as correlates. For example, higher rumination and impulsivity put individuals at even higher risk for developing BD compared to developing MDD only.20, 21 Neuropsychological batteries and neuroimaging tools might also detect discrepancies in functioning and may offer pathophysiological correlates, thus could be a useful way to distinguish between subgroups. Specific deficits in neuropsychological functioning are hallmarks of MDD and BD even when the individual is currently well,22, 23 although there appears to be substantial overlap in these cognitive markers. Differences in these measurements may be useful to delineate and characterize different mood disorder groupings, or better understand the neurobiology that is affected in different subgroups.
Indeed, disruption in the functional circuits in the brain, particularly those supporting emotion processing, emotion regulation and reward processing, have been observed in those with BD.24 One particular region of interest in these functional circuits, and particularly within resting-state functional connectivity analyses, has been the ventral striatum, involved in reward processing, anhedonia, and behavioral activation. The VS regions, and these related constructs, are all potential sources of difference between MDD and BD. In particular, this region has shown differences in function and structure in those with BD and those at risk for the development of BD.6 A recent review has noted increased volume, decreased grey matter in those at risk, and increased activity at rest in the ventral striatum.7 Disrupted connectivity between the VS and other regions of the brain may represent an early neural marker of BD.6 Abnormalities and disruptions to this region may correspond to the onset and risk for developing BD.
In the present study, the goal is to identify subtypes of MDD, particularly subtypes that are at-risk for developing BD. We hypothesize that different subgroups based on mood symptoms of this young adult sample can be defined using cluster-analysis. We also expected that these subgroups would display different cognitive vulnerabilities, neuropsychological functioning, and neural connectivity related to the ventral striatum, particularly as it hones in on lifetime hypo/mania vulnerability.
Methods
Study participants (N=78) from the ages of 18–23 were recruited from the Chicago, IL and Ann Arbor, MI communities. Age range was restricted to young adulthood to better highlight any emerging subgroup patterns at an optimal point to minimize variance in development and effects of disease progression. Additionally, this age range allows testing the hypotheses that some young adults already show subthreshold manic symptomatology, and that the MOODS-SR might be useful in identifying those at risk for later diagnosis of BD. The present study was a secondary sub-analysis, and the sample was under - powered to explore the optimal number of disease subtypes, and is merely an exploratory illustration. Recruiting currently remitted patients with MDD minimized current symptom load, as they were not currently meeting threshold for a major depressive episode. Diagnoses were made based on DSM-IV criteria using the Diagnostic Interview for Genetic Studies25 after informed consent was completed, consistent with the Declaration of Helsinki and approved by the Institutional Review Boards of the University of Illinois at Chicago, and the University of Michigan at Ann Arbor. Remitted major depressive disorder (rMDD) participants met criteria for history of MDD and were allowed to have current or past co-morbid anxiety disorders. HCs were excluded for any current or past Axis I or Axis II disorder. Participants diagnosed with rMDD typically had 1 or 2 previous episodes (mode of 1 episode). Diagnosis was confirmed with family interview (parent or older sibling) using modified Family Interview for Genetic Studies.26 All data reported was collected over separate intake, cognitive testing and fMRI sessions.
Mood Spectrum Self-Report (MOODS-SR).
The MOODS-SR has 161 yes or no questions regarding whether the participant has experienced various situations now or in the past, which are then summed into factors.8,18,19
Clinical Variables.
Clinical variables of interest regarding illness were collected through the DIGS, including age of first episode, number of depressive episodes, length of longest depressive episode, and Global Assessment of Functioning (GAF).25 Family history was ascertained as part of the DIGS, adapted FIGS, and Longitudinal Interval Follow-up Evaluations (LIFE), coded for presence of any mood disorder.
Questionnaire Measures.
The Ruminative Responses Scale (RRS) is a self-report measure of rumination.27 Two motivational systems were measured by the BIS/BAS.28 The Barratt Impulsiveness Scale (BIS-11) is a self-report measure of impulsive behavior and preferences.29 The NEO-Personality Inventory-Revised (NEO-PI-R) was administered to assess five major domains of personality: Openness to Experience, Conscientiousness, Extraversion, Agreeableness, and Neuroticism, .30
Neuropsychological Measures.
Estimated Intelligence Quotient was assessed using the Synonym Knowledge Task to determine if subtypes differ in global cognitive ability.31 The Facial Emotion Perception Test (FEPT) is a test of accuracy and speed in identification of facial expressions including accuracy for fear, anger, happy, sad and neutral faces.32 The Modified, Titrated Monetary Incentive Delay Task (mMID) is a simple, contingent reward sensitivity task dependent upon responding to a rapid response window. The task is titrated based off of the participant’s accuracy and speed to optimize performance to perceived difficulty ratios.33 Money earned during the last two runs was used as the dependent variable. The Parametric Go/No-Go Task captures sustained attention, inhibitory control, and processing speed to target cues.32, 34 Reaction time and percent correct inhibition was assessed for both 2 and 3 target trials. Controlled Oral Word Association Test (COWA) provides a measure of verbal fluency to confrontation based upon cues of the first consonant in words.35 The Wisconsin Card Sorting Test provides a measure of executive functioning, including inferential problem solving using error percentiles.36 Trail Making A/B and C/D were also administered, capturing visual speed and switching respectively.37 The Purdue Pegboard provides a measure of bimanual dexterity.
fMRI Acquisition
The University of Michigan scan consisted of an eyes-open resting state scan acquired over eight minutes using a 3.0 T GE Signa scanner (Milwaukee, WI). We used T2*-weighted single shot reverse spiral sequences with the following parameters: 90 degree flip, field-of-view 20, matrix size =64*64, slice thickness =4 mm, 30 ms echo time, 29 slices. At the University of Illinois, we collected eyes-open, eight minute resting scans using a 3.0 T GE Discovery scanner (Milwaukee, WI), using parallel imaging with ASSET and T2* gradient-echo axial EPI. We used the following parameters: 90 degree flip, field-of-view = 22 * 22 cm, matrix size =64 * 64, slice thickness = 3 mm (0 mm gap), 22.2 ms echo time, 44 slices. At both locations, high-resolution anatomic T1 scans were obtained for spatial normalization. Motion was minimized using foam pads, and/or cross on the display, and participants were told the importance of staying still. Additionally, a visual tracking line was used at the University of Illinois. For both sites, TRs of 2000 ms were used, with a total of 240 TRs.
fMRI Preprocessing
We took several steps to reduce effects of noise and artifact. Slice time correction was completed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/doc/), and we applied motion detection and correction algorithms using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). We used coregistration of structural images to functional images. Next we used spatial normalization of the coregistered T1-spgr to the Montreal Neurological Institute (MNI) template. The normalization matrix was applied to the slice-time-corrected, time series data. The result, normalized T2* timeseries data, was spatially smoothed with a 5 mm Gaussian kernel producing T2* images with isotropic voxels, 2 mm on a side.
fMRI Cross-Correlation Analysis
Time series data were detrended and mean-centered. Additionally, physiologic correction was performed by regressing out white matter and cerebral spinal fluid signals.38 Motion parameters were regressed out.39 Global signal was not regressed due to collinearity violations with gray matter signal, challenging mis-estimates of anticorrelations40 and non-linear impact upon distance-micromovement relationships.39 Time-series were band-pass filtered over 0.01 – 0.10 Hz. Seeds were derived based on previous literature examining resting state connectivity of the ventral striatum.41 The following Montreal Neurologic Institute (MNI) coordinates were used based upon prior work: right superior ventral striatum (RVSs; 10, 15, 0), right inferior ventral striatum (RVSi; 9, 9, −8), left superior ventral striatum (LVSs; −10, 15, 0), and left inferior ventral striatum (LVSi; −10, 15, 0). The VSi is what is traditionally considered Nucleus Accumbens in humans, and the VSs is ventral caudate, and we used these pre-existing foci to enable comparisons with prior studies. 41
Statistical Analyses
K-Means Cluster Analysis to Determine Homogeneous Subsets.
Utilizing the factors developed by Cassano and colleagues,8,18 nine mania and six depression factors from the MOODS-SR were entered into a k-cluster analysis to divide participants, with an optimal solution of 3 clusters based upon sample size and AIC criteria (AIC = 668.74, chosen to maintain statistical power while allowing investigation into more homogeneous subgroupings. The 2 (AIC = 687.56) and 4 (AIC = 676.22) cluster solutions were equivalent in silhouette measure of cohesion and separation (.4 for all 3 solutions). Moroever a 2 cluster solution recapitulated the case-control group membership, making it a less ideal solution. The 4 cluster solution had one cell which was too small (n=6) for subsequent comparisons, rendering the 3 cluster solution optimal for the hypotheses put forth. The three-cluster solution was derived from differences in factor scores from both depression and mania subscales of the MOODS-SR. Based upon clinical characteristics and the scores on the MOODS-SR factors, the k-clusters were then labeled Minimal (Min), Moderate (Mod), and Severe (Sev; Table 1).
Table 1.
Demographics and MOOD-SR Clusters
| Min (N=31) |
Mod (N=34) | Sev (N=13) |
Statistical Analysis | ||
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | F | p-value | |
| Demographics | |||||
| Males (N) | 9 | 9 | 5 | 0.66 | .72 |
| rMDD (N) | 3 | 34 | 13 | 66.23 | <.001 |
| Race (N Caucasian) | 21 | 18 | 8 | 15.04 | .52 |
| Age | 20.71 (1.62) | 21.26 (1.54) | 20.92 (1.75) | 0.978 | .38 |
| Education | 14.61 (1.45) | 14.71 (1.36) | 13.85 (1.52) | 1.81 | .17 |
| Verbal IQ | 104.45 (9.57) | 106.73 (8.48) | 101.62 (10.82) | 1.47 | .24 |
| Longest MDE | 8.00 (0.00) | 27.39 (30.33) | 26.5 (36.71) | 0.18 | .84 |
| GAF at Intake | 91.52 (4.42) | 82.54 (8.74) | 77.73 (11.48) | 17.13 | < .001a |
| Age of First Episode | 17.5 (2.12) | 16.47 (4.33) | 15.75 (4.18) | 0.20 | .82 |
| Number of MDE | 0.15 (0.38) | 1.71 (1.29) | 2.92 (2.81) | 9.43 | < .001 b |
| MOOD-SR Clusters | |||||
| Depression Factors | |||||
| Depressed Mood | 1.87 (2.09) | 16.29 (2.8) | 16.92 (4.96) | 217.95 | < .001 b |
| Psychomotor Retardation | 1.13 (1.73) | 8.53 (3.58) | 11.60 (1.86) | 92.14 | < .001 b, c |
| Suicidality | 0.26 (0.68) | 2.24 (1.76) | 2.38 (1.98) | 17.54 | < .001b |
| Drug/Illness Related Depression | 0.00 (0.00) | 0.56 (0.89) | 1.23 (1.09) | 13.46 | <.001b,c |
| Psychotic Features | 0.48 (0.96) | 2.15 (1.23) | 3.46 (1.13) | 37.4 | <.001 b,c |
| Neurovegetative Symptoms | 1.35(1.72) | 5.15 (2.28) | 7.77 (1.88) | 55.06 | <.001 b,c |
| Mania Factors | |||||
| Psychomotor Activation | 1.06 (1.61) | 2.97 (2.46) | 9.54 (2.5) | 70.78 | <.001 b,c |
| Creativity | 2.2 (2.23) | 5.29 (2.46) | 8.69 (1.25) | 42.27 | <.001 b,c |
| Mixed Instability | 0.42 (0.62) | 1.18 (0.83) | 2.85 (1.99) | 24.63 | <.001 b,c |
| Sociability/Extraversion | 2.19 (1.94) | 2.26 (1.8) | 4.15(1.63) | 5.99 | .004 d |
| Spirituality/Mysticism/Psychoticism | 0.23 (0.76) | 0.47 (1.13) | 1.77 (1.64) | 9.22 | <.001 d |
| Mixed Irritability | 0.46 (0.77) | 1.91 (1.22) | 4.38 (1.5) | 57.21 | <.001 b,c |
| Inflated Self-Esteem | 0.16 (0.45) | 0.91 (1.11) | 3.23 (1.59) | 42.13 | <.001 b,c |
| Euphoria | 0.81 (1.08) | 1.89 (1.51) | 4.08 (1.04) | 29.97 | <.001 b,c |
| Wastefulness/Recklessness | 0.74 (1.09) | 0.85 (1.10) | 2.46 (0.88) | 13.22 | <.001 d |
Min= Minimal, Mod= Moderate (Unipolar), Sev = Severe, M= Mean, SD= Standard Deviation, IQ= Intelligence Quotient, MDE=Major Depressive Episode in weeks, GAF= Global Assessment of Functioning, rMDD= remitted Major Depression.
= Min > Mod, Sev
= Mod, Sev > Min
= Sev > Mod
= Sev>Min, Mod
Cluster Group Comparisons
A series of ANOVAs were computed between the 3 cluster groups. For questionnaire measures, and neuropsychological tests, significant ANOVAS were followed by post-hoc tests with Bonferroni corrections to determine specific differences between patient clusters. For rs-fMRI, a threshold of p < 0.005 and cluster extent of 57 voxels was used (p < .01 corrected for each model) based upon the updated 2016 version of 3dClustsim.42 Data from significant areas of group differences in connectivity were extracted using Marsbar (http://marsbar.sourceforge.net) and compared using post-hoc t-tests (Bonferroni corrected).
Results
Cluster Analysis
The Min cluster consisted of a mixture of HC (N=28) and rMDD (N=3), while the Mod and Sev clusters consisted only of rMDD (N=34; N=13 respectively; Table 1). There was a main effect of group on all measures of the MOODS-SR (Table 1). Post-hoc tests were run to determine pairwise differences. The Min cluster was significantly lower than Sev on all factors (p <.001). Min was significantly lower than Mod on all factors (all p <0.02) except Sociability/Extraversion, Spirituality/Mysticism/Psychoticism, and Wastefulness. Sev was higher than Mod on all factors except for Depressed Mood and Suicidality (Table 1). Clinically, Min was higher in GAF than Mod and Sev (p<0.001; p<.001;Table 1). Those in Sev group were numerically, but not significantly more likely to have a positive family history of mood disorder (6/12) relative to Mod (12/32) and Min (5/27), (X = 4.58 (2), p = .11), all but one (hx of BD) of whom were positive history of MDD. Among rMDD, those without family history were significantly higher in Depression (F(1,46) = 4.39, p = .04) and Suicidal ideation (F(1,46) = 4.90, p = .03) factors, but did not differ in any other factors (ps > .11).
Connectivity to RVSs
Results are reported in Supplemental Table 1 and Figure 2. The areas of connectivity that differed amongst the MOODS-SR groups were in regions posited to be part of the cognitive control network - middle and inferior frontal gyri, precuneus, and anterior cingulate cortex. Post-hoc Bonferroni tests were run to determine pairwise differences. Connectivity was higher in Mod versus Sev in middle and inferior frontal gyri. Connectivity was higher in Min, Mod versus Sev for anterior cingulate cortex. It was lower in the precuneus in Min versus Mod, and Sev.
Figure 2. Differences in Connectivity to RVSs seed.
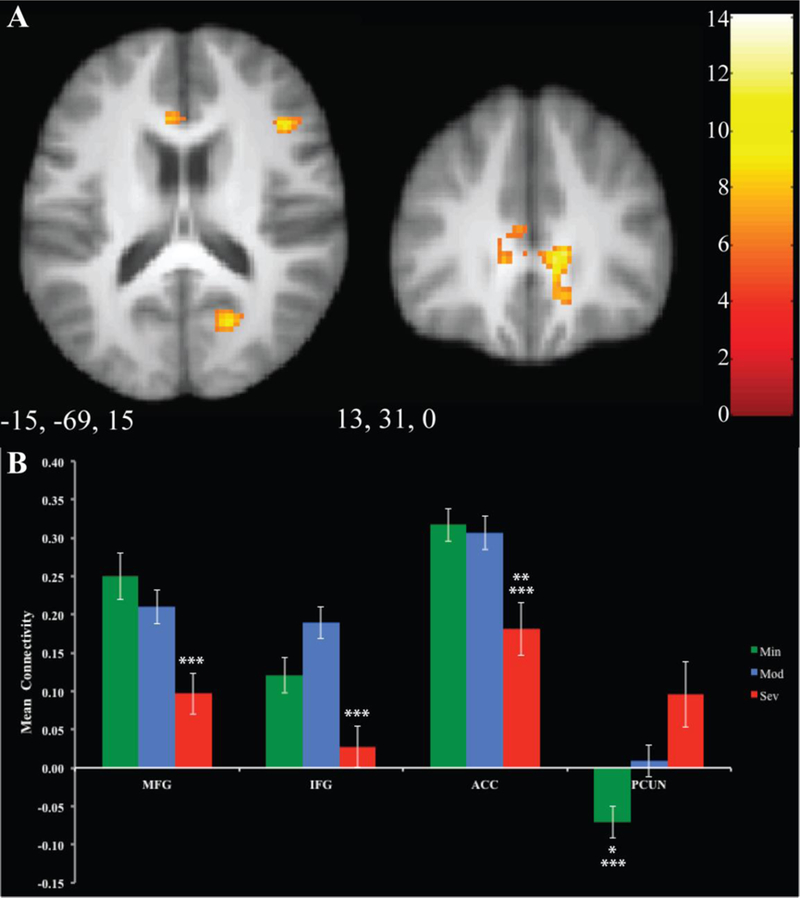
Regions of significant differences between groups are displayed in Panel A. Mean connectivity for each group is illustrated for each cluster in Panel B. Error bars represent 1 standard error. Significant relationships between clusters are denoted by asterisks.: ** =p<.05 between Min and Sev, ***= p<.05 between Mod and Sev, MFG=Middle Frontal Gyrus, IFG=Inferior Frontal Gyrus, ACC=Anterior Cingulate Cortex, PCUN= Precuneus
Connectivity to LVSs
Results are reported in Supplemental Table 2 and Figure 3. There is a main effect of group in a large number of networks, including cognitive control, default mode, and secondary visual regions. Post-hoc Bonferroni tests were run to determine pairwise differences. Mod was higher than Sev in the middle frontal gyrus. Mod showed significantly higher connectivity than Min in the inferior frontal gyrus, supramarginal gyrus, and cuneus. For the supramarginal gyrus, posterior cingulate, and caudate, Mod was significantly higher than Min and Sev. Connectivity in the lingual gyrus was significantly higher in Sev versus Min and Mod. For the fusiform gyrus, Sev was significantly higher than Min. In the anterior cingulate gyrus and for the other cluster in the posterior cingulate gyrus, Min was significantly lower than Mod and Sev.
Figure 3. Differences in Connectivity to LVSs seed.
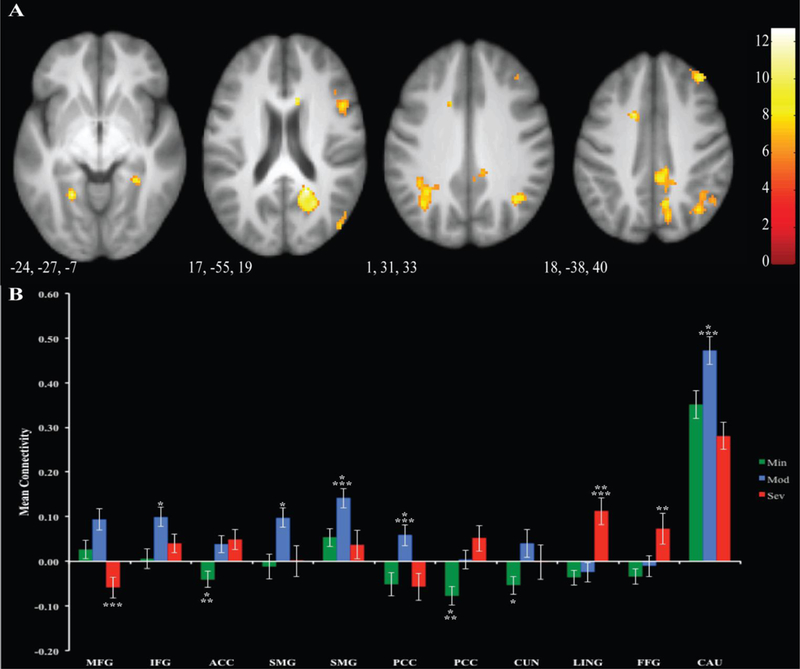
Regions of significant differences between groups are displayed in Panel A. Mean connectivity for each group is illustrated for each cluster in Panel B. Error bars represent 1 standard error. Significant relationships between clusters are denoted by asterisks.: *=p<.05 between Min and Mod ** =p<.05 between Min and Sev, ***= p<.05 between Mod and Sev, MFG=Middle Frontal Gyrus, IFG=Inferior Frontal Gyrus, ACC=Anterior Cingulate, SMG=Supramarginal Gyrus, PCC= Posterior Cingulate Cortex, LING= Lingual Gyrus, FFG=Fusiform Gyrus, CAU=Caudate
Connectivity to RVSi
Results are reported in Supplemental Table 3 and Figure 4. There was a main effect of group for the precuneus and the vermis. Post-hoc Bonferroni tests were run to determine pairwise differences. In the precuneus, Sev was significantly higher than Min and Mod. Connectivity with the vermis was significantly higher in Sev versus Min and Mod.
Figure 4. Differences in Connectivity to RVSi seed.
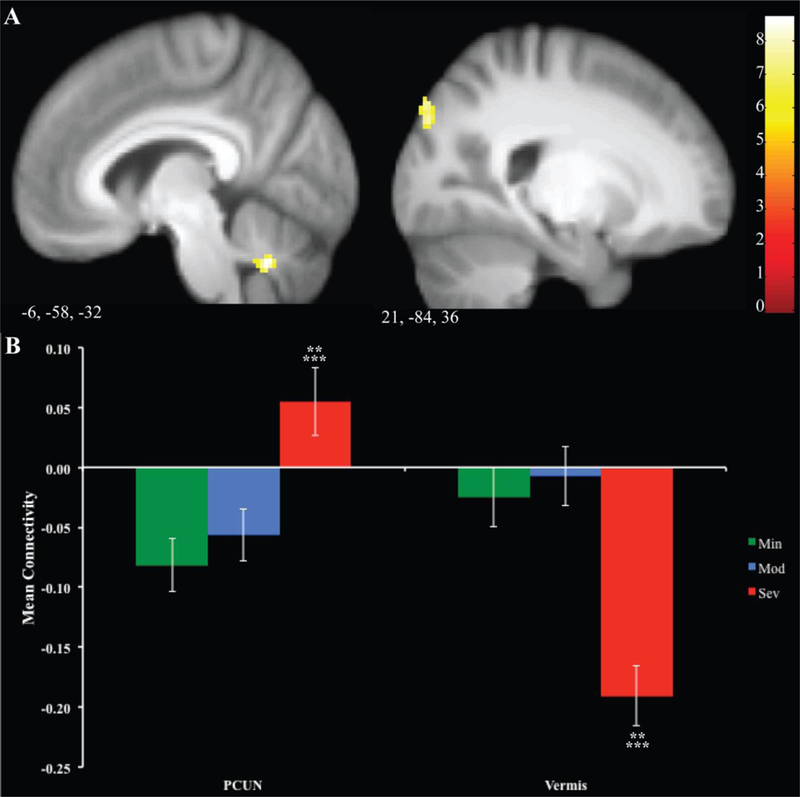
Regions of significant differences between groups are displayed in Panel A. Mean signal for each group is illustrated for each cluster in Panel B. Error bars represent 1 standard error. ** =p<.05 between Min and Sev, ***= p<.05 between Mod and Sev
Connectivity to LVSi
There were no significant main effect of group observed for LVSi.
Questionnaire Measures
There was a significant main effect of group on the RRS, BIS-11 Total, BAS Total, Neuroticism, Extraversion, Openness, and Consciousness (Table 2). Post-hoc Bonferroni tests were run to determine pairwise differences. On the RRS, Min was lower than Mod and Sev. Mod was lower than Sev on BIS-11. Min was lower than Mod for the BAS total only. Min and Mod were significantly lower than Sev on Neuroticism, and higher in Conscientiousness . Min was higher in extraversion than Mod and Sev. Sev was significantly higher in Openness relative to Mod.
Table 2.
Cluster Differences in Questionnaire Measures
| Min | Mod | Sev | Statistical Analysis |
||
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | F | p-value | |
| RRS Total | 30.4 (8.82) | 46.90 (16.13) | 51.67 (14.16) | 16.61 | <.001 a |
| BIS-11 Total | 53.23 (10.38) | 49.00 (11.00) | 60.08 (8.47) | 5.47 | .01 b |
| BIS Total | 18.97 (2.82) | 20.35 (3.46) | 18.77 (3.30) | 1.98 | .15 |
| BAS Total | 36.90 (11.47) | 43.74 (4.04) | 41.85 (9.93) | 5.14 | .01 c |
| NEO-PI Neuroticism | 71.69 (17.88) | 81.97 (23.52) | 107.15 (13.65) | 12.04 | <.001d |
| NEO-PI Extraversion | 128.86 (18.45) | 113.21 (20.81) | 109.23 (119.36) | 6.440 | .003 e |
| NEO-PI Openness | 121.03 (17.15) | 128.03 (16.16) | 139.31 (14.56) | 5.923 | .004 b |
| NEO-PI Agreeableness | 123.24 (14.22) | 123.09 (15.19) | 115.31 (12.93) | 1.830 | .17 |
| NEO-PI Conscientiousness | 125.83 (16.89) | 123.82 (19.75) | 111.62 (15.81) | 4.268 | .02 e |
Min= Minimal, Mod= Moderate (Unipolar), Sev = Severe, M= Mean, SD= Standard Deviation, RRS= Ruminative Responses Scale, BIS-11= Barrett Impulsiveness Scale, BIS= Behavioral Inhibition System, BAS= Behavioral Activation System
= Mod, Sev > Min
= Sev > Mod
= Min < Mod
= Sev > Min, Mod
Min > Mod, Sev
Neuropsychological Variables.
There was a significant main effect of group on fear and anger accuracy (Table 3). Post-hoc Bonferroni tests were run to determine pairwise differences. Sev performed significantly better on fear accuracy than Min and Mod, and better in anger accuracy than Min. All other neuropsychological tests did not show a statistically significant main effect of group.
Table 3.
Cluster Differences in Neuropsychological Measures
| Min | Mod | Sev | Statistical Analysis |
||
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | F | p-value | |
| Trail Making Test | |||||
| A/C (Visual Processing) in secs | 21.9 (7.93) | 22.50 (7.17) | 22.00 (6.53) | 0.60 | .94 |
| B/D (Switching) in secs | 53.35 (14.75) | 51.85 (17.37) | 62.08 (37.56) | 1.14 | .33 |
| Purdue Pegboard | |||||
| (Bimanual Dexterity) | 10.47 (1.72) | 10.54 (1.88) | 9.47 (1.47) | 1.89 | .16 |
| Controlled Oral Word | |||||
| (Fluency) Percentiles | 75.13 (23.78) | 73.76 (23.21) | 76.31 (29.61) | 0.06 | .94 |
| Wisconsin Card Sort | |||||
| Errors (Executive Functioning) | 53.16 (26.84) | 59.15 (25.97) | 60.31 (36.39) | 0.47 | .63 |
|
Modified Titrated
Monetary Incentive Delay |
|||||
| $ (Reward Processing) | 38.01 (11.75) | 36.42 (11.06) | 33.8 (13.61) | 0.48 | .62 |
| Facial Emotion Perception | |||||
| Fear Accuracy | 0.81 (0.11) | 0.81 (0.13) | 0.93 (0.07) | 4.15 | .02 a |
| Anger Accuracy | 0.66 (0.22) | 0.76 (0.19) | 0.91 (0.11) | 6.56 | .003 b |
| Happy Accuracy | 0.96 (0.08) | 0.97 (0.06) | 0.96 (0.06) | 0.42 | .66 |
| Sad Accuracy | 0.77 (0.19) | 0.84 (0.14) | 0.80 (0.15) | 1.27 | .29 |
| Neutral Accuracy | 0.77 (0.22) | 0.68 (0.2) | 0.72 (0.08) | 1.55 | .22 |
| Parametric Go/No-go | |||||
| 2 Target RT | 424.51 (37.22) | 423.15 (46.31) | 436.06 (66.38) | 0.38 | .69 |
| 2 Target Inhibition | 0.77 (0.19) | 0.77 (0.18) | 0.69 (0.20) | 0.99 | .38 |
| 3 Target RT | 498.89 (51.99) | 491.66 (53.22) | 509.69 (52.93) | 0.57 | .57 |
| 3 Target Inhibition | 0.58 (0.19) | 0.66 (0.19) | 0.54 (0.16) | 2.12 | .13 |
a Bonferroni corrections were used. All significant p-values are shown.
Min= Minimal, Mod= Moderate, Sev = Severe, M= Mean, SD= Standard Deviation, sec= seconds, COWA= Controlled Oral Word Association Test, WSCT= Wisconsin Card Sorting Test, mtMID= Modified Monetary Incentive Delay, FEPT= Facial Emotion Perception Task, PGNG= Parametric Go/No-Go, RT= Reaction Time.
Sev > Min, Mod
= Sev > Min
Discussion
The present study was conducted to ascertain whether lifetime symptoms of depression and mania might aid in detecting more homogenous subgroups of MDD. This study yielded three different cluster groups based upon lifetime symptoms within an otherwise homogenous sample of remitted MDD young adults and age matched healthy comparison adults. We were able to detect a group with minimal mood symptomology (primarily HCs and at a lower risk of developing mood pathology), a group with moderate and primarily unipolar symptomology (Mod), and a group that was more severe in both elevated manic and depressive symptomology (Sev). We were able to illustrate differences that delineated the Mod and Sev clusters, including differences in connectivity with the VS, facial perception performance, self-reported impulsivity, and self-reported extraversion and conscientiousness. This appears to be the first study to examine the MOODS-SR in a remitted sample, and to highlight that a bipolar risk subgroup could potentially be defined with MOODS-SR early in the course of MDD.
There was a small group of individuals currently remitted for MDD who endorsed significantly higher lifetime mania symptoms, although not clinically elevated to the point that it would be captured in diagnostic interviews. This finding is consistent with the presence of many prodromal symptoms before the development and awareness of BD, including mood lability and elation, swings or cyclothymic features, racing thoughts, irritability, and psychomotor activation, (many of the mania factors in the MOODS-SR).43,44 This group also endorsed more severe lifetime depressive symptoms in four factors. Sev may represent a distinct MDD group with substhreshold manic symptomology, who may never go on to develop BD. Alternatively, this group’s elevation in lifetime mania symptoms may put them at risk to develop BD, or more likely reflects a subtype of MDD with elements of BD symptomatology at the subthreshold level (Benvenuti et al., 2015; Fagiolini et al., 2006; Jules & Giovanni, 2005). We add that the Sev group was nominally more likely to have a positive family history of MDD (50%), relative to the Mod and Min groups. Future work can determine whether family history may be linked to more mixed lifetime symptoms and different connectivity, neuropsychological functioning, and trait factors. As all but one of these individuals with positive family history was for MDD and not for BD, family history was not likely to be definitive.
The Moderate group defined by cluster analysis presented as more moderate in lifetime symptomatology, and endorsed more unipolar symptoms relative to bipolar symptoms. The key difference between this group and the more severe group was endorsement of manic symptoms. They also differed on six of eight depressive symptom factor scores, with Sev higher than Mod.
Several facets stand out as important distinguishing constructs between the Sev group and the other two clusters of rMDD. Sev showed higher trait impulsivity than Mod and Min on reported impulsivity. Higher trait impulsivity tends to represent a marker for developing BD, although it has also been linked to MDD and ADHD. Both higher neuroticism and lower conscientiousness distinguished Sev from the other clusters in this study, which may be a potential subtyping feature for risk for BD, consistent with the literature in BD.45 Further research must be done to determine if impulsivity, trait neuroticism and trait low conscientious are a useful way to discriminate those who are at increased risk to develop BD from those who are more likely to remain unipolar.
Surprisingly, the Sev group was better at correctly identifying fear and anger facial expressions. One meta-analysis has found less impairment for identifying emotions in faces in those with BD than those with schizophrenia and with MDD,46 while another found the degree of impairment to be comparable between BD and MDD.47 Prior studies of those with BD may illustrate impaired emotion processing due to active symptoms or disease scar.47 This study’s emotion processing differences cannot be accounted for by differences in attention, executive function, visual memory or verbal memory. The superior detection of negative emotions in bipolar risk versus more unipolar rMDD individuals may perhaps be useful in distinguishing the two subgroups and may be useful at detecting subsyndromal manic features early in the course of illness. In other words, individuals with MDD who do not possess impairment in facial perception may represent a special group to follow as they may present with mania symptomatology.
Across studies, reduced connectivity has been found in prefrontal and limbic brain regions for BD and MDD.48, 50 Connectivity studies have supported a model positing dysfunction of subcortical-prefrontal networks and limbic regions in BD, where disruption of mood may be caused by reduced prefrontal modulation of subcortical and medial temporal structures within the anterior limbic system.50 Within the current study, connectivity analyses revealed disrupted connectivity with the VS in the Sev group, which could be useful to delineate those at risk for developing BD versus those who show primarily unipolar symptomology. Compared to Mod, Sev had reduced connectivity between the RVSs and several anterior cognitive control regions, perhaps highlighting diminished regulatory capacity for approach behaviors. In contrast, for the left VSs a different pattern was evident of elevated connectivity for Mod relative to Sev and Min in a widespread set of regions including cognitive control regions, and emotion processing. A few regions showed elevation in Sev relative to Min and Mod, including secondary visual processing regions, including those for facial emotion. In the right VSi, there was increased connectivity in Sev with the precuneus, and decreased connectivity with the vermis compared to Mod. These connectivity disruptions may represent early and sensitive risk markers, particularly for the left VSs, for those at high-risk for developing BD.
Developing homogeneous subgroupings can aid in better diagnosis, and potentially, better treatment for the mood disorder spectrum. While antidepressants are highly effective for those with solely depressive symptomatology, up to 50% of people who are diagnosed with unipolar MDD are resistant to antidepressants, and have subthreshold or threshold manic symptoms.51 Depending on the length of the observation period, 15–30% of people who were previously diagnosed as unipolar progress to BD.52 Thus, not only could use of the MOODS-SR in this age range lead to designation of individuals in “high risk” categories, it may also lead to earlier treatment and improved prevention efforts. Indeed, there may be increased specificity in the nature and types of treatments that could work for these subgroups. Identification of neural, neuropsychological, or personality features that aid in risk determination could lead to earlier and more effective treatment.
This study has a few limitations to cover. A strength of the study was that multiple methods were used to validate subgroups; however, future studies will be needed to further validate links to BD by recruiting groups with elevated lifetime mania symptoms with and without a history of BD. Additionally, these studies would benefit from larger sample sizes to increase power, as the present study was a secondary data analysis with a sample of convenience. The sample size was not large enough to form additional cluster subgroups (to evaluate what might be an optimal cluster number) because sufficient power would have been lost to determine trait differences between smaller cluster groups. The three-cluster solution was only marginally lower in AIC relative to the 2 and 4 cluster solutions and was identical in the silhouette measure. A larger sample size would be beneficial in determining further homogenous subgroupings/clusters in those with mood disorders. In addition, some measures expected to vary across mood clusters did not show significant differences between our clusters, such as inhibition or bimanual dexterity. This may have been due to a focus on lifetime mood symptomology in a currently remitted sample, which is more likely to highlight trait rather than state differences. Finally, we were unable to prospectively follow this sample to determine final diagnostic outcomes, and thus empirically testing conversion from MDD diagnosis to BD remains an active area of research. Alternatively, comparison to a group with diagnosis of BD could have clarified whether the BD and Sev groups were similar on a number of neuropsychological, trait and brain imaging features. Future studies will need to determine the course of illness in such groups, and to determine if these groups are a risk group for BD or if they represent a subtype of MDD with subthreshold mania symptomology.
In conclusion, the MOOD-SR is a useful tool to reduce homogeneity within young, remitted MDD samples. It also may be a useful tool to identify those at risk for developing BD, even in those whose illness clinically and historically presents as unipolar. There may be a subgroup of people with depression histories who have been improperly diagnosed or are at risk of developing more severe pathology in the future. It is more likely that there is an MDD subgroup with some subthreshold manic symptomatology, and these individuals may show a different clinical course with different optimal treatments.
Supplementary Material
Figure 1. Example of distribution for a depression and mania factors of the MOOD-SR.
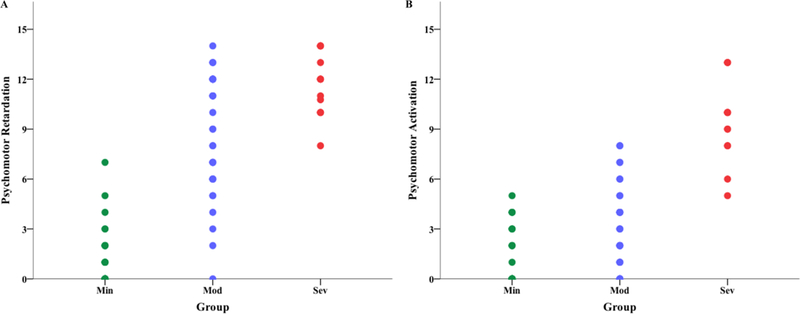
Psychomotor Retardation (A) and Psychomotor Activation (B) is shown separated by group. There is very little overlap of the Min and Sev groups. Min= Minimal, Mod= Moderate, Sev = Severe
Acknowledgements
We would like to thank the individuals that participated in this study. We thank the Multifaceted Explorations of the Neurobiology of Depressive Disorders laboratory for assistance in data collection and diagnostic interviews.
Funding
This work was supported by NIMH grant MH 091811 (SAL), and UIC Clinical and Translational Science Awards Program NCATS UL1TR000050 and 1S10RR028898.
References:
- 1.Cassano GB, Rucci P, Frank E, Fagiolini A, Dell’Osso L, Shear MK, Kupfer DJ. The mood spectrum in unipolar and bipolar disorder : Arguments for a unitary approach. Am J Psychiatry 2004;161(7):1264–9. [DOI] [PubMed] [Google Scholar]
- 2.Fournier JC, Keener MT, Almeida J, Kronhaus DM, Phillips ML. Amygdala and whole‐brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disord 2013;15(7):741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Das T, Deng W, Wang Q, Li Y, Zhao L, Ma X, Wang Y, Yu H, Li X, Meng Y. Clinical utility of a short resting‐state MRI scan in differentiating bipolar from unipolar depression. Acta Psychiatrica Scandinavica 2017. [DOI] [PubMed]
- 4.Alloy LB, Olino T, Freed RD, Nusslock R. Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behav Ther 2016;47(5):600–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González DA, Jenkins SR. Cross-measure equivalence and communicability in the assessment of depression : a focus on factor-based scales. Assessment 2014;21(6):731–41. [DOI] [PubMed] [Google Scholar]
- 6.Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults : identifying neural markers for new interventions. Transl Psychiatry 2017;7(e1096):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: Toward DSM-V. Schizophr Bull 2007;33(4):893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassano GB, Mula M, Rucci P, Miniati M, Frank E, Kupfer DJ, et al. The structure of lifetime manic – hypomanic spectrum. J Affect Disord 2009;112(1):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RMA. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994;31(4):281–94. [DOI] [PubMed] [Google Scholar]
- 10.Angst J, Cassano GB. The mood spectrum: improving the diagnosis of bipolar disorder. Bipolar Disord 2005;7:4–12. [DOI] [PubMed] [Google Scholar]
- 11.Akiskal HS, Bourgeois ML, Angst J, Post R, Möller HJ, Hirschfeld R. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord 2000;59:S5–30. [DOI] [PubMed] [Google Scholar]
- 12.Benazzi F Frequency of bipolar spectrum in 111 private practice depression outpatients. Eur Arch Psychiatry Clin Neurosci 2003;253(4):203–8. [DOI] [PubMed] [Google Scholar]
- 13.Ten Have M, Lamers F, Wardenaar K, Beekman A, De Jonge P, Van Dorsselaer S, et al. The identification of symptom-based subtypes of depression: A nationally representative cohort study. J Affect Disord 2016;190:395–406. [DOI] [PubMed] [Google Scholar]
- 14.Parker G Classifying depression: Should paradigms lost be regained? Am J Psychiatry 2000;157(8):1195–203. [DOI] [PubMed] [Google Scholar]
- 15.Michopoulos I, Zervas IM, Papakosta VM, Tsaltas E, Papageorgiou C, Manessi T, Papakostas YG, Lykouras L, Soldatos CR. Set shifting deficits in melancholic vs. non-melancholic depression: preliminary findings. Eur Psychiatry 2006;21(6):361–3. [DOI] [PubMed] [Google Scholar]
- 16.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 2016;173(4):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, et al. Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC study. Neuropsychopharmacology 2016;41(2):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassano GB, Benvenuti A, Miniati M, Calugi S, Mula M, Maggi L, et al. The factor structure of lifetime depressive spectrum in patients with unipolar depression. J Affect Disord 2009;115(1): 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dell’Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, et al. Measuring mood spectrum: Comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr Psychiatry 2002;43(1):69–73. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Yu BH, Lee DS, Kim JH. Ruminative response in clinical patients with major depressive disorder, bipolar disorder, and anxiety disorders. J Affect Disord 2012;136:e77–81. [DOI] [PubMed] [Google Scholar]
- 21.Ryan KA, Vederman AC, Mcfadden EM, Weldon AL, Kamali M, Langenecker SA, et al. Differential executive functioning performance by phase of bipolar disorder. Bipolar Disord 2012;14(5):527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust New Zeal J Psychiatry 2009;43(12):1105–17. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz MM, Gerraty RT. A Meta-analytic Investigation of Neurocognitive Deficits in Bipolar Illness: Profiles and Effects of Clinical State. Neuropsychology 2009;23(5):551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips ML, Swartz HA. A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. Am J Psychiatry 2014;171(8):829–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies: Rationale, unique features, and training. Arch Gen Psychiatry 1994;51(11):849–59. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell ME. Manual for the FIGS Bethesda (MD: ): Clinical Neurogenetics Branch, National Institute of Mental Health; 1992. [Google Scholar]
- 27.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination Reconsidered: A Psychometric Analysis. Cognit Ther Res 2003;27(3):247–59. [Google Scholar]
- 28.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol 1994;67(2):319–33. [Google Scholar]
- 29.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51(6):768–74. [DOI] [PubMed] [Google Scholar]
- 30.Costa PTJ, McCrae RR. The revised neo personality inventory (neo-pi-r). The SAGE handbook of personality theory and assessment London: SAGE Publications Ltd; 2008. p. 179–98. [Google Scholar]
- 31.Langenecker SA, Giordani B. Synonym Knowledge Test (Version 1) Ann Arbor: BBRINMMAP; 2003. [Google Scholar]
- 32.Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta J-K, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol 2005(3);27:320–33. [DOI] [PubMed] [Google Scholar]
- 33.DelDonno SR, Weldon AL, Crane NA, Passarotti AM, Pruitt PJ, Gabriel LB, et al. Affective personality predictors of disrupted reward learning and pursuit in major depressive disorder. Psychiatry Res 2015;230(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langenecker SA, Zubieta J-K, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test-retest reliability of the Parametric Go/No-Go Test. J Clin Exp Neuropsychol 2007;29(8):842–53. [DOI] [PubMed] [Google Scholar]
- 35.Benton AL, Hamsher K de S, Sivan AB. Multilingual Aphasia Examination Iowa City: AJA Associate; 1994. [Google Scholar]
- 36.Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease ofshifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol 1948;38(4):404–11. [DOI] [PubMed] [Google Scholar]
- 37.Army Individual Test Battery. Manual of Directions and Scoring Washington, DC: War Department, Adjutant General’s Office; 1944. [Google Scholar]
- 38.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. Elsevier Inc; 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, et al. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J Appl Math 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox MD, Zhang D, Snyder AZ, Raichle ME. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J Neurophysiol 2009;101(6):3270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 2008;18(12):2735–47. [DOI] [PubMed] [Google Scholar]
- 42.Cox RW, Reynolds RC, Taylor PA. AFNI and clustering: false positive rates redux. BioRxiv 2016. [DOI] [PMC free article] [PubMed]
- 43.Bechdolf A, Ratheesh A, Cotton SM, Nelson B, Chanen AM, Betts J, et al. The predictive validity of bipolar at-risk (prodromal) criteria in help-seeking adolescents and young adults: A prospective study. Bipolar Disord 2014;16(5):493–504. [DOI] [PubMed] [Google Scholar]
- 44.Homish GG, Marshall D, Dubovsky SL, Leonard K. Predictors of later bipolar disorder in patients with subthreshold symptoms. J Affect Disord 2013;144(1):129–33. [DOI] [PubMed] [Google Scholar]
- 45.Jabben N, Penninx BWJH, Beekman ATF, Smit JH, Nolen WA. Co-occurring manic symptomatology as a dimension which may help explaining heterogeneity of depression. J Affect Disord 2011;131:224–32. [DOI] [PubMed] [Google Scholar]
- 46.Rocca CC, Heuvel E, Caetano SC, Lafer B. Facial emotion recognition in bipolar disorder: A critical review. Rev Bras Psiquiatr 2009;31(2):171–80. [DOI] [PubMed] [Google Scholar]
- 47.Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Res 2011;188(3):303–9. [DOI] [PubMed] [Google Scholar]
- 48.Wessa M, Kanske P, Linke J. Bipolar disorder: A neural network perspective on a disorder of emotion and motivation. Restor Neurol Neurosci 2014;32(1):51–62. [DOI] [PubMed] [Google Scholar]
- 49.Stange JP, Bessette KL, Jenkins LM, Peters AT, Feldhaus C, Crane NA, et al. Attenuated intrinsic connectivity within cognitive control network among individuals with remitted depression: Temporal stability and association with negative cognitive styles. Hum Brain Mapp 2017;38(6):2939–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychtiatry 2005;10(1):105–16. [DOI] [PubMed] [Google Scholar]
- 51.Sharma V, Khan M, Smith A. A closer look at treatment resistant depression: Is it due to a bipolar diathesis? J Affect Disord 2005(2);84:251–7. [DOI] [PubMed] [Google Scholar]
- 52.Faravelli C, Amedei SG, Scarpato MA, Faravelli L. Bipolar Disorder: an impossible diagnosis. Clin Pract Epidemiol Ment Heal 2009;5(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benvenuti A, Miniati M, Callari A, Giorgi Mariani M, Mauri M, & Dell’Osso L (2015). Mood Spectrum Model: Evidence reconsidered in the light of DSM-5. World Journal of Psychiatry, 5(1), 126–137. 10.5498/wjp.v5.i1.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagiolini A, Dell’osso L, Pini S, Armani A, Bouanani S, Rucci P, … Frank E. (2006). Validity and reliability of a new instrument for assessing mood symptomatology: the Structured Clinical Interview for Mood Spectrum (SCI‐MOODS). Int J Methods Psychiatr Res, 8(2), 71–82. 10.1002/mpr.58 [DOI] [Google Scholar]
- 55.Jules A, & Giovanni C (2005). The mood spectrum: improving the diagnosis of bipolar disorder. Bipolar Disorders, 7(s4), 4–12. 10.1111/j.1399-5618.2005.00210.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


