Summary
CD4+ helper T cells are essential for immune responses and differentiate in the thymus from CD4+CD8+ ‘double-positive’ (DP) thymocytes. The transcription factor Runx3 inhibits CD4+ T-cell differentiation by repressing Cd4 gene expression; accordingly, Runx3 is not expressed in DP thymocytes or developing CD4+ T cells. The transcription factor Thpok is up-regulated in CD4-differentiating thymocytes and required to repress Runx3. However, how Runx3 is controlled at early stages of CD4+ T-cell differentiation, before the onset of Thpok expression, remains unknown. Here we show that Gata3, a transcription factor preferentially and transiently up-regulated by CD4+ T-cell precursors, represses Runx3 and binds the Runx3 locus in vivo. Accordingly, we show that high-level Gata3 expression and expression of Runx3 are mutually exclusive. Furthermore, whereas Runx3 represses Cd4, we show that Gata3 promotes Cd4 expression in Thpok-deficient thymocytes. Thus, in addition to its previously documented role in promoting CD4-lineage gene-expression, Gata3 represses CD8-lineage gene expression. These findings identify Gata3 as a critical pivot of CD4-CD8 lineage differentiation.
Keywords: CD4-CD8 differentiation, Gata3, Runx3, T-cell development, transcription
1. Introduction
Helper T cells are essential for immune responses. They recognize peptide antigens bound to class II major histocompatibility complex (MHC-II) molecules and express the CD4 ‘coreceptor’ [1]. These attributes distinguish helper T cells from cytotoxic T cells, which are MHC-I restricted and express the CD8 coreceptor. Helper and cytotoxic cells differentiate in the thymus from precursors that express both CD4 and CD8 (double positive, DP) [2, 3]. The divergence of these two lineages takes place in thymocytes that have undergone positive selection, i.e. that have been rescued from programmed cell death upon engagement of their T-cell antigen receptor (TCR) by MHC-peptide complexes expressed on the thymic stroma.
MHC II-signaled thymocytes must maintain CD4 expression during positive selection, because CD4 helps MHC-II recognition and signaling by the T-cell receptor (TCR) [4, 5]. Accordingly, the Cd4 gene is tightly regulated, and transcriptional repression is critical for such regulation. Most notably, the transcription factors Runx1 and Runx3 limit Cd4 expression to DP and MHC II-restricted cells [6, 7]. Runx1 represses Cd4 in early thymocytes, before the DP stage. In contrast, Runx3 represses Cd4 in CD8-differentiating cells, in which it is specifically expressed, and thereby contributes to CD8-lineage commitment [8, 9]. Runx3 is also important for expression of cytotoxic genes, a hallmark of the CD8 lineage [10, 11] and therefore control multiple aspects of CD8-lineage differentiation. Because ectopic Runx3 expression represses Cd4 and impairs CD4CD4+ T cell differentiation [12, 13], the differentiation of CD4+ T cells requires expression of Runx3 to be limited to thymocytes undergoing MHC I-induced positive selection. How this is achieved remains poorly understood.
Two transcription factors, Ets1 and Stat5, have been proposed to promote Runx3 expression [14, 15]. However, both are expressed throughout T-cell development, raising the question of how they could limit Runx3 expression to MHC I-restricted thymocytes. Stat5 is activated in thymocytes in response to signaling by IL-7, and is therefore inactive in DP thymocytes which do not express the IL-7 receptor (IL-7R). However, IL-7R is expressed in both MHC-I and MHC II-selected thymocytes [16], and it is unclear how Stat5 could activate Runx3 in the former but not the latter. Reciprocally, the transcription factor Thpok, specifically expressed in MHC II-restricted cells and required for CD4+ T cell differentiation, represses Runx3 [10, 17–20]. However, Thpok is not expressed in DP cells and is expressed at low levels in CD4+CD8int ‘transitional’ cells, the precursors of CD4+-lineage thymocytes. Thus, the transcriptional control of Runx3 expression in early CD4+-lineage precursor cells remains unclear.
Here, we show that a Thpok-independent mechanism represses Runx3 in MHC II-restricted thymocytes, and we present evidence that it involves the transcription factor Gata3, previously shown to promote CD4+-lineage differentiation [21–23]. These studies identify a novel, repressive, function of Gata3 during CD4+-lineage differentiation in the thymus.
2. Results
Thpok-independent Runx3 repression during CD4+ cell differentiation in the thymus
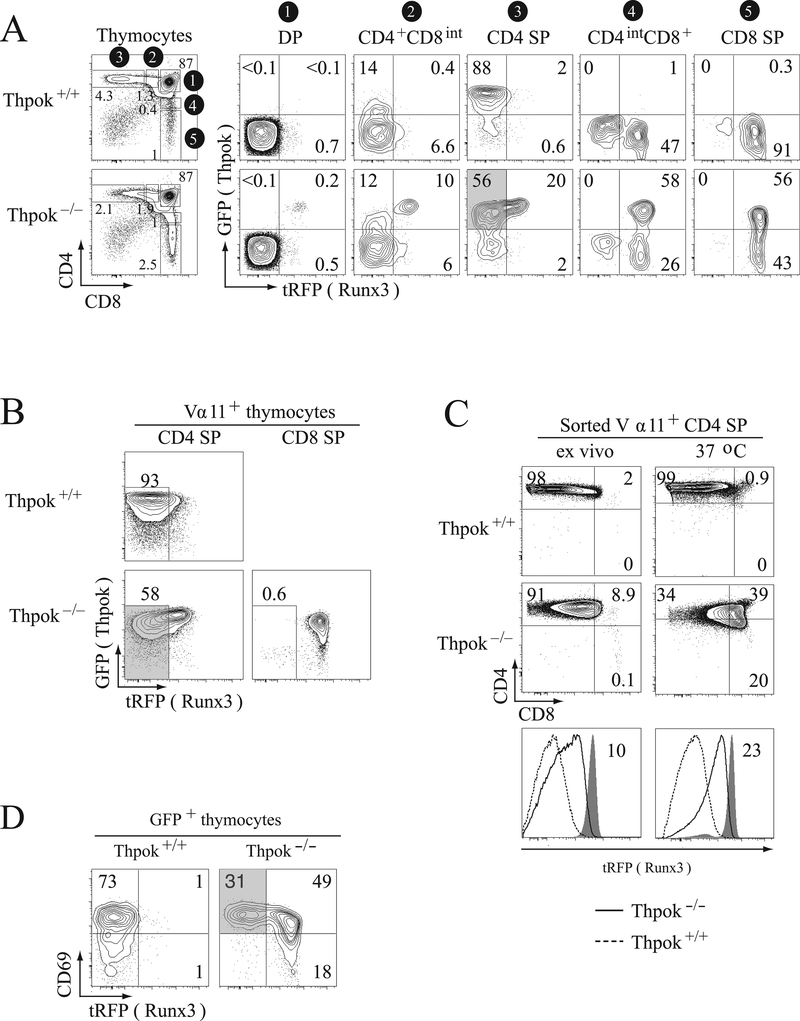
To study the kinetics of Thpok and Runx3 up-regulation in the thymus, we set up an experimental system using a GFP-based BAC reporter for the gene expressing Thpok (Zbtb7b, thereafter called Thpok), and a tRFP-based reporter for Runx3. Both reporters, ThpokGFP and Runx3tRFP respectively, have been previously shown to appropriately track expression of the respective gene [10, 14]. In wild-type mice, DP thymocytes express neither ThpokGFP nor Runx3tRFP (Fig. 1A, top, column 1); in contrast, thymocytes that have undergone positive selection (CD4+CD8− and CD4−CD8+ single positive (SP)) express ThpokGFP if CD4 SP and Runx3tRFP if CD8 SP (Fig. 1A, top, columns 3 and 5). Most CD4+CD8int ‘transitional’ thymocytes, which are either MHC-I or MHC II-restricted cells undergoing positive selection, failed to express either reporter (Fig. 1A, top, column 2), consistent with the fact that most of them are not yet committed to either lineage [4]. In contrast, the CD4intCD8+ subset was enriched in CD8-committed Runx3-expressing cells (Fig. 1A, top, column 4).
Figure 1. Runx3 repression during CD4-lineage differentiation.
(A) Contour plots show expression of ThpokGFP and Runx3tRFP reporters in the indicated thymocyte subsets from Thpok+/+ and Thpok−/− mice (gating on the left, gate numbers shown on a black background). Note the expression of ThpokGFP in the CD4intCD8+ and CD8 SP subsets in Thpok−/− mice, identifying MHC II-restricted ‘redirected’ thymocytes. The gray-shaded box in column 3 (bottom) contains ThpokGFP+ Runx3tRFP– cells. (B) Contour plots of ThpokGFP and Runx3tRFP expression in Vα11+ CD4 or CD8 SP thymocytes from Thpok−/− and Thpok+/+ mice carrying the MHC-II-restricted AND TCR transgene; gates are defined in Supporting Information Fig. 1. The gray-shaded area identifies ThpokGFP+ Runx3tRFP– cells. (C) Sorted CD4 SP thymocytes from Thpok+/+ or Thpok−/− AND mice carrying the Runx3tRFP reporter were placed in single cell suspension culture overnight and analyzed for expression of surface CD4 and CD8 (top) and tRFP (bottom) before (left) and after (right) culture. Overlaid histograms (bottom) show Runx3tRFP expression in Thpok−/− (solid line histogram) or Thpok+/+ (dashed line histogram) cells. Gray-filled histograms show tRFP fluorescence in CD8 SP cells from AND Thpok−/− mice analyzed in parallel. Numbers in bottom panels indicate tRFP fluorescence relative to CD8 SP controls (gray-shaded histograms) set to 100. (A-C) In each panel, data shown are representative of at least three experiments. (D) Contour plots of CD69 vs. tRFP expression gated on GFP+ thymocytes from Thpok+/+ or Thpok−/− mice carrying both the ThpokGFP and Runx3tRFP reporters. The gray-shaded area identifies the CD69hi GFP+ tRFP– subset in Thpok−/− mice.
While Thpok disruption impairs the development of mature CD4 SP cells, it does not prevent the positive selection of MHC II-restricted cells [17]. In Thpok−/− mice, MHC II-restricted DP thymocytes initially down-regulate CD8 expression and adopt a ‘CD4 SP-like’ surface phenotype (CD4+CD8int/lo) (Supporting Information Fig. 1A). Consistent with previous results [19], most Thpok−/− CD4 SP-like cells expressed ThpokGFP (Fig. 1A, bottom, column 3), indicating that Thpok protein is not needed to initiate Thpok gene expression. However, unlike Thpok+/+ CD4 SP thymocytes which differentiate into CD4+ T cells, Thpok−/− CD4 SP-like thymocytes terminate CD4 and reinitiate CD8 expression, thereby being ‘redirected’ to the CD8-lineage [24]. Their persistent GFP expression identifies such redirected cells in the CD8 SP compartment of Thpok−/− mice (Fig. 1A, bottom, columns 4 and 5).
Because Thpok was previously shown to repress Runx3 [10, 19, 20], we predicted that Thpok−/− CD4 SP-like thymocytes, which make no Thpok protein, would express the Runx3 reporter. Unexpectedly, while a few CD4 SP-like thymocytes expressed Runx3tRFP, most of them failed to do so (Fig. 1A, bottom, column 3, shaded area). Similar observations were made in Thpok−/− mice carrying the MHC II-restricted AND TCR transgene (Fig. 1B, bottom left, shaded area), indicating that such a ThpokGFP+ Runx3tRFP− population indeed resulted from MHC-II signaling.
It was possible that MHC II-signaled Thpok−/− cells failed to express Runx3 because Runx3 up-regulation is a late event in thymocyte maturation, requiring signals that these cells had not yet received. A non mutually exclusive possibility was that Runx3 was repressed by Thpok-independent intrathymic signals. The latter but not the former hypothesis predicted that removing Thpok−/− CD4 SP-like thymocytes from their intrathymic environment would relieve such repression and therefore enhance, rather than reduce, Runx3 expression. Experimental evidence supported this conclusion (Fig. 1C, bottom): whereas a substantial subset of Thpok−/−CD4 SP-like thymocytes expressed little or no Runx3 (tRFP) ex vivo, they all did after overnight single-cell suspension culture to disrupt thymocyte-stroma interactions. Accordingly, mutant cells also down-regulated the Runx3 target CD4 (Fig. 1C). In contrast, Thpok-sufficient CD4 SP cells remained tRFP− and CD4hi in these same conditions. These findings supported the idea that intrathymic signals, not mediated by Thpok, restrained Runx3 expression in MHC II-signaled thymocytes. We therefore decided to explore this possibility.
Gata3 represses Runx3
The Thpok-deficient cells that expressed ThpokGFP but not Runx3tRFP expressed CD69 (Fig. 1D, gray shaded), a molecule up-regulated by intrathymic TCR engagement [25]. This raised the possibility that intrathymic TCR signaling repressed Runx3 in a Thpok-independent manner. The transcription factor Gata3 is up-regulated by TCR signaling in thymocytes [26, 27], whereas its expression is down-regulated when thymocytes are removed from their intrathymic environment (Supporting Information Fig. 1B). This pattern of expression was reciprocal to that of Runx3, prompting us to consider the possibility that high-level Gata3 expression would repress Runx3. Previous analyses have shown that Gata3 is required for CD4 but not CD8-lineage differentiation, and that in the absence of Gata3 MHC II-restricted thymocytes are ‘redirected’ into CD8 SP cells [21, 22]. These previous findings are consistent with the hypothesis that Gata3 represses Runx3. Indeed, this hypothesis predicts that, in the absence of Gata3, MHC II-restricted thymocytes would express Runx3, repress Cd4 and be ‘redirected’ to a CD8-lineage fate. While it was not possible to directly evaluate the hypothesis by inactivating Gata3 specifically in cells with high Gata3 expression (CD4+CD8int thymocytes, see below), we reasoned that ectopic Gata3 expression should impair Runx3 up-regulation. To assess this prediction, we used a Gata3 transgene that expresses Gata3 protein at the ‘high’ physiological set point (the peak level during positive selection) in all thymocytes (Fig. 2A and S2A) [28]. At this level, the Gata3 transgene had little or no effect on the differentiation of wild-type (Thpok-sufficient) MHC II-restricted thymocytes (Supporting Information Fig. 2B).
Figure 2. Enforced Gata3 expression represses Runx3 in MHC II-restricted thymocytes.
(A) Expression of intra-cellular Gata3 was analyzed by flow cytometry in Gata3 transgenic thymocyte subsets (solid line histogram) or their non-transgenic counterparts (gray-shaded histograms). The vertical dotted line indicates the peak of Gata3 expression in wild type CD4+CD8int thymocytes. Data are from two mice analyzed in a single experiment, and representative of three independent determinations. (B) Plots depict expression of Runx3tRFP vs. CD69 in post-selection (TCRhi) thymocytes from Gata3 transgenic and control Thpok−/− mice. Numbers below plots show the mean tRFP fluorescence intensity (arbitrary units) in the top and bottom right quadrants; the leftmost plot shows background fluorescence in mice that do not carry the Runx3tRFP reporter. (C) The ratio of tRFP+/tRFP– cells among TCRhi CD69hi thymocytes, either Gata3-transgenic (right) or non-transgenic controls (left) is shown. Lines link mice analyzed within the same experiment. (D) Plots show CD4 and CD8 expression in TCRhi CD69hi Thpok−/− thymocytes from the same mice as in (B). (B-D) Data shown are from five pairs of mice analyzed in five distinct experiments.
To evaluate Gata3 repression of Runx3, we examined how the Gata3 transgene affected Runx3tRFP expression in Thpok−/− thymocytes. Because the predicted effects on Runx3 might affect Cd4 and Cd8, it was essential not to restrict these analyses to particular subsets defined by CD4 and CD8 expression, but rather to examine the whole population of TCRhi post-selection Thpok−/− cells. In that subset, the Gata3 transgene substantially impaired Runx3 up-regulation, although it did not prevent it (Fig. 2B). Most TCRhi Gata3-transgenic cells failed to express tRFP, whereas 60% of their non-transgenic counterparts did so; furthermore, the Gata3 transgene reduced tRFP fluorescence intensity in reporter-expressing cells by almost 60% (Fig. 2B, bottom). Both effects were most pronounced in CD69hi cells (Fig. 2B, C), suggesting that Runx3 repression by Gata3, whether transgenic or endogenous, was specific of TCR-signaled cells. We conclude from these experiments that Gata3 represses Runx3 in Thpok−/− thymocytes.
In Thpok−/− mice, both MHC II- and MHC I-restricted thymocytes express Runx3. To determine if the Gata3 transgene repressed Runx3 in MHC I-restricted cells, we examined its effects on Runx3tRFP in Thpok+/+ mice, in which only MHC I-restricted cells express Runx3. We found that the Gata3 transgene had little if any effect on Runx3tRFP expression in these cells (Supporting Information Fig. 2C): both the frequency of RFP-expressing cells and RFP fluorescence intensity were similar in transgenic and control cells. We had similar results when analyzing Runx3tRFP expression on gated CD8 SP thymocytes (data not shown). These analyses support the conclusion that Runx3 repression by Gata3 is specific of MHC II-signaled thymocytes. Of note, this indicates that the effects shown in Thpok−/− cells (Fig. 2B, C), which are a mix of MHC-I and MHC II-specific cells, probably underestimate the actual Gata3-mediated Runx3 repression in the latter.
We next examined whether Gata3-mediated Runx3 repression had physiological consequences. Because Runx3 represses Cd4, we predicted that repression of Runx3 would result in sustained CD4 expression by Gata3-transgenic cells. We evaluated this prediction in the TCRhi CD69+ subset from Thpok−/− mice, in which Gata3-mediated Runx3 repression was the most prominent. Indeed, the fraction of CD4lo cells in this subset was reduced in Thpok−/− Gata3 transgenic mice compared with that in their non-transgenic counterparts (Fig. 2D), demonstrating that Gata3-mediated Runx3 repression improved CD4 expression. We also noted that the Gata3 transgene impaired CD8 expression in CD8 SP cells (Figs. 3 and S2D). However, this effect is unlikely to be Runx3-dependent, because it was observed in MHC I-restricted cells, in which Gata3 had little or no effect on Runx3 (Supporting Information Fig. 2C), as well as in DP thymocytes, which do not express Runx3 [29]. Accordingly, previous studies had shown that Runx3 has little effect on CD8 expression in the thymus, unlike in effector T cells [29, 30].
Figure 3. Gata3 represses expression of CD8.
Overlaid histograms show surface CD8 expression on gated pre-selection DP and TCRhi CD8 SP thymocytes from Gata3-transgenic (solid line histogram) and control (gray-filled histogram) thymocytes. Data shown are representative of five experiments.
High-level Gata3 and Runx3 expression are mutually exclusive
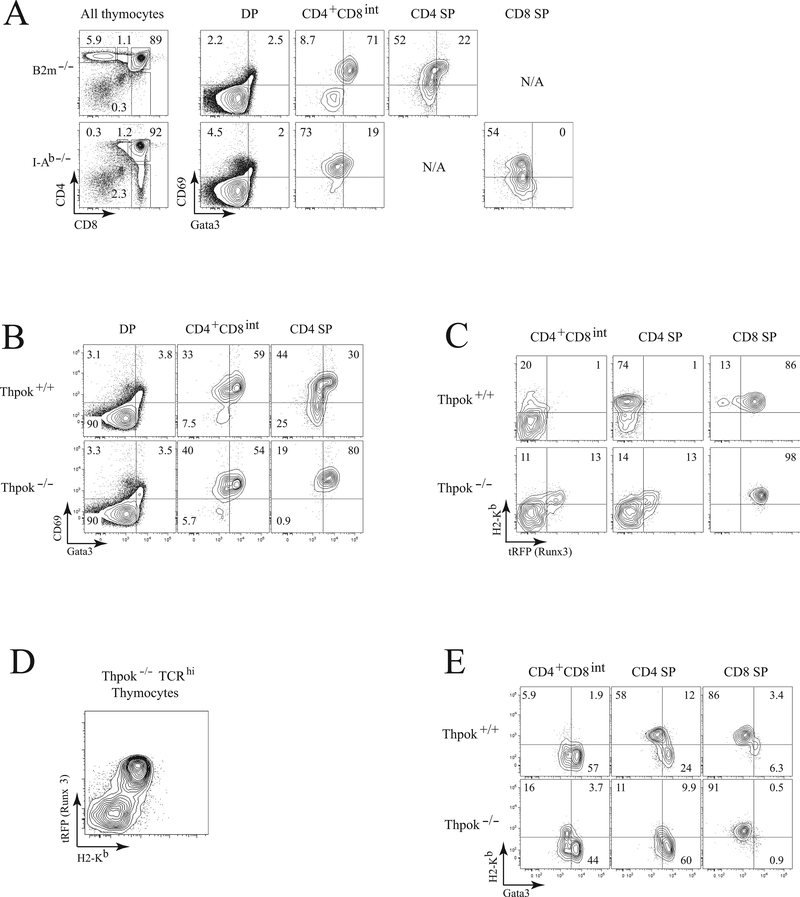
If the conclusion that Gata3 represses Runx3 is correct, high-level Gata3 and Runx3 expression should be mutually exclusive. To evaluate this prediction, we used intra-cellular staining to assess Gata3 expression. We preferred this procedure to GFP reporter mice [27] because GFP, due to its long half-life [31], does not accurately reflect transient changes in Gata3 protein expression. We compared Gata3 expression in MHC-II and MHC-I restricted thymocytes obtained from B2m−/− and I-Ab−/− mice respectively. Consistent with previous studies [26, 32], Gata3 expression was highest in CD69+ CD4+CD8int MHC II-restricted cells (Fig. 4A, compare top and bottom rows). In Thpok+/+ mice these cells do not express Runx3 (Fig. 1A), consistent with the idea of Gata3 repression of Runx3. In Thpok−/− mice however, both CD4+CD8int and CD4 SP-like subsets contained Runx3tRFP+ and Gata3hi cells (compare Figs. 1A and 4B respectively). Unfortunately, we could not use the same staining procedure to directly compare Gata3 and Runx3tRFP expression at the single-cell level, because the cell fixation required to measure intra-cellular Gata3 abolishes RFP fluorescence.
Figure 4. Runx3 and high-level Gata3 expression are mutually exclusive in- thymocytes.
(A) Contour plots (right) show intra-cellular Gata3 and surface CD69 expression in MHC II- (top, from B2m−/− mice) and MHC I-restricted (bottom, from MHC-II-deficient mice) thymocyte subsets as gated on the left. (B) Contour plots show intracellular Gata3 and surface CD69 expression in Thpok+/+ (top) and Thpok−/− (bottom) thymocyte subsets gated as in (A). (C) Expression of H-2Kb and Runx3tRFP in Thpok+/+ and Thpok−/− thymocytes gated as indicated in (A). (D) Plots show surface H-2Kb vs. Runx3tRFP expression in gated TCRhi thymocytes from Thpok−/− mice. (E) Plots show intracellular Gata3 vs. surface H-2Kb expression in CD4+CD8int, CD4 SP and CD8 SP thymocytes from Thpok+/+ and Thpok−/− mice. Data shown are representative of two or more experiments.
To overcome this obstacle, we used the MHC-I molecule H-2Kb as a surrogate for Runx3 expression. This marker is normally up-regulated in immature post-selection cells (TCRhi CD69+) and remains expressed in more mature cells (Supporting Information Fig. 3), and its expression is not affected by the Gata3 transgene (data not shown). In wild-type CD4 SP thymocytes, which do not express Runx3, H-2Kb and Runx3 expression are not correlated (Fig. 4C, middle column, top). In contrast, expression of H-2Kb parallels that of Runx3 in wild-type CD8-lineage cells (Fig. 4C, top right). Importantly, there was an excellent correlation between H-2Kb and Runx3tRFP expression in all subsets of Thpok−/− thymocytes, CD4+CD8int, CD4 SP and CD8 SP (Fig. 4C, bottom), and among all post-selection (TCRhi) cells (Fig. 4D). Thus, H-2Kb can be used as a surrogate for Runx3 expression in cells that do not express Thpok, i.e. wild-type CD8-lineage cells and all post-selection cells in Thpok−/− mice.
Consequently, we plotted H-2Kb vs. intra-cellular Gata3 expression in thymocytes (Fig. 4E). In Thpok−/− thymocytes, there was no substantial population in the top right quadrant of each plot, where would be cells with high Gata3 and H-2Kb expression. Thus, Gata3 protein levels are low in H-2Kb-expressing cells, from which we conclude that Gata3hi cells do not express Runx3, consistent with our finding that Gata3 represses Runx3. In summary, these studies document that Gata3 expression peaks in CD69hi MHC II-restricted thymocytes that are H-2Kb−, whereas Runx3 is expressed in H-2Kb+ cells.
Gata3 delays the CD8-lineage redirection of Thpok-deficient cells
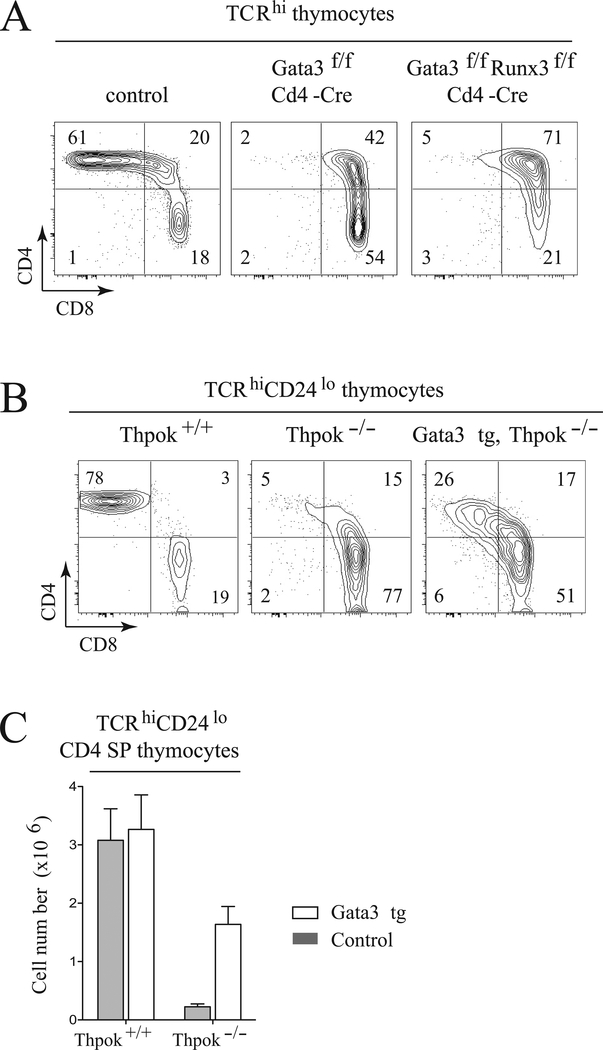
Previous studies had shown that Gata3 is needed for CD4 T cell development [21, 26], and notably for Thpok expression [22, 33]. The repression of Runx3 by Gata3, together with previous findings that Gata3 antagonizes Runx3 protein function in mature T cells [34], raised the possibility that unrestrained Runx3 expression was responsible for the impaired CD4-differentiation of Gata3-deficient thymocytes. If that were the case, disruption of Runx3 should restore the CD4-differentiation in Gata3-deficient mice. To evaluate this possibility, we generated mice conditionally deleting both Gata3 and Runx3 in DP cells. As expected because of Runx3 inactivation, a large fraction of double-deficient CD8-lineage thymocytes failed to terminate CD4 expression (Fig. 5A, right), resulting in the appearance of CD4+CD8+ post-selection thymocytes. This indicated that Cd4 silencing in Gata3-deficient thymocytes remained largely Runx3-dependent. However, we found no restoration of CD4 SP cell development (Fig. 5A), indicating that Gata3 is required for CD4+ T-cell development independently of its effect on Runx3.
Figure 5. Gata3 delays the CD8-redirection of Thpok-deficient thymocytes.
(A) Plots show CD4 and CD8 surface expression in TCRhi thymocytes from wild-type (control) mice, Gata3f/f or Runx3f/f Gata3f/f Cd4-Cre mice; data shown are from one experiment representative of three performed. (B) CD4 and CD8 expression in TCRhi CD24lo thymocytes from Thpok+/+ mice, and from Gata3-transgenic and control Thpok−/− mice. Data shown are from one experiment representative of three performed. (C) The number of TCRhi CD24lo CD4 SP thymocytes from the indicated mice is shown as mean + SD. Data pooled from five independent experiments analyzing 14 Thpok−/− mice (7 each Gata3 transgenic and control) and 8 Thpok+/+ mice (4 each Gata3 transgenic and control).
Conversely, it was possible that, by repressing Runx3 and CD8, sustained Gata3 expression would rescue in part the CD4-developmental block of Thpok-deficient thymocytes and prevent their redirection to the CD8 lineage. To evaluate this possibility, we examined the most mature thymocytes, which have both up-regulated TCR and down-regulated the maturation marker CD24 (Heat stable antigen, HSA). Whereas these cells normally are either CD4 or CD8 SP, the CD4 SP component is nearly absent in Thpok−/− mice, because the CD8-redirection of MHC II-restricted cells occurs before their terminal maturation (Fig. 5B) [10, 17, 19, 20]. The Gata3 transgene greatly increased the number of TCRhi CD24lo thymocytes with a CD4 SP-like phenotype (Fig. 5B). However, despite their large numbers (half that of mature CD4 SP cells in Thpok-sufficient mice, Fig. 5C), these CD4 SP-like cells did not terminate CD8 expression and there was no reconstitution of the peripheral CD4+ cell population (data not shown). Thus, as expected from its repression of Runx3, enforced Gata3 expression delays the CD8-lineage redirection of Thpok−/− thymocytes; however, it does not restore their differentiation into CD4+ T cells, presumably because its repression of Runx3 is transient only.
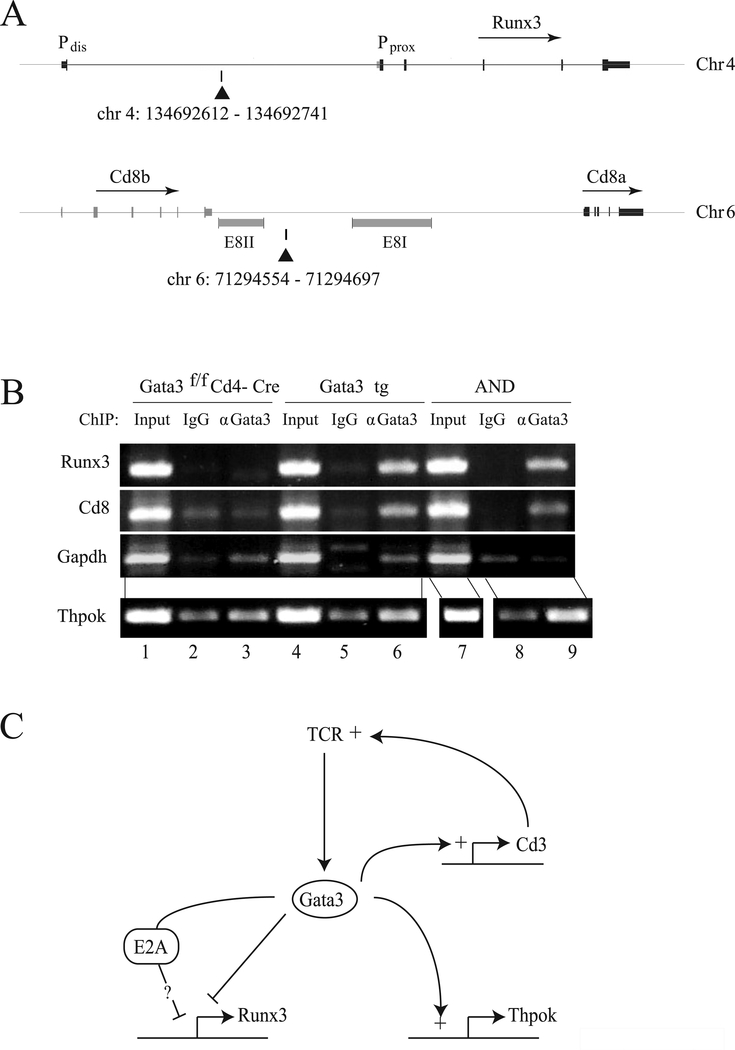
Gata3 molecules bind the Runx3 locus
Genome-wide analyses of Gata3 DNA binding have identified a Gata3 site within the Runx3 locus, occupied in CD4−CD8− double negative (DN) thymocytes (Fig. 6A) [35, 36]. While binding to this site was not detected in DP or naïve CD4+ T cells, this could be because Gata3 levels in these cells are lower than in DN or MHC II-signaled thymocytes. Consequently, we performed Chromatin Immunoprecipitation (ChIP) from AND TCR transgenic thymocytes, most of which are MHC II-signaled. We detected the target Runx3 sequence in Gata3 immunoprecipitates from AND thymocytes (Fig. 6B, top row, lanes 7–9), but not from Gata3-deficient cells (lanes 1–3), or in immunoprecipitates prepared with an isotype control antibody (lane 8). Of note, Gata3 also bound a site downstream of the Cd8b gene, consistent with the reduced expression of CD8 in Gata3-transgenic cells (Fig. 6A and B, middle row). Similar results were obtained from Gata3 transgenic thymocytes (Fig. 6B, lanes 4–6). These findings support the idea that Gata3 binding to Runx3 and Cd8 contribute to its repressive effect.
Figure 6. Gata3 binds to Runx3 and Cd8 loci.
(A) Chromosomal coordinates and schematic representations of Runx3 and Cd8 loci were obtained from the UCSC genome browser (mm9 mouse genome assembly). Location of PCR amplicons identifying Gata3 binding sites in each locus are shown by black triangles. Pdis and Pprox refer to the distal and proximal Runx3 promoters respectively. Note the Gata3 binding site location immediately downstream of the Cd8 E8(II) enhancer active throughout T-cell development [6]. (B) ChIP analyses of Gata3 binding in unseparated thymocytes from AND TCR transgenic, Gata3 transgenic or Gata3-deficient mice. Agarose-gel bands show PCR amplicons on the Cd8, Runx3, Gapdh (as a control for background) and Thpok (as a positive control, site A in Ref. [22]) loci from anti-Gata3 or control IgG immunoprecipitates, or from unfractionated chromatin (input). Data shown are representative of at least two separate experiments (two distinct mice of each genotype). (C) Central position of Gata3 in the circuitry of CD4-lineage differentiation. Gata3 (i) preserves bi-potency of MHC II-signaled thymocytes by repressing Runx3 (this study, left), (ii) promotes TCR signaling, possibly through binding to genes encoding CD3 subunits (top right, [35, 40]), and (iii) promotes Thpok expression (bottom right, [22]).
In summary, our study provides evidence for a novel function of Gata3 during the differentiation of CD4 lineage T cells in the thymus, namely transiently repressing Runx3 expression, that comes in addition to the previously reported functions of this factor in promoting CD4-lineage differentiation (schematized in Fig. 6C).
3. Discussion
Our study identifies a novel function of Gata3, whereby this factor represses Runx3 before Thpok up-regulation in MHC II-signaled thymocytes. It was previously proposed that MHC II-induced TCR signals repress Runx3 in cells that do not yet express Thpok [4], and our findings identify Gata3 as an important contributor to this effect.
We have previously shown that Gata3 is required in MHC II-restricted thymocytes for CD4-lineage specification, including for the expression of Thpok [22]. The current study points to a distinct function of Gata3. While it participates in the logic of CD4-lineage differentiation, it involves repressing a CD8-lineage specific gene, Runx3. Runx3 activity is critical to CD8-lineage differentiation [7, 37]. Runx3 promotes both the cessation of Cd4 and Thpok expression (therefore ensuring CD8-lineage commitment) and the initiation of CD8-lineage gene expression, even though such functions are in part masked by the redundancy between Runx1 and Runx3. Thus, by repressing Runx3, Gata3 controls a key factor in CD8-lineage differentiation.
Unlike Runx3 repression by Thpok, which persists in mature thymocytes and T cells and is a key component of CD4-lineage commitment [10, 20, 29], the repression of Runx3 by Gata3 is transient and therefore does not induce CD4-lineage commitment. From a developmental perspective, Gata3 repression of Runx3 would serve to preserve the CD4-CD8 ‘bi-potency’ of thymocytes while they undergo TCR signaling; this would notably be effected by ensuring the sustained Cd4 expression required for proper CD4+ cell development [5]. Bi-potency is resolved if Thpok is up-regulated, resulting in CD4-lineage commitment, or if TCR signaling ceases without Thpok up-regulation, resulting in Runx3 up-regulation and CD8-lineage commitment.
The repression of Runx3 by Gata3 was specific of MHC II-signaled cells in the thymus. Similarly, we did not observe any specific Runx3 repression in peripheral CD8 cells of Gata3 transgenic mice (Y.X. and R.B., unpublished observation). What accounts for this specificity remains to be determined. Because Gata3 functions are exquisitely dependent on its expression level [38], it is possible that expression of the Gata3 transgene is insufficient to bring Gata3 levels in MHC I-restricted cells to the peak seen in MHC II-specific transitional cells (see Fig. 2A). A non-mutually exclusive possibility is that Runx3 repression requires stage-specific post-translational modifications of Gata3, or its cooperation with other factors, such as IRF4 [39]. Indeed, genome-wide studies of Gata3 binding suggest that the outcomes of Gata3 recruitment to DNA are context-dependent [35]. Of note, our observations do not exclude that high-level Gata3 expression represses Runx3 in other contexts. Previous studies have pointed to such a possibility during the differentiation of mature CD4+ T cells into Th2 effectors [34].
Regardless of its mechanism, the fact that Gata3-mediated repression of Runx3 is specific of MHC II-signaled cells has two separate and important correlates. First, Gata3 repression of Runx3 is not caused by Gata3 repression of Cd8. Indeed, MHC II-restricted cells, in which Gata3 represses Runx3, do not need CD8 molecules to signal. Second, it explains the apparent paradox that the Gata3 transgene did not inhibit the development of wild-type CD8-lineage cells, which are MHC I-restricted and in which Gata3 does repress Runx3.
Our findings suggest a possible mechanism underpinning the repression of Runx3 by Gata3, by showing direct binding of Gata3 molecules to the Runx3 locus. However, Gata3 recruitment to Runx3 does not imply a direct regulatory function, and determining the role of such binding in Runx3 repression will require the identification of the cis-regulatory elements that control Runx3, which are not yet known. In addition, given the pleiotropic effects of Gata3 during positive selection, it is possible that additional mechanisms are involved in Gata3-mediated Runx3 repression. In particular, Gata3 has been proposed to activate expression of the transcription factor E2A [40], an E-box binding protein that serves as a ‘gate-keeper’ of positive selection redundantly with the related factor HEB [41, 42]. Inactivation of both E2A and HEB promotes the differentiation of CD8-lineage cells, and there is evidence that these factors repress Runx3, directly or indirectly [41, 42]. Thus, Gata3 could repress Runx3 through E2A.
We also noted that the Gata3 transgene repressed IL-7Rα expression (data not shown), an effect more pronounced in CD8 than CD4 SP cells. While it had been proposed that IL-7 promoted Runx3 expression in CD8-differentiating thymocytes, a recent report has shown IL-7, and γc-cytokines in general, to be dispensable for Runx3 up-regulation in these cells [43]. Thus, the effect of Gata3 on IL-7Rα expression does not account for repression of Runx3 by Gata3.
The inhibition of CD8 expression by the Gata3 transgene mirrored the slightly increased CD8 expression of Gata3-deficient thymocytes [22, and Y.X. and R.B., unpublished observations]. In contrast, the conclusion on Runx3 repression relies on transgenic Gata3 expression only. As with all gain-of-function approaches, this raises the question of whether it also applies to endogenous Gata3. In support of our conclusion, the CD8 redirection’ of Gata3-deficient MHC II-restricted thymocytes [22] is fully consistent with the idea that Gata3 represses Runx3. That is, our current results predict that Gata3 inactivation, by promoting expression of Runx3, would redirect MHC II-restricted cells into the CD8 lineage, which is indeed the case [22]. The fact that Runx3 disruption fails to restore the CD4-lineage differentiation of Gata3-deficient thymocytes supports the concept that Gata3 is required for the specification of the CD4 lineage, in addition to its effects on Runx3.
This novel repressive role of Gata3 adds to the panoply of functions this factor performs during CD4-lineage differentiation. We previously reported that Gata3 is required for Thpok expression both in conventional MHC II-restricted thymocytes and in iNK T cells [22, 33]. Gata3 promotes other aspects of CD4-lineage differentiation, as enforced Thpok expression does not rescue the CD4-differentiation of Gata3-deficient cells [22]. What these functions include remains to be determined. Gata3 binds to genes encoding the CD3 subunits of TCR complexes or TCR signaling intermediates [35, 40], and may therefore be required for efficient TCR signal transduction. This could explain a stronger requirement for Gata3 in CD4- than CD8-lineage differentiation, as sustained TCR signaling is required for the former but not for the latter [4]. It is also possible that Gata3 promotes the expression of other CD4-lineage specific transcription factors, whose identity would have remained elusive. The recent observation that Gata3 expression is targeted by the Ras-Erk and calcium-calcineurin signaling pathways [27], both downstream of the TCR, further emphasizes its function as a critical pivot in the choice between CD4 and CD8 lineages.
4. Materials and Methods
Mice
Thpok−/−, Gata3f/f, ThpokGFP and Runx3tRFP mice were previously described [14, 22, 44]. B2m−/− and I-Ab−/− mice and mice carrying the AND or P14 TCR transgenes were obtained from Jax. Gata3 transgenic mice were generated in our laboratory [28]. Briefly, a Gata3 cDNA was inserted into the transgenic expression vector p29Δ2, driven by the promoter of human CD2 gene [45]; the resulting DNA was microinjected to generate Gata3 transgenic mice using previously described procedures [46]. Transgenic founders were identified by Southern blotting; transgenic animals were subsequently identified by PCR from tail DNA using the following primers: 5’ CTC GAC TTA CAT CCG AAC CCG GTA 3’ and 5’ CGC TCT TGC TCT CTG TGT ATG 3’. Animal procedures used in this study were approved by the National Cancer Institute Animal Care and Use Committee.
Antibodies
Flow cytometry antibodies against CD4 (RM4.5), CD8 (53–6.7), TCRβ (H57–597), CD24 (M1/69), CD44 (IM7), CD69 (H1.2F3), H-2Kb (AF6–120.1) and anti-Gata3 (L50–823) were from BD Biosciences or eBioscience.
Cell preparation and staining
Thymocytes and spleen cells were prepared and stained as described [22], and analyzed by flow cytometry on LSRII or LSR Fortessa cytometers (BD Biosciences). Dead cells were excluded by forward light-scatter gating and DAPI staining. Data was analyzed with Flowjo software. Gata3 intracellular staining was performed on cells fixed and permeabilized (using eBioscience kit 00–5523-00) after surface staining; dead cells were excluded using the live/dead fixable staining (Invitrogen L-23105).
Thymocytes were sorted as described [22] and placed (5 × 106/ml) in RPMI 1640 medium, supplemented with 10% FCS, Glutamine and antibiotics. Cells were analyzed by immunofluorescence and flow cytometry after 18 hours at 37°C.
Gata3 chromatin precipitation (ChIP)
Anti-Gata3 ChIP was performed using the EZChIP kit (Millipore 17–371) as described [22], with modifications. Briefly, thymocytes from Gata3f/f Cd4-Cre mice, Gata3 transgenic mice and AND TCR transgenic mice were fixed with 1% formaldehyde and lysed in 1% SDS lysis buffer. The crosslinked DNA was sonicated to 200–500bp (Ultrasonic processor XL, Misonix Inc), and then diluted with the kit ‘dilution buffer’ to a concentration of 107 cell-equivalents per ml. 20 μl of sonicated chromatin (~2 × 105 cells) was set aside as input. For each ChIP reaction, 3 ml chromatin (~3 × 107 cells) were incubated overnight with protein G Dynalbeads (Invitrogen) coupled antibodies (5μg): anti-Gata3 (BD Bioscience 558686) or control mouse IgG. The immunoprecipitated DNA was retrieved and washed according to the manufacturer’s recommendations. Input and ChIP DNA were then subject in parallel to cross-linking reversal, phenol/chloroform extraction and ethanol precipitation. 1% of ChIP DNA (~3 × 105 cells) or input DNA (~2 × 103 cells) was used for conventional PCR and analyzed on 1.5% agarose gel. The primer pairs for Cd8, Runx3 and Gapdh for ChIP-PCR were as follows: Cd8: 5’CAACTTCCACTGGTTGGATTTACG3’; 5’ TTGATGCCCCGCTTTTGAAG3’;
Runx3: 5’ CTCCAGGCAGGCAGGATCTG 3’; 5’ GGTCTGGGTAGCTGAGCCCTG 3’
Gapdh: 5’ GAGGACAATAAGGCTCAAGG 3’; 5’ CTCTCGGCTGGGTGGAGTG-3’.
Supplementary Material
Acknowledgments
We thank E. Rothenberg for insightful discussions and for sharing unpublished results, B. Taylor and S. Banerjee for cell sorting, T.-A. Lewis for mouse technical support, and Andrea C. Carpenter, B.J. Fowlkes and Melanie Vacchio for reading the manuscript.. Supported by the Intramural Research Programs of the National Cancer Institute, Center for Cancer Research, and of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations:
- DP:
double positive
- SP:
single positive
Footnotes
The authors declare no financial or commercial conflict of interest.
References
- 1.Paul WE, (ed). Fundamental Immunology, 6th Edition. Lippincott Williams & Wilkins, Philadelphia: 2008. [Google Scholar]
- 2.Bosselut R, CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol 2004. 4:529–540. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter AC and Bosselut R, Decision checkpoints in the thymus. Nat Immunol 2010. 11:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer A, Adoro S and Park JH, Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol 2008. 8:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adoro S, McCaughtry T, Erman B, Alag A, Van Laethem F, Park JH, Tai X et al. , Coreceptor gene imprinting governs thymocyte lineage fate. EMBO J 2011. 31:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniuchi I, Ellmeier W and Littman DR, The CD4/CD8 lineage choice: new insights into epigenetic regulation during T cell development. Adv Immunol 2004. 83:55–89. [DOI] [PubMed] [Google Scholar]
- 7.Collins A, Littman DR and Taniuchi I, RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol 2009. 9:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y et al. , Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell 2002. 111:621–633. [DOI] [PubMed] [Google Scholar]
- 9.Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y et al. , Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A 2003. 100:7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Wildt KF, Castro E, Xiong Y, Feigenbaum L, Tessarollo L and Bosselut R, The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity 2008. 29:876–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y et al. , Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med 2009. 206:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grueter B, Petter M, Egawa T, Laule-Kilian K, Aldrian CJ, Wuerch A, Ludwig Y et al. , Runx3 Regulates Integrin {alpha}E/CD103 and CD4 Expression during Development of CD4-/CD8+ T Cells. J Immunol 2005. 175:1694–1705. [DOI] [PubMed] [Google Scholar]
- 13.Kohu K, Sato T, Ohno S, Hayashi K, Uchino R, Abe N, Nakazato M et al. , Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol 2005. 174:2627–2636. [DOI] [PubMed] [Google Scholar]
- 14.Zamisch M, Tian L, Grenningloh R, Xiong Y, Wildt KF, Ehlers M, Ho IC et al. , The transcription factor Ets1 is important for CD4 repression and Runx3 up-regulation during CD8 T cell differentiation in the thymus. J Exp Med 2009. 206:2685–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY et al. , Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol 2010. 11:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H and Nishikawa S, Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc Natl Acad Sci U S A 1993. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W et al. , The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 2005. 433:826–833. [DOI] [PubMed] [Google Scholar]
- 18.Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P et al. , The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol 2005. 6:373–381. [DOI] [PubMed] [Google Scholar]
- 19.Egawa T and Littman DR, ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol 2008. 9:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muroi S, Naoe Y, Miyamoto C, Akiyama K, Ikawa T, Masuda K, Kawamoto H et al. , Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat Immunol 2008. 9:1113–1121. [DOI] [PubMed] [Google Scholar]
- 21.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH and Ho IC, Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 2003. 19:863–875. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, Paul WE et al. , Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol 2008. 9:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho IC, Tai TS and Pai SY, GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol 2009. 9:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Park K, Wang H, He X, Zhang Y, Hua X, Li Y et al. , CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 2008. 28:346–358. [DOI] [PubMed] [Google Scholar]
- 25.Swat W, Dessing M, von BH and Kisielow P, CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol 1993. 23:739–746. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV and Alberola-Ila J, GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity 2003. 19:83–94. [DOI] [PubMed] [Google Scholar]
- 27.Gimferrer I, Hu T, Simmons A, Wang C, Souabni A, Busslinger M, Bender TP et al. , Regulation of GATA-3 Expression during CD4 Lineage Differentiation. J Immunol 2011. 186:3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesourne R, Uehara S, Lee J, Song KD, Li L, Pinkhasov J, Zhang Y et al. , Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol 2009. 10:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egawa T, Tillman RE, Naoe Y, Taniuchi I and Littman DR, The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med 2007. 204:1945–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan H, Sakaguchi S, Tenno M, Kopf A, Boucheron N, Carpenter AC, Egawa T et al. , Cd8 enhancer E8I and Runx factors regulate CD8alpha expression in activated CD8+ T cells. Proc Natl Acad Sci U S A 2011. 108:18330–18335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCaughtry TM, Wilken MS and Hogquist KA, Thymic emigration revisited. J Exp Med 2007. 204:2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendriks RW, Nawijn MC, Engel JD, van DH, Grosveld F and Karis A, Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol 1999. 29:1912–1918. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Carr T, Xiong Y, Wildt KF, Zhu J, Feigenbaum L, Bendelac A et al. , The sequential activity of Gata3 and Thpok is required for the differentiation of CD1d-restricted CD4(+) NKT cells. Eur J Immunol 2010. 40:2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagi R, Junttila IS, Wei G, Urban JFJ, Zhao K, Paul WE and Zhu J, The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity 2010. 32:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, Narlikar L et al. , Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 2011. 35:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JA, Mortazavi A, Williams BA, Wold BJ and Rothenberg EV, Dynamic Transformations of Genome-wide Epigenetic Marking and Transcriptional Control Establish T Cell Identity. Cell 2012. 149:467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L and Bosselut R, CD4-CD8 lineage differentiation: Thpok-ing into the nucleus. J Immunol 2009. 183:2903–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taghon T, Yui MA and Rothenberg EV, Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat Immunol 2007. 8:845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y, Li H, Sun Y, Chen X, Liu H, Gao X and Liu X, Interferon regulatory factor 4 regulates thymocyte differentiation by repressing Runx3 expression. Eur J Immunol 2010. 40:3198–3209. [DOI] [PubMed] [Google Scholar]
- 40.Ling KW, van Hamburg JP, de Bruijn MJ, Kurek D, Dingjan GM and Hendriks RW, GATA3 controls the expression of CD5 and the T cell receptor during CD4 T cell lineage development. Eur J Immunol 2007. 37:1043–1052. [DOI] [PubMed] [Google Scholar]
- 41.Jones ME and Zhuang Y, Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity 2007. 27:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones-Mason ME, Zhao X, Kappes D, Lasorella A, Iavarone A and Zhuang Y, E Protein Transcription Factors Are Required for the Development of CD4(+) Lineage T Cells. Immunity 2012. 36:348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A et al. , Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med 2012. 209:2263–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N et al. , Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol 2004. 5:1157–1165. [DOI] [PubMed] [Google Scholar]
- 45.Shores EW, Huang K, Tran T, Lee E, Grinberg A and Love PE, Role of TCR zeta chain in T cell development and selection. Science 1994. 266:1047–1050. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Adams A, Wildt KF, Aronow B, Feigenbaum L and Bosselut R, Restricting Zap70 expression to CD4+CD8+ thymocytes reveals a T cell receptor-dependent proofreading mechanism controlling the completion of positive selection. J Exp Med 2003. 197:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.