Abstract
Background and Objectives:
Gastric cancer outcomes differ between Asian and Western countries, even when controlling for contributing factors, but whether this difference holds true for China remains inadequately studied. We sought to compare the presentation, treatment, and outcomes of patients with gastric cancer (GC) undergoing curative intent (R0) resection between the U.S. and China, and to ascertain whether geography/ institution is an independent predictor of DSS.
Methods:
Data were analyzed from patients with GC undergoing R0 resection at high-volume cancer centers in the U.S. (Memorial Sloan Kettering Cancer Center [MSKCC], n=1,378) and China (Fujian Medical University Union Hospital [FMUUH], n=4,262) between 2000 and 2014. Factors associated with disease-specific survival (DSS) were examined by multivariate analysis.
Results:
The 5-year DSS (p < 0.001) for all patients was better at MSKCC than at FMUUH, even among patients not receiving preoperative chemotherapy (p < 0.001), but stratification by sub-stage eliminated this difference (p > 0.05). Factors independently associated with DSS included age, histology, tumor size, T category, N category, gastrectomy type, and preoperative chemotherapy, but not institution.
Conclusions:
Although the presentation of GC patients between MSKCC and FMUUH differs, survival of patients with curatively resected GC, when matched for clinical stage, is comparable.
Keywords: Stomach neoplasms, stomach cancer, perioperative chemotherapy, prognosis, Eastern and Western, China, United States
INTRODUCTION
Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide, and half of all GC cases arise in East Asia.[1] Examining differences in GC presentation,[2–6] management,[7,8] and outcomes[6,9–12] between GC patients in Eastern and Western countries could shed light on the relative effectiveness of various treatment strategies, as standards vary among global regions.
A preliminary step towards correlating differences in GC management with outcomes is to determine whether survival does indeed differ between countries or regions. Previous studies comparing GC survival outcomes between individual institutions in the U.S. and Asian countries have reached differing conclusions. One analysis of data from MSKCC and Yokohama City University found that the more favorable outcomes for GC patients at the Japanese institution were attributable to differences in tumor location and T category.[10] A series of two comparisons of GC outcomes at MSKCC with those at Seoul St. Mary’s Hospital in South Korea led to contrasting conclusions. While the first study found that survival was greater among Korean patients stage-for-stage,[13] the second, in which there were fewer differences between institutions in surgical approach, found that survival was similar.[11] Finally, only one study has compared outcomes between patients in the U.S. (again at MSKCC) and China (at Beijing Cancer Hospital [BCH]), and indicated that survival outcomes were worse at BCH, even when controlling for stage.[12]
To further survey potential differences in gastric cancer survival between the U.S. and China, we compared the presentation, treatment, pathology and outcomes of patients who underwent R0 resection at another high-volume cancer center in China, Fujian Medical University Union Hospital (FMUUH) in Fuzhou, to those of patients treated at MSKCC.
MATERIALS AND METHODS
Data Collection
This study was approved by the Institutional Review Boards (IRB) of both institutions. We collected data for patients who underwent gastrectomy between January 1, 2000, and December 31, 2014 at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, NY, US or Fujian Medical University Union Hospital (FMUUH) in Fuzhou, Fujian Province, China from the institutions’ prospectively maintained GC databases. Inclusion criteria were diagnosis of primary GC, removal of all residual macroscopic or microscopic disease (R0 resection); and more than 15 harvested lymph nodes. Exclusion criteria included other malignancy; distant metastasis; wedge, endoscopic mucosal, or endoscopic submucosal resection, and missing data. TNM category was reconfirmed using original pathologic data based on the 7th edition of the TNM staging system (AJCC/UICC, 2010).[14] Data regarding patient presentation was obtained through review of the patient’s history, physical examination, laboratory tests, chest radiography, upper GI endoscopy, and abdominal CT scans.
Follow-up after Resection
Follow-up after R0 resection consisted of a history and physical, as well as CT or PET/CT of the chest, abdomen, and pelvis and complete blood counts, chemistry profiles, and esophagogastroduodenoscopy (EGD) as clinically indicated, every 3-6 months for 1-2 years, every 6-12 months for 3-5 years, and then every year thereafter for at least 5 years. For patients who received neoadjuvant and/or adjuvant chemotherapy, CT or PET/CT of the chest/abdomen/pelvis with oral and IV contrast was obtained every 6-12 months for the first 2 years, then annually up to 5 years. In the MSKCC dataset, disease status at last follow-up was based on retrospective review of medical records and review of the Social Security Death Index. In the FMUUH dataset, disease status at last follow-up was based on the information of the Department of Gastric Surgery or the National Statistical Office.
Statistical Analysis
Disease-specific survival (DSS) was measured from the time of surgery to death from GC. Continuous variables were evaluated as means ± standard deviation using the t test, and interval values are presented as medians. Differences in proportions between the two countries were compared using the chi-squared test. DSS was estimated using the Kaplan-Meier method. Survival distributions were compared using the log-rank test. Cox proportional hazard regression models of DSS were established for both countries. All statistical analyses were carried out using SPSS version 18 (SPSS, Chicago, IL). P values less than 0.05 in a two-sided test were considered statistically significant.
RESULTS
Demographic and Clinicopathological Characteristics
Our database review identified 4,262 patients at FMUUH and 1,378 patients at MSKCC who underwent R0 gastric resection for primary gastric tumors between 2000 and 2014. The mean age, body mass index (BMI), and number of comorbidities were higher in US patients (Table 1). GC patients treated at MSKCC were much more ethnically diverse than those at FMUUH. Tumors were more often proximal at MSKCC (50% vs 27%) and more often distal at FMUUH (30% vs 41%). Total and distal gastrectomy were performed more often at FMUUH (56% vs. 25% and 43% vs. 38%, respectively). Patients treated at FMUUH had tumors that invaded deeper than those of patients at MSKCC (most frequent depth, T4 vs. T1), as well as more metastatic lymph nodes (mean of 7 vs. 2), and more advanced disease (stage III, 58.2% vs. 25.0%) (p < 0.001 for all comparisons). More lymph nodes were retrieved from patients at FMUUH (32 vs. 26 from patients at MSKCC; p < 0.001). Patients treated at MSKCC were more likely to have received preoperative chemotherapy (47% vs 2%, p < 0.001), while patients treated at FMUUH more often received postoperative chemotherapy (38% vs 22%, p < 0.001) (Table 1). Perioperative chemotherapy was generally administered to patients with advanced GC and usually consisted of fluoropyrimidine-based combinations with platinum at both institutions.
Table 1.
Demographics and clinicopathological characteristics
| Parameters | FMUUH (n=4,262) | MSKCC (n=1,378) | p |
|---|---|---|---|
| Age | 60 ± 11 | 64 ± 13 | <0.001 |
| Male gender | 3201 (75.1) | 881 (63.9) | <0.001 |
| BMI | 22.2 ± 2.9 | 27.7 ± 5.2 | <0.001 |
| Ethnicity | <0.001 | ||
| White | 0 (0) | 1096 (79.5) | |
| Black | 0 (0) | 87 (6.3) | |
| Asian | 4262 (100.0) | 155 (11.2) | |
| Others | 0 (0) | 40 (2.9) | |
| Comorbidities present | 1139 (26.7) | 562 (40.8) | <0.001 |
| Tumor locationa | <0.001 | ||
| Proximal | 1156 (27.1) | 685 (49.7) | |
| Middle | 804 (18.9) | 250 (18.1) | |
| Lower | 1737 (40.8) | 410 (29.8) | |
| Mixed | 565 (13.3) | 33 (2.4) | |
| Undifferentiated histologyb | 3089 (72.5) | 830 (60.2) | <0.001 |
| Tumor size (cm) | 5.1 ± 2.8 | 3.4 ± 2.8 | <0.001 |
| Depth of invasionc | <0.001 | ||
| T1 | 807 (18.9) | 539 (39.1) | |
| T2 | 452 (10.6) | 196 (14.2) | |
| T3 | 920 (21.6) | 408 (29.6) | |
| T4a | 1630 (38.2) | 227 (16.5) | |
| T4b | 453 (10.5) | 8 (0.6) | |
| No. of metastatic lymph nodesd | <0.001 | ||
| 0 | 1327 (31.1) | 771 (56.0) | |
| 1-2 | 580 (13.6) | 261 (18.9) | |
| 3-6 | 738 (17.3) | 183 (13.3) | |
| 7-15 | 939 (22.0) | 150 (10.9) | |
| ≥16 | 678 (15.9) | 13 (0.9) | |
| TNM stagee | |||
| I | 984 (23.1) | 617 (44.8) | <0.001 |
| II | 799 (18.7) | 417 (30.3) | |
| III | 2479 (58.2) | 344 (25.0) | |
| D2 lymphadenectomyf | 4133 (97.0) | 1333 (96.7) | 0.656 |
| No. of lymph nodes retrieved | 32 ± 12 | 26 ± 11 | <0.001 |
| No. of positive lymph nodes | 7 ± 9 | 2 ± 4 | <0.001 |
| No. of negative lymph nodes | 25 ± 13 | 24 ± 10 | 0.001 |
| Type of gastrectomy | <0.001 | ||
| Total | 2365 (55.5) | 342 (24.8) | |
| Distal | 1827 (42.9) | 527 (38.2) | |
| Proximal | 70 (1.6) | 509 (36.9) | |
| Received pre-op chemotherapy | 91 (2.1) | 645 (46.8) | <0.001 |
| Received post-op chemotherapy | 1618 (38.0) | 299 (21.7) | <0.001 |
Adenocarcinoma of the esophagogastric junction within the stomach was categorized as proximal third gastric cancer;
Histology subtype was categorized as differentiated (well-differentiated and moderately differentiated adenocarcinoma) or undifferentiated (poorly differentiated adenocarcinoma and signet ring cell carcinoma);
Depth of invasion includes p-depth of invasion and yp-depth of invasion;
No. of metastatic LNs includes p- No. of metastatic LNs and yp- No. of metastatic LNs;
TNM stage includes p-TNM stage and yp-TNM stage. p refers to the postoperative pathology for patients receiving surgery without preoperative chemotherapy. yp refers to the postoperative pathology for patients receiving surgery with preoperative chemotherapy.
All other patients underwent D1 lymphadenectomy.
Survival Analysis
AT MSKCC and FMUUH, median follow-up times were 38 (range, 0-184) and 43 (range, 0-147) months, respectively. The 5-year DSS was 72% at MSKCC, and 60% at FMUUH (p < 0.001) (Supplemental Table 1; Fig. 1 illustrates DSS over time). The numbers of deaths from other causes are provided to address potential underestimation of risk of cancer-associated death (Supplemental Table 1). At MSKCC the probability of death due to other causes was higher than at FMUUH (10% vs. 3%).
Figure 1.
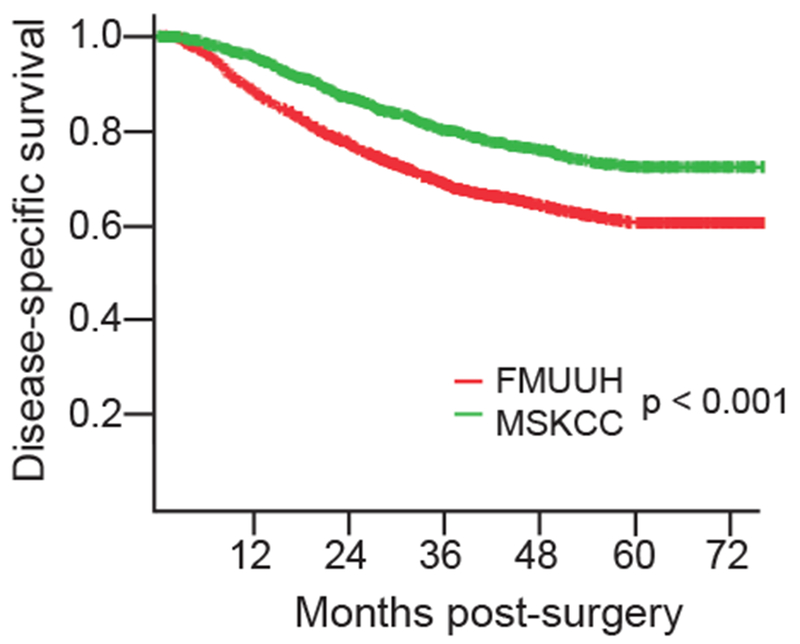
Disease-specific survival of all patients.
To eliminate the potential for biased down-staging due to the more frequent use of preoperative chemotherapy at MSKCC, we compared DSS in patients receiving surgery without preoperative chemotherapy between the two institutions. The 5-year DSS was still higher at MSKCC (80% vs. 61%; p < 0.001) (Table 2, Fig. 2A). Comparing survival within sub-stages and procedures, 5-year DSS was higher at MSKCC for patients with advanced T (T3-T4) and N (N3) category cancer, and for those who underwent proximal or total gastrectomy.
Table 2.
Five-year disease-specific survival in patients receiving surgery without preoperative chemotherapy by subgroup
| FMUUH (n=4,171) | MSKCC (n=733) | p | |||
|---|---|---|---|---|---|
| 5-year DSS | 95% CI | 5-year DSS | 95% CI | ||
| All patients | 61% | 0.590-0.630 | 80% | 0.761-0.839 | <0.001 |
| TNM stage | |||||
| Stage IA | 95% | 0.930-0.970 | 96% | 0.940-0.980 | 0.920 |
| Stage IB | 92% | 0.881-0.959 | 87% | 0.792-0.948 | 0.139 |
| Stage IIA | 85% | 0.811-0.889 | 85% | 0.752-0.948 | 0.813 |
| Stage IIB | 78% | 0.741-0.819 | 67% | 0.552-0.788 | 0.288 |
| Stage IIIA | 63% | 0.571-0.689 | 61% | 0.473-0.747 | 0.777 |
| Stage IIIB | 50% | 0.461-0.539 | 57% | 0.433-0.707 | 0.227 |
| Stage IIIC | 30% | 0.280-0.320 | 35% | 0.174-0.526 | 0.229 |
| T category | |||||
| T1 | 94% | 0.920-0.960 | 93% | 0.891-0.969 | 0.508 |
| T2 | 84% | 0.801-0.879 | 82% | 0.722-0.918 | 0.905 |
| T3 | 62% | 0.581-0.659 | 75% | 0.672-0.828 | 0.008 |
| T4 | 43% | 0.410-0.450 | 49% | 0.392-0.588 | 0.034 |
| N category | |||||
| N0 | 90% | 0.880-0.920 | 91% | 0.871-0.949 | 0.512 |
| N1 | 76% | 0.721-0.799 | 76% | 0.662-0.858 | 0.731 |
| N2 | 59% | 0.551-0.629 | 63% | 0.493-0.767 | 0.445 |
| N3 | 32% | 0.300-0.340 | 48% | 0.362-0.598 | 0.001 |
| Histology | |||||
| Differentiated | 77% | 0.750-0.790 | 89% | 0.851-0.929 | <0.001 |
| Undifferentiated | 55% | 0.530-0.570 | 75% | 0.711-0.789 | <0.001 |
| Tumor location | |||||
| Proximal | 57% | 0.531-0.609 | 78% | 0.721-0.839 | <0.001 |
| Middle | 59% | 0.551-0.629 | 77% | 0.692-0.848 | <0.001 |
| Lower | 68% | 0.660-0.700 | 83% | 0.771-0.889 | <0.001 |
| Mixed | 47% | 0.431-0.509 | 86% | 0.703-1.017 | 0.005 |
| Type of gastrectomy | |||||
| Total | 53% | 0.510-0.550 | 76% | 0.682-0.838 | <0.001 |
| Distal | 71% | 0.690-0.730 | 83% | 0.791-0.869 | <0.001 |
| Proximal | 69% | 0.572-0.808 | 78% | 0.702-0.858 | 0.198 |
Figure 2.
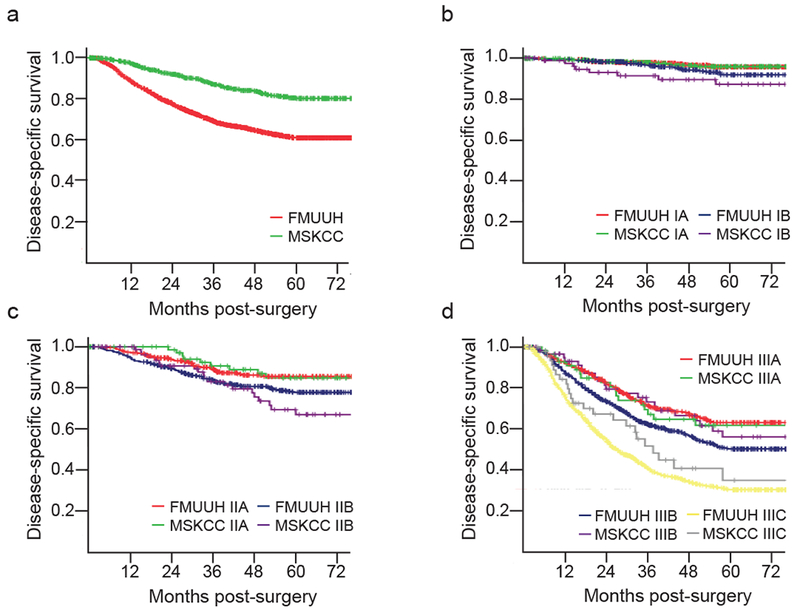
Comparison of disease-specific survival in patients in patients receiving surgery without preoperative chemotherapy between MSKCC and FMUUH. a, all stages; b, stage I; c, stage II; d, stage III.
Identification of Factors Contributing to Disease-specific Survival
Our unadjusted single-factor analysis identified 10 factors as significantly associated with DSS (Table 3). Adjusted multivariate analysis narrowed the list of significantly contributing factors to age (p < 0.001), histology (p < 0.001), tumor size (p = 0.006), depth of invasion (p = 0.009), number of metastatic LNs (p < 0.001), number of negative LNs (p < 0.001), gastrectomy type (p < 0.001), and preoperative chemotherapy (p < 0.001), and eliminated institution (p = 0.449) (Table 3).
Table 3.
Risk factors for disease-specific survival for all patients
| Variable | Unadjusted HR (95%CI) | p | Adjusted HR (95% CI) | p |
|---|---|---|---|---|
| MSKCC vs. FMUUH | 0.606 (0.533-0.688) | <0.001 | 0.844 (0.544-1.309) | 0.449 |
| Ethnicity (vs. white) | <0.001 | 0.675 | ||
| Black | 0.881 (0.546-1.423) | 0.605 | 1.176 (0.721-1.918) | 0.516 |
| Asian | 1.528 (1.332-1.752) | <0.001 | 1.011 (0.644-1.588) | 0.961 |
| Others | 0.654 (0.270-1.585) | 0.347 | 0.629 (0.258-1.532) | 0.307 |
| Female vs. male | 0.856(0.768-0.954) | 0.005 | 0.898(0.803-1.005) | 0.061 |
| Age (vs. <45) | 0.001 | <0.001 | ||
| 45-70 | 0.953 (0.806-1.127) | 0.574 | 1.063 (0.895-1.262) | 0.489 |
| >70 | 1.179 (0.980-1.417) | 0.080 | 1.555 (1.278-1.893) | <0.001 |
| Comorbidities vs. none | 0.888 (0.799-0.987) | 0.028 | 0.972 (0.868-1.089) | 0.626 |
| BMI (vs. <25) | <0.001 | 0.374 | ||
| 25-28 | 0.826 (0.721-0.946) | 0.006 | 0.917 (0.797-1.056) | 0.231 |
| ≥28 | 0.611 (0.515-0.725) | <0.001 | 0.903 (0.737-1.106) | 0.324 |
| Tumor location (vs. proximal)a | <0.001 | 0.544 | ||
| Middle | 0.942 (0.822-1.079) | 0.389 | 0.944 (0.814-1.094) | 0.444 |
| Lower | 0.697 (0.620-0.783) | <0.001 | 1.035 (0.857-1.249) | 0.722 |
| Mixed | 1.582 (1.371-1.825) | <0.001 | 0.920 (0.785-1.078) | 0.302 |
| Undifferentiated vs. differentiated histologyb | 2.310 (2.041-2.614) | <0.001 | 1.291 (1.133-1.472) | <0.001 |
| Tumor size (vs. <3.0 cm) | <0.001 | 0.006 | ||
| 3.0-5.0 | 2.962 (2.481-3.537) | <0.001 | 1.259 (1.027-1.545) | 0.027 |
| >5.0 | 6.080 (5.123-7.216) | <0.001 | 1.396 (1.129-1.725) | 0.002 |
| Depth of invasion (vs. T1)c | <0.001 | <0.001 | ||
| T2 | 2.537 (1.881-3.420) | <0.001 | 1.517 (1.108-2.078) | 0.009 |
| T3 | 6.000 (4.714-7.637) | <0.001 | 2.245 (1.702-2.962) | <0.001 |
| T4a | 10.807 (8.598-13.585) | <0.001 | 3.122 (2.358-4.133) | <0.001 |
| T4b | 13.551 (10.557-17.394) | <0.001 | 3.193 (2.353-4.334) | <0.001 |
| No. of metastatic LNs (vs. 0)d | <0.001 | <0.001 | ||
| 1-2 | 2.566(2.092-3.147) | <0.001 | 1.775(1.434-2.196) | <0.001 |
| 3-6 | 4.301(3.592-5.150) | <0.001 | 2.375(1.942-2.905) | <0.001 |
| 7-15 | 8.347(7.086-9.831) | <0.001 | 3.828(3.113-4.707) | <0.001 |
| ≥16 | 13.731(11.589-16.270) | <0.001 | 4.928(3.769-6.443) | <0.001 |
| D2 vs. D1 lymphadenectomy | 7.770 (3.389-15.561) | <0.001 | 1.886 (0.931-3.819) | 0.078 |
| No. of LNs retrieved (vs. 15-25) | 0.016 | 0.386 | ||
| 25-35 | 1.166 (1.041-1.305) | 0.008 | 1.030 (0.894-1.187) | 0.678 |
| 35-45 | 1.199 (1.047-1.373) | 0.009 | 1.137 (0.928-1.393) | 0.214 |
| ≥45 | 1.042 (0.883-1.231) | 0.626 | 1.001 (0.768-1.304) | 0.996 |
| No. of negative LNs (vs. <10) | <0.001 | <0.001 | ||
| 10-20 | 0.338 (0.296-0.385) | <0.001 | 0.773 (0.662-0.902) | 0.001 |
| 20-30 | 0.200 (0.174-0.230) | <0.001 | 0.609 (0.497-0.747) | <0.001 |
| ≥30 | 0.143 (0.123-0.167) | <0.001 | 0.473 (0.359-0.624) | <0.001 |
| Gastrectomy type (vs. total) | <0.001 | <0.001 | ||
| Distal | 0.502 (0.452-0.557) | <0.001 | 0.779 (0.655-0.925) | 0.005 |
| Proximal | 0.658 (0.555-0.781) | <0.001 | 1.505 (1.199-1.891) | 0.002 |
| Pre-op chemotherapy (n=736) (vs. none, n=4904) | 1.104 (0.957-1.273) | 0.176 | 2.104 (1.724-2.569) | <0.001 |
| Post-op chemotherapy (n=1917) (vs. none, n=3723) | 1.320 (1.200-1.453) | <0.001 | 0.908 (0.820-1005) | 0.062 |
Tumor location: Adenocarcinoma of the esophagogastric junction within the stomach was categorized as proximal third gastric cancer;
Histology subtype was categorized as differentiated type (well-differentiated and moderately differentiated adenocarcinoma) or undifferentiated type (poorly differentiated adenocarcinoma and signet ring cell carcinoma);
Depth of invasion includes p- depth of invasion and yp-depth of invasion;
No. of metastatic LNs includes p- No. of metastatic LNs and yp- No. of metastatic LNs; p refers to the postoperative pathology for patients receiving surgery without preoperative chemotherapy. yp refers to the postoperative pathology for patients receiving surgery with preoperative chemotherapy.
Among patients receiving surgery without preoperative chemotherapy, adjusted multivariate analysis identified a similar list of survival-influencing factors as for the whole population, with the addition of gender (p=0.039), lymphadenectomy (p=0.025), and postoperative chemotherapy (p=0.024), (Supplemental Table 2). Among patients receiving surgery without either pre- or postoperative chemotherapy, fewer factors were identified; compared to the list of factors for the whole population, the only addition was lymphadenectomy (p=0.025), and the exceptions were tumor size and gastrectomy (Supplemental Table 3).
DISCUSSION
Here, we show that stage-adjusted survival outcomes are similar for GC patients at a high-volume institution in China and a similar center in the U.S. This conclusion contrasts with that of a recent study comparing GC outcomes at another high-volume cancer treatment center in China, Beijing Cancer Hospital (BCH), to those at the same U.S. institution (MSKCC), in which survival was worse at the Chinese center, even for patients with the same stage cancer.[12]
Many factors may help explain the distinct conclusions of the current study and that of the prior U.S.-China comparison.[12] The most likely contributor is the much greater number of lymph nodes retrieved at FMUUH (median 32 vs. 16 at BCH). Greater lymph node retrieval has been associated with better survival in numerous studies,[15,16] including one in China.[17]
Another key difference is that the prior study included patients with fewer than 15 LNs retrieved, which may have resulted in underestimation of N category preferentially among Chinese cases, where retrieval of few lymph nodes was more frequent.[12] Such stage migration would make outcomes appear worse for cancers classified as early-stage but which were actually more advanced.
The comparison with BCH also only included patients who did not receive preoperative chemotherapy.[12] That choice could have eliminated some patients with stage II or greater GC from the MSKCC population because of the fact that preoperative chemotherapy is standard in the U.S. while postoperative chemotherapy is more common in China. However, such patient selection is unlikely to account for the difference in conclusions between that study and the current one, as institution was not a prognostic factor even when patients receiving preoperative chemotherapy were eliminated from the analysis. Further, preoperative chemotherapy has been associated with enhanced survival compared with postoperative chemotherapy,[18] so excluding patients who received chemotherapy prior to surgery would make outcomes appear worse, not better. Confirming the benefit of preoperative chemotherapy, our risk factor analysis found that preoperative, but not postoperative, chemotherapy provides a survival advantage.
The fact that stage-specific DSS was comparable between institutions, while DSS for the entire cohort was better for patients treated at MSKCC, is consistent with the greater frequency with which FMUUH patients presented with more advanced disease. The high rate of late diagnosis at FMUUH likely reflects multiple factors. As FMUUH is a tertiary cancer treatment center, patients treated there are socioeconomically and geographically diverse, so many patients have limited access to primary care. Cultural reluctance to seek treatment for cancer may also contribute.[19] This hesitance is understandable in light of the historically low survival rate for gastric cancer; even in 2005, only 27% of GC patients in China survived 5 years.[20]
The current study has several limitations. This is a retrospective study comparing data from two different institutions in disparate regions of the world, so the analysis is vulnerable to both confounding factors and selection bias despite our best efforts to adjust for differences between the two groups. Our data also spans a time period of 15 years, so treatments may have changed over time, which could impact survival.
Our findings may not be representative of GC outcomes across China. As FMUUH is a university hospital in an urban area that treats a high volume of cancer patients, outcomes are probably better than those at smaller or more rural hospitals.[21,22] Similarly, our conclusions are not meant to describe the state of GC across East Asia, as is clear from their distinction from those of comparisons with other institutions in Japan and South Korea. Better outcomes in those countries are generally attributable to earlier diagnosis,[10,11] which is possible because of large-scale government-sponsored screening programs.[23,24] A similar screening program has been implemented in parts of China with especially high GC prevalence;[25,26] expanding these efforts could improve nationwide outcomes in the long term. In Asian countries without screening, survival appears to be similar to that in the West,[5,27] though few in-depth studies have compared outcomes stage by stage.
While our investigation suggests that GC survival is governed by well-established prognostic variables such as stage and lymph node positivity rather than geography, the disparate findings of these two analyses highlight the need for further investigation to define and understand potential differences in GC presentation, etiology, and treatment among different geographic locations.
CONCLUSIONS
Marked discrepancies exist in clinicopathologic presentation of GC patients between high-volume cancer centers in the US and China. After adjusting for relevant prognostic factors, however, stage-specific DSS is similar and is governed by extent of disease after resection.
Supplementary Material
Synopsis:
There are wide discrepancies in contributing factors for GC in the U.S. and China. We analyzed patient data from two high-volume centers who underwent R0 gastrectomy and found that survival outcomes depend on stage rather than geography/institution.
Acknowledgments
Financial Support: This research was funded, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748, the National Key Clinical Specialty Discipline Construction Program of China (No. [2012] 649), and scientific and technological innovation joint capital projects of Fujian Province (2016Y9031).
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. : GLOBOCAN 2012 v1.1, Cancer Incidence and Mortality Worldwide 2015; 11:http://globocan.iarc.fr/. [DOI] [PubMed] [Google Scholar]
- 2.Gill S, Shah A, Le N, Cook EF, Yoshida EM: Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol 2003;21(11):2070–2076. [DOI] [PubMed] [Google Scholar]
- 3.Davis PA, Sano T: The difference in gastric cancer between Japan, USA and Europe: What are the facts? What are the suggestions? Crit Rev Oncol Hematol 2001;40(1):77–94. [DOI] [PubMed] [Google Scholar]
- 4.Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H: Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer 2000;89(9):1883–1892. [DOI] [PubMed] [Google Scholar]
- 5.Rahman R, Asombang AW, Ibdah JA: Characteristics of gastric cancer in Asia. World J Gastroenterol 2014;20(16):4483–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shim JH, Song KY, Jeon HM, et al. : Is gastric cancer different in Korea and the United States? Impact of tumor location on prognosis. Ann Surg Oncol 2014;21(7):2332–2339. [DOI] [PubMed] [Google Scholar]
- 7.Russo A, Li P, Strong VE: Differences in the multimodal treatment of gastric cancer: East versus west. J Surg Oncol 2017;115(5):603–614. [DOI] [PubMed] [Google Scholar]
- 8.Kim R, Tan A, Choi M, El-Rayes BF: Geographic differences in approach to advanced gastric cancer: Is there a standard approach? Crit Rev Oncol/Hematol 2013;88(2):416–426. [DOI] [PubMed] [Google Scholar]
- 9.Verdecchia A, Mariotto A, Gatta G, et al. : Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer 2003;39(11):1603–1609. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi Y, Yoshikawa T, Tsuburaya A, et al. : Is gastric carcinoma different between Japan and the United States? Cancer 2000;89(11):2237–2246. [PubMed] [Google Scholar]
- 11.Strong VE, Song KY, Park CH, et al. : Comparison of disease-specific survival in the United States and Korea after resection for early-stage node-negative gastric carcinoma. J Surg Oncol 2013;107(6):634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strong VE, Wu AW, Selby LV, et al. : Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015;112(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strong VE, Song KY, Park CH, et al. : Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251(4):640–646. [DOI] [PubMed] [Google Scholar]
- 14.Washington K: 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17(12):3077–3079. [DOI] [PubMed] [Google Scholar]
- 15.Baiocchi GL, Tiberio GA, Minicozzi AM, et al. : A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 2010;252(1):70–73. [DOI] [PubMed] [Google Scholar]
- 16.Mirkin KA, Hollenbeak CS, Wong J: Greater Lymph Node Retrieval Improves Survival in Node-Negative Resected Gastric Cancer in the United States. J Gastric Cancer 2017;17(4):306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng J, Yamashita H, Seto Y, Liang H: Increasing the Number of Examined Lymph Nodes is a Prerequisite for Improvement in the Accurate Evaluation of Overall Survival of Node-Negative Gastric Cancer Patients. Ann Surg Oncol 2017;24(3):745–753. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald TL, Efird JT, Bellamy N, et al. : Perioperative chemotherapy versus postoperative chemoradiotherapy in patients with resectable gastric/gastroesophageal junction adenocarcinomas: A survival analysis of 5058 patients. Cancer 2017;123(15):2909–2917. [DOI] [PubMed] [Google Scholar]
- 19.Zong L, Abe M, Seto Y, Ji J: The challenge of screening for early gastric cancer in China. Lancet 2016;388(10060):2606. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H, Zheng R, Guo Y, et al. : Cancer survival in China, 2003–2005: A population-based study. Int J Cancer 2015;136(8):1921–1930. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SW, Yang ZX, Zheng RS, et al. : [Incidence and mortality of stomach cancer in China, 2013]. Zhonghua zhong liu za zhi [Chin J Oncol] 2017;39(7):547–552. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Zheng R, Baade PD, et al. : Cancer statistics in China, 2015. CA Cancer J Clin 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 23.Hamashima C, Shibuya D, Yamazaki H, et al. : The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38(4):259–267. [DOI] [PubMed] [Google Scholar]
- 24.Bae JM, Shin SY, Kim EH: Optimal Interval for Repeated Gastric Cancer Screening in Normal-Risk Healthy Korean Adults: A Retrospective Cohort Study. Cancer Res Treat 2015;47(4):564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Mao X, Xu K, et al. : Massive Endoscopic Screening for Esophageal and Gastric Cancers in a High-Risk Area of China. PLoS One 2015;10(12):e0145097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Yu L, Hao CQ, et al. : Effectiveness of endoscopic gastric cancer screening in a rural area of Linzhou, China: results from a case-control study. Cancer Med 2016;5(9):2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redaniel MT, Laudico A, Mirasol-Lumague MR, et al. : Cancer survival discrepancies in developed and developing countries: comparisons between the Philippines and the United States. Br J Cancer 2009;100(5):858–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


