Abstract
The concordance rate for developing autoimmune disease in identical twins is around 50% demonstrating that gene and environmental interactions contribute to disease etiology. The environmental contribution to autoimmune disease is a wide-ranging concept including exposure to immunotoxic environmental chemicals. Because the immune system is immature during development suggests that adult-onset autoimmunity may originate when the immune system is particularly sensitive. Among the pollutants most closely associated with inflammation and/or autoimmunity include Bisphenol-A, mercury, TCDD, and trichloroethylene. These toxicants have been shown to impart epigenetic changes (e.g., DNA methylation) that may alter immune function and promote autoreactivity. Here we review these autoimmune-promoting toxicants and their relation to immune cell epigenetics both in terms of adult and developmental exposure.
Keywords: Autoimmunity, Epigenetics, CD4+ T cells, Development, Environmental toxicants
1. Introduction
The immune system is designed to recognize and eliminate foreign antigens. If the immune system instead attacks self-antigens, autoimmune diseases may occur. Approximately 24 million Americans have one or more autoimmune disease. These chronic, incurable disorders disproportionately affect females, and are among the leading causes of death for young and middle-age women [1]. Twin studies have shown that although an individual’s genome may increase susceptibility, environmental triggers are required to initiate disease. Defining how the environment promotes autoimmunity will enhance understanding of so-called idiopathic autoimmune disease. The elevated prevalence and incidence rates of autoimmune disease parallel the documented increase in environmental pollutants leading to an appreciation of environmental toxicants common to industrialized nations as important riggers for autoimmunity [2].
Enhanced sensitivity of the immune system to environmental perturbations during key developmental events occurring prenatally and/or postnatally are critical for later life function [3–6]. The cells of the innate immune system (i.e. neutrophils, dendritic cells, NK cells, and macrophages) provide the first line of defense against pathogens. Their relative functional immaturity at birth means that innate immunity is weak in the newborn compared to an adult. The second line of defense is mediated by the cells of the adaptive immune system. T cells derived in the thymus are abundant at birth, and they need to undergo further maturation in the periphery to become fully functional. Peripheral B cells in the newborn are similarly immature, and require further maturation to respond to antigens. Thus, due to the vulnerability of the developing immune system, developmental exposures may influence adult autoimmunity [7].
When contemplating how developmental toxicant exposure “programs” the host for autoimmunity, one likely scenario involves epigenetic alterations such as aberrant DNA methylation. The epigenome consists of modifications of the genome that do not alter DNA base sequences, but can regulate gene expression and phenotype. While it is understood that the epigenome is regulated by several epigenetic mechanisms other than DNA methylation (e.g., histone acetylation and micro-RNA expression), of the various forms of epigenetic modifications, DNA methylation is the most thoroughly investigated. Maturation of immune cells are largely controlled by DNA methylation events that occur most often in early life that are functionally evident in later life and potentially to additional generations [8]. Auto-immune diseases [(lupus, rheumatoid arthritis, type 1 diabetes (T1D), and multiple sclerosis)] associated with environmental toxicants are also linked to abnormal methylation [9] and may represent a mechanism by which environmental triggers promote autoimmunity. Thus, the review will focus on toxicant-induced effects on DNA methylation in autoimmune disease.
2. DNA methylation changes at various stages of immune development
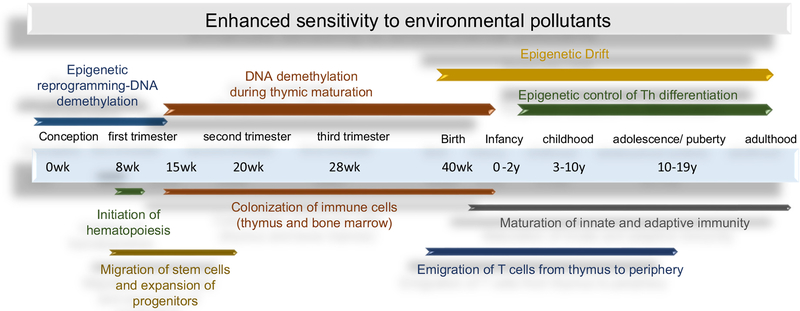
Autoimmune disease, whether antibody-or T cell-mediated is largely driven by CD4+ T cells. As shown in Fig. 1, epigenetic events play an important role in immune cell development and correspond to several key cellular maturational events. Prior to immune system development, genome-wide global epigenetic reprogramming in early embryonic development occurs immediately after fertilization to ensure loss of DNA methylation allowing for global repression and gene expression in all cells [10]. In later stages, CpG methylation coincides with general developmental life stages with a reported global trend of demethylation during Tcell development in the thymus closely related to the development of TCR function [11].
Fig. 1.
Key human immune system developmental checkpoints that correspond with important changes in DNA methylation. General steps of human immune system development spans from gestation to early postnatal life. These events in particular are epigenetically regulated via DNA methylation and represent a sensitive window for perturbation due to environmental insults that may later manifest in later life autoimmune disease in certain individuals with genetic susceptibility or lifestyle factors.
Another DNA methylation mechanism identified as an emerging concept in toxicology is epigenetic drift (i.e., drift) [12]. Drift is the divergence of the epigenome as a function of age due to stochastic changes in methylation. Under normal circumstances drift occurs because the fidelity of maintaining CpG methylation in mammalian cells (about 97–99% per cell division) is not absolute [13]. The small but significant error rate creates opportunity for changes in the methylome to occur and accumulate in constantly dividing cells, such as self-renewing effector/memory CD4+ T cells [14]. Drift involves both involves both hypo- and hyper-methylation events, and can encompass as much as 2.2% of total CpG sites, and 5–25% of specific genes over time [15,16]. Drift can impact promoter methylation status and gene expression, and has been used to explain the subset heterogeneity of memory CD4+ T cells that occurs during aging. In terms of autoimmune disease (e.g., T1D) results from twin studies suggest that drift causes heterogeneity in disease onset, severity, and predisposition to secondary complications [17]. The events that dysregulate drift are unclear, but appear to involve environmental exposures [18,19]. Importantly, although drift appears soon after birth, it occurs at a higher rate of change in children compared with adults [20]. Thus, although drift is still an understudied area of epigenetics, environmental influences may perturb this process in early life to promote autoimmunity.
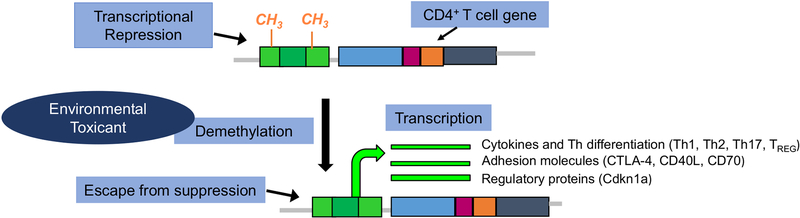
One stage of vulnerability mediated by epigenetic changes is CD4+ T cell differentiation. Beginning in early life, the phenotype of differentiated CD4+ T cell subsets are normally controlled by carefully maintained levels of DNA methylation in the promoters of pertinent regulatory genes [21,22] (Fig. 2). The development of autoimmune disease can disrupt the methylation patterns of differentiated CD4+ T cells, resulting in the demethylation of genes that encode immunomodulatory factors as reported in juvenile arthritis [23]. Subsets of differentiated CD4+ Tcells (i.e., Th1/Th17) have been shown to promote autoimmune disease in part to their persistence as effector/memory CD4+ T cells. The dysregulated methylome in autoimmune disease is associated with increased heterogeneity or plasticity in these subsets [24,25].
Fig. 2.
The phenotype of differentiated CD4+ T cells are normally controlled by carefully maintained levels of DNA methylation in the promoters of pertinent genes. Development of autoimmune disease can disrupt methylation patterns of differentiated CD4+ T cells resulting in the demethylation of genes that encode pro-inflammatory cytokines, chemokines, adhesion molecules, or subset differentiation. Toxicants have been associated with DNA demethylation in CD4+ T cells tied to increased expression of critical pro-inflammatory genes, cell cycle molecules/regulatory proteins and adhesion molecules within biological pathways with known links to autoimmunity.
While several key maturational and differentiation events in Tcells are regulated by DNA methylation, it is not known whether these events promote autoreactivity. While studies have shown that the function of autoreactive CD4+ T cells can be mediated by epigenetic processes, most of this work has been done in lupus models. Normal activated CD4+ Tcells treated with the DNA methyltransferase drug/inhibitor 5-azacytidine in vitro hypomethylates certain genes important in autoimmunity that become autoreactive upon adoptive transfer [26]. Whether environmental toxicants drive autoimmune-promoting epigenetic events in CD4+ T cells similar to 5-azacytidine is not known.
Identification of environmental pollutants that promote autoimmunity has been studied extensively [27,28]. However, there are a few toxicants associated with autoimmunity in the context of developmental exposure that have been identified and that may also impart autoimmune-promoting effects, at least in part, by aberrant DNA methylation. These substances are briefly reviewed below.
3. Mercury
Human exposure to mercury is common due primarily to its anthropogenic release from industrial use. Evidence that mercury promotes autoimmunity appears to be more straightforward in mouse models where it stimulates ANAs and induces immune complex-mediated lupus nephritis. Developmental exposure to subclinical doses of HgCl2 in maternal drinking water in mice has been shown to cause adverse immune effects in offspring. This includes increased number of activated CD4+ T cells and increased levels of brain-reactive antibodies [29].
In humans, mercury exposure was associated with sub-clinical autoimmunity as measured by increased ANAs among reproductive-age women [30]. Prenatal methyl-mercury exposure was associated with reduced levels of B cells and CD4+ T cells [31]. With regard to autoimmune disease, Sardinia has the second highest incidence of T1D in the world. The especially elevated levels of heavy metals, including mercury, in this country has led to the assumption that exposure to mercury, in the context of metals and other co-exposures during development promotes generation of this early-life autoimmune disease [32].
The potential autoimmune-promoting capacity of mercury is undoubtedly complex and likely involves other factors that enhance disease risk. Mercury can promote oxidative stress by depletion of anti-oxidant glutathione in immune cells [33]. This aspect of toxicity is functionally relevant to DNA methylation effects because glutathione pathway intermediates direct methionine metabolism to increase or decrease methyl donors to execute cellular methylation reactions. Direct evidence that mercury impacts DNA methylation in immune cells was shown in humans, where prenatal exposure to mercury detected in infant toenails correlated with hypermethylation of CpG islands of cord blood leukocytes [34]. These data highlight the potential for DNA methylation events in mercury-induced immunotoxicity or autoimmunity.
4. Bisphenol A
Bisphenol A (BPA) is a xenoestrogenic compound used to manufacture polycarbonate plastics and epoxy resins that can be detected in human blood and tissues [35]. There is evidence that developmental BPA exposure alters the immune system later in life and may be a potential autoimmune trigger. Studies report associations between urinary levels of BPA and allergic asthma [36]. In mice gestation, only exposure to BPA increased the development of adult experimental autoimmune encephalomyelitis (EAE) [37]. In a different study, gestational and lactational exposure to BPA failed to exacerbate adult EAE [38]. Diabetic (NOD) mice given BPA during gestation generated female offspring with an increased incidence of diabetes [39]. Perinatal BPA exposure accelerated inflammation in a mouse model of virally-induced multiple sclerosis [40].
Although the effects of BPA in mouse models were potentially relevant to human disease, most used concentrations of BPA that were higher than the typical concentration found in drinking water (<1 ppb). Developmental exposure to BPA at the US EPA oral reference dose (50 μg/kg/day) did not increase the severity of experimental inflammatory colitis in mice [41]. In humans, serum BPA in adults has shown consistent association with autoantibodies associated with thyroid- and neuron-specific antigens [42,43]. An association between urinary BPA concentrations and asthma in a cohort of inner-city children was reported implying developmental/early life exposures promoted inflammatory disease [44]. These results suggest that BPA may enhance autoimmunity depending on dose, model, and window of exposure.
DNA methylation changes with BPA exposure have been reported in the actual tissues that are targeted by the autoimmune response as in perinatal BPA exposure and altered DNA methylation in liver [45]. In NOD mice, BPA exposure increased the number of TREGS, while also increasing production of IL-17 [39]. Thus, BPA-mediated DNA methylation events may play a role in effector cell generation since differentiation of naïve CD4 Tcells into these subsets is regulated in part by DNA methylation. Even if toxicants do not induce uni-directional changes in the methylation state of specific genes, they may support epigenetic drift as shown in a longitudinal study of early life exposure to BPA [46]. These data suggest the potential role for DNA methylation in BPA-mediated immunotoxicity.
5. Trichloroethylene
One toxicant linked to the development of autoimmunity is the industrial solvent and water pollutant, trichloroethylene (TCE). Chronic TCE exposure (mostly occupational, but sometimes environmental) has been linked to a variety of autoimmune diseases including lupus [47], autoimmune hepatitis [48], and scleroderma [49]. Signs of immune activation and alterations in lymphocyte subsets have been associated with chronic environmental exposure to drinking water contaminated by TCE or via dermal or inhalational occupational exposure [50–54]. We and others have reported that chronic TCE exposure in drinking water during adulthood induces autoimmune hepatitis in autoimmune-prone MRL +/+ mice, and that this disease development is associated with several changes in CD4+ T cells [55,56].
Developmental exposure to TCE is a concern based on the ability of TCE and its metabolites to cross the placenta and its detection in breastmilk [57]. We and others have shown that continuous developmental exposure to TCE in mice (gestation, lactation, and early life) generated CD4+ Tcell alterations and/or early signs of tissue inflammation in both normal and autoimmune-susceptible mouse strains [58,59]. Similar immune-altering effects were observed with gestation- or postnatal-only exposure in young adult mice [60,61]. We recently reported continuous chronic exposure to low-level TCE beginning at gestation generated autoimmune hepatitis at postnatal day 259 even when TCE exposure was stopped 15 weeks earlier [62]. Because the effects persisted after TCE was removed from the drinking water suggested that programming events played a role in disease pathology. Indeed TCE altered the DNA methylation profile of the IFN-γ promoter in mouse CD4+ T cells [63]. In a genome-wide DNA methylation and gene expression study, TCE exposure in vivo differentially methylated CpGs in regions that bind polycomb group proteins [64] whose function is to regulate T effector cell expansion and differentiation [65]. Chronic exposure to TCE increased epigenetic drift in CD4+ T cells that corresponded with immune pathology [66]. These results support evidence that epigenetic events may play a role in TCE-induced immunotoxicity and autoimmunity.
6. TCDD/AHR ligands
While several environmental pollutants bind the aryl hydrocarbon receptor (AHR), 2,3,7,8-tetrachlorodibenzo-p-dioxin or TCDD is the prototypical ligand. Developmental exposure to TCDD and other AHR-binding xenobiotics alter the immune system. In contrast to the primarily immunosuppressive nature of adult TCDD exposure, developmental exposure to TCDD generated immune dysfunction with a different result. Adult C57BL/6 and autoimmune-prone SNF (1) mice exposed to TCDD on gestational day 12 demonstrated autoimmune glomerulonephritis including increased levels of anti-dsDNA in adulthood [67,68]. Neonatal exposure to TCDD increased pro-inflammatory cytokine and autoantibody production in a mouse model of Sjogren’s syndrome through signaling events in the neonatal thymus [69]. More recently, the novel autoimmune-prone Gnaq± mice were used to examine the autoimmune-promoting effects of TCDD on CD4+ Tcells. Exposure to TCDD generated offspring which developed lupus-like disease sooner and at a higher frequency than the offspring of vehicle-exposed dams [70]. Thus, unlike adult exposure, developmental exposure to AHR ligands appear to promote autoimmunity. It is plausible to hypothesize that epigenetic events which are more relevant during developmental periods may play a role in this disparity.
Studies have shown that DNA methylation plays a role in immunoregulation of AHR ligands. When the methylation status of CpG islands present in Foxp3 and IL-17 promoters following AHR activation were examined for their methylation/demethylation status, demethylation of CpG islands present in the Foxp3 promoter and increased methylation of CpG islands of the IL-17 promoter, following activation of AHR during colitis was observed [71]. In a different model, TCDD changed DNA methylation patterns in CD8+ T cells in the context of host resistance to viral infection [72].
7. Conclusion and future research needs
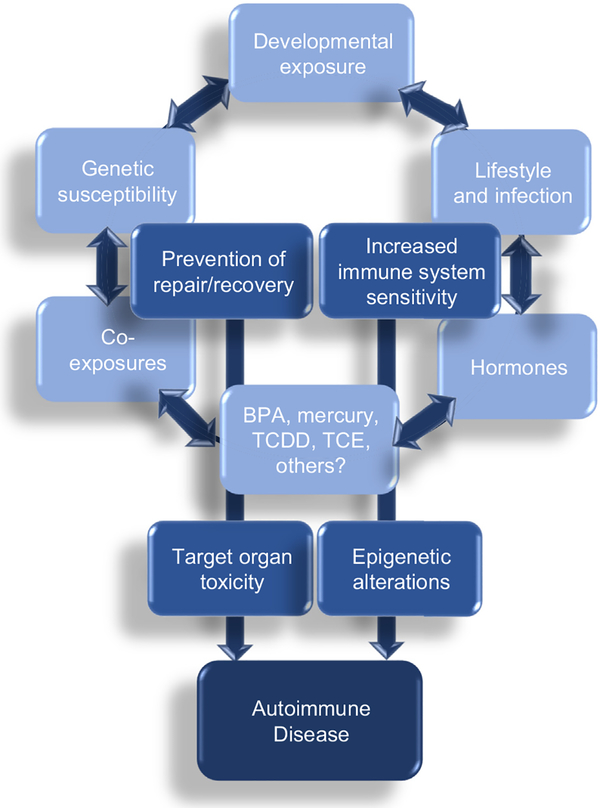
This review summarized several environmental chemicals studied for their potential to alter immune function, DNA methylation, and promote autoimmunity after developmental exposure. Although many autoimmune-promoting immunotoxicants appear to target CD4+ T cells, end organ pathology may also occur from toxicant-induced damage that enhances immunogenicity of proteins in certain target tissues or by disrupting repair or anti-oxidant systems designed to promote regeneration/recovery. Autoimmunity is complex and may be affected by lifestyle factors and genetic susceptibility (Fig. 3). In particular, the role of co-exposures, whether other toxicants or lifestyle factors that also impair immune function/DNA methylation should be an important research consideration for future study. For example, 50% of American women of childbearing age are overweight or obese [73]. Data from animal studies demonstrated adverse outcomes in models of autoimmunity and allergic sensitization models of maternal and postnatal obesity [74,75]. In humans, maternal obesity influenced programing of the neonatal immune system [76] potentially enhancing risk to inflammatory disease such as asthma in offspring [77]. Thus certain risk factors may synergistically act on an already sensitive developing immune system by modifying the methylome and downstream gene expression leading to enhanced susceptibility to autoimmunity. There are only a few studies with toxicants outlined in this review that addressed diet/obesity co-exposures [(e.g., BPA [12] and TCE (manuscript in preparation)]. Future studies should aim to interrogate DNA methylation and gene expression events to confirm the role of epigenetic modifications when considering both exogenous and/or endogenous risk factors in the development of autoimmune disease.
Fig 3.
Conceptual diagram of hypothesized factors associated with autoimmunity. Autoimmune diseases result from complex interplay of gene–environmental interactions. The developmental period represents an enhanced time of epigenetic plasticity that may facilitate functional changes in an already sensitive maturing immune system. Multiple exposures to several factors together with impaired toxicant-induced regeneration/repair mechanisms converge with these factors to promote autoreactivity and disease.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Walsh SJ, Rau LM: Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health 2000, 90(9):1463–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parks CG, Miller FW, Pollard KM, Selmi C, Germolec D, Joyce K, Rose NR, Humble MC: Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci 2014, 15(8): 14269–14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns-Naas LA, Hastings KL, Ladics GS, Makris SL, Parker GA, Holsapple MP: What’s so special about the developing immune system? Int J Toxicol 2008, 27(2):223–254. [DOI] [PubMed] [Google Scholar]
- 4.Dietert RR: Developmental immunotoxicology: focus on health risks. Chem Res Toxicol 2009, 22(1):17–23. [DOI] [PubMed] [Google Scholar]
- 5.Dietert RR: Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human studies. Adv Med 2014, 2014:867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holsapple MP, O’Lone R: Juvenile immunotoxicology. Toxicol Pathol 2012, 40(2):248–254. [DOI] [PubMed] [Google Scholar]
- 7.Colebatch AN, Edwards CJ: The influence of early life factors on the risk of developing rheumatoid arthritis. Clin Exp Immunol 2011, 163(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST: Age-associated DNA methylation in pediatric populations. Genome Res 2012, 22(4):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun B, Hu L, Luo ZY, Chen XP, Zhou HH, Zhang W: DNA methylation perspectives in the pathogenesis of autoimmune diseases. Clin Immunol 2016, 164:21–27. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Jacobsen SE, Reik W: Epigenetic reprogramming in plant and animal development. Science 2010, 330(6004): 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez RM, Suarez-Alvarez B, Mosen-Ansorena D, Garcia-Peydro M, Fuentes P, Garcia-Leon MJ, Gonzalez-Lahera A, Macias-Camara N, Toribio ML, Aransay AM, Lopez-Larrea C: Regulation of the transcriptional program by DNA methylation during human alphabeta T-cell development. Nucleic Acids Res 2015, 43(2):760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochmanski J, Montrose L, Goodrich JM, Dolinoy DC: Environmental deflection: the impact of toxicant exposures on the aging epigenome. Toxicol Sci 2017, 156(2):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ushijima T, Watanabe N, Okochi E, Kaneda A, Sugimura T, Miyamoto K: Fidelity of the methylation pattern and its variation in the genome. Genome Res 2003, 13(5):868–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacoby M, Gohrbandt S, Clausse V, Brons NH, Muller CP: Interindividual variability and co-regulation of DNA methylation differ among blood cell populations. Epigenetics 2012, 7(12):1421–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, Zhang N, Liang S, Donehower LA, Issa JP: Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res 2010, 20(3):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zampieri M, Ciccarone F, Calabrese R, Franceschi C, Burkle A, Caiafa P: Reconfiguration of DNA methylation in aging. Mech Ageing Dev 2015, 151:60–70. [DOI] [PubMed] [Google Scholar]
- 17.MacFarlane AJ, Strom A, Scott FW: Epigenetics: deciphering how environmental factors may modify autoimmune type 1 diabetes. Mamm Genome 2009, 20(9–10):624–632. [DOI] [PubMed] [Google Scholar]
- 18.Busche S, Shao X, Caron M, Kwan T, Allum F, Cheung WA, Ge B, Westfall S, Simon MM, Barrett A, Bell JT, et al. : Population whole-genome bisulfite sequencing across two tissues highlights the environment as the principal source of human methylome variation. Genome Biol 2015, 16 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah S, McRae AF, Marioni RE, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, et al. : Genetic and environmental exposures constrain epigenetic drift over the human life course. Genome Res 2014, 24(11):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones MJ, Goodman SJ, Kobor MS: DNA methylation and healthy human aging. Aging Cell 2015, 14(6):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto S, Ogoshi K, Sasaki A, Abe J, Qu W, Nakatani Y, Ahsan B, Oshima K, Shand FH, Ametani A, Suzuki Y, et al. : Coordinated changes in DNA methylation in antigen-specific memory CD4 T cells. J Immunol 2013, 190(8):4076–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.• Komori HK, Hart T, LaMere SA, Chew PV, Salomon DR: Defining CD4 T cell memory by the epigenetic landscape of CpG DNA methylation. J Immunol 2015, 194(4):1565–1579.The authors used targeted bisulfite sequencing to examine 2100 genes (56,000 CpGs) in human naïve and memory CD4+ T cells. One hundred thirty two genes (456 CpGs) displayed differential metylation between naïve and memory cells. A majority of these genes correlated with differential gene expression following activation. This work is signiificant in that it reveals mechanisms for priming of memory T cells via epigenetic mechanisms that may be relevant in pathologic situations including autoimmunity.
- 23.Meyer B, Chavez RA, Munro JE, Chiaroni-Clarke RC, Akikusa JD, Allen RC, Craig JM, Ponsonby AL, Saffery R, Ellis JA: DNA methylation at IL32 in juvenile idiopathic arthritis. Sci Rep 2015, 5:11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirahara K, Nakayama T: CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol 2016, 28(4):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova EA, Orekhov AN: T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. Biomed Res Int 2015, 2015:327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quddus J, Johnson KJ, Gavalchin J, Amento EP, Chrisp CE, Yung RL, Richardson BC: Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest 1993, 92(1): 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Germolec D, Kono DH, Pfau JC, Pollard KM: Animal models used to examine the role of the environment in the development of autoimmune disease: findings from an NIEHS expert panel workshop. J Autoimmun 2012, 39(4): 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard KM, Hultman P, Kono DH: Toxicology of autoimmune diseases. Chem Res Toxicol 2010, 23(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Gao D, Bolivar VJ, Lawrence DA: Induction of autoimmunity to brain antigens by developmental mercury exposure. Toxicol Sci 2011, 119(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somers EC, Ganser MA, Warren JS, Basu N, Wang L, Zick SM, Park SK: Mercury exposure and antinuclear antibodies among females of reproductive age in the United States: NHANES. Environ Health Perspect 2015, 123(8):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oulhote Y, Shamim Z, Kielsen K, Weihe P, Grandjean P, Ryder LP, Heilmann C: Children’s white blood cell counts in relation to developmental exposures to methylmercury and persistent organic pollutants. Reprod Toxicol 2017, 68:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Songini M, Mannu C, Targhetta C, Bruno G: Type 1 diabetes in Sardinia: facts and hypotheses in the context of worldwide epidemiological data. Acta Diabetol 2017, 54(1):9–17. [DOI] [PubMed] [Google Scholar]
- 33.Thompson SA, Roellich KL, Grossmann A, Gilbert SG, Kavanagh TJ: Alterations in immune parameters associated with low level methylmercury exposure in mice. Immunopharmacol Immunotoxicol 1998, 20(2):299–314. [DOI] [PubMed] [Google Scholar]
- 34.Cardenas A, Koestler DC, Houseman EA, Jackson BP, Kile ML, Karagas MR, Marsit CJ: Differential DNA methylation in umbilical cord blood of infants exposed to mercury and arsenic in utero. Epigenetics 2015, 10(6):508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C: Lead and bisphenol A concentrations in the Canadian population. Health Rep 2010, 21(3):7–18. [PubMed] [Google Scholar]
- 36.Vaidya SV, Kulkarni H: Association of urinary bisphenol A concentration with allergic asthma: results from the national health and nutrition examination survey 2005–2006. J Asthma 2012, 49(8):800–806. [DOI] [PubMed] [Google Scholar]
- 37.• Rogers JA, Mishra MK, Hahn J, Greene CJ, Yates RM, Metz LM, Yong VW: Gestational bisphenol-A exposure lowers the threshold for autoimmunity in a model of multiple sclerosis. Proc Natl Acad Sci U S A 2017, 114(19):4999–5004.In a model of MS, the authors found that exposure during gestation, but not adulthood, increased the development of autoimmune encephalomyelitis in male but not female mice. An important role of Granulocyte-colony stimulating factor (GCSF) was found and highlights an important role for the innate immune sytstem in the development of autoimmunity in this model.
- 38.Krementsov DN, Katchy A, Case LK, Carr FE, Davis B, Williams C, Teuscher C: Studies in experimental autoimmune encephalomyelitis do not support developmental bisphenol a exposure as an environmental factor in increasing multiple sclerosis risk. Toxicol Sci 2013, 135(1):91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodin J, Bolling AK, Becher R, Kuper F, Lovik M, Nygaard UC: Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol Sci 2014, 137(2):311–323. [DOI] [PubMed] [Google Scholar]
- 40.Brinkmeyer-Langford C, Rodrigues A, Kochan KJ, Haney R, Rassu F, Steelman AJ, Young C, Riggs P, Storts R, Meagher MW, Welsh CJ: Consequences of perinatal bisphenol A exposure in a mouse model of multiple sclerosis. Autoimmunity 2014, 47(1):57–66. [DOI] [PubMed] [Google Scholar]
- 41.Roy A, Gaylo A, Cao W, Saubermann LJ, Lawrence BP: Neither direct nor developmental exposure to bisphenol A alters the severity of experimental inflammatory colitis in mice. J Immunotoxicol 2013, 10(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B: The association of serum bisphenol a with thyroid autoimmunity. Int J Environ Res Public Health 2016, 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharrazian D, Vojdani A: Correlation between antibodies to bisphenol A, its target enzyme protein disulfide isomerase and antibodies to neuron-specific antigens. J Appl Toxicol 2017, 37(4):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, Canfield S, Resnick D, Calafat AM, Perera FP, Whyatt RM: Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 2013, 131(3):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson OS, Kim JH, Peterson KE, Sanchez BN, Sant KE, Sartor MA, Weinhouse C, Dolinoy DC: Novel epigenetic bio-markers mediating bisphenol a exposure and metabolic phenotypes in female mice. Endocrinology 2017, 158(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.• Kochmanski J, Marchlewicz EH, Savidge M, Montrose L, Faulk C, Dolinoy DC: Longitudinal effects of developmental bisphenol A and variable diet exposures on epigenetic drift in mice. Reprod Toxicol 2017, 68:154–163.Using mouse tail tissue, methylation was quantified at repetitive elements (IAP and LINE1) as well as imprinted genes, Igf2 and H19 in mice exposed to vehicle or BPA. Diet was introduced as a relevant co-exposure (mediterranean or western diet). The study demonstrated DNA methylation drift across age and nutritional exposures.
- 47.Parks CG, De Roos AJ: Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus 2014, 23(6):527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper GS, Makris SL, Nietert PJ, Jinot J: Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect 2009, 117(5):696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao JH, Duan Y, Wang YJ, Huang XL, Yang GJ, Wang J: The influence of different solvents on systemic sclerosis: an updated meta-analysis of 14 case-control studies. J Clin Rheumatol 2016, 22(5):253–259. [DOI] [PubMed] [Google Scholar]
- 50.Bassig BA, Zhang L, Vermeulen R, Tang X, Li G, Hu W, Guo W, Purdue MP, Yin S, Rappaport SM, Shen M, et al. : Comparison of hematological alterations and markers of B-cell activation in workers exposed to benzene, formalde-hyde and trichloroethylene. Carcinogenesis 2016, 37(7): 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosgood HD 3rd, Zhang L, Tang X, Vermeulen R, Qiu C, Shen M, Smith MT, Ge Y, Ji Z, Xiong J, He J, et al. : Decreased numbers of CD4(+) naive and effector memory T cells, and CD8(+) naive T cells, are associated with trichloroethylene exposure. Front Oncol 2011, 1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilburn KH, Warshaw RH: Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinu-clear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ Res 1992, 57(1):1–9. [DOI] [PubMed] [Google Scholar]
- 53.Lan Q, Zhang L, Tang X, Shen M, Smith MT, Qiu C, Ge Y, Ji Z, Xiong J, He J, Reiss B, et al. : Occupational exposure to trichloroethylene is associated with a decline in lymphocyte subsets and soluble CD27 and CD30 markers. Carcinogenesis 2010, 31(9):1592–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo M, Yamagiwa T, Kobayashi R, Ikeda K, Satoh M, Inagaki N, Nagai H, Nagase H: Augmentation of antigen-stimulated allergic responses by a small amount of trichloroethylene ingestion from drinking water. Regul Toxicol Pharmacol 2008, 52(2):140–146. [DOI] [PubMed] [Google Scholar]
- 55.Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA: Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol Appl Pharmacol 2008, 228(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR: Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology 2000, 46(2):123–137. [DOI] [PubMed] [Google Scholar]
- 57.Toxicological review of trichloroethylene In support of summary information on the Integrated Risk Information System (IRIS). 2011. Press Release. [Google Scholar]
- 58.Blossom SJ, Doss JC: Trichloroethylene alters central and peripheral immune function in autoimmune-prone MRL(+/+) mice following continuous developmental and early life exposure. J Immunotoxicol 2007, 4(2):129–141. [DOI] [PubMed] [Google Scholar]
- 59.Peden-Adams MM, Eudaly JG, Heesemann LM, Smythe J, Miller J, Gilkeson GS, Keil DE: Developmental immunotoxicity of trichloroethylene (TCE): studies in B6C3F1 mice. J Environ Sci Health A Tox Hazard Subst Environ Eng 2006, 41(3): 249–271. [DOI] [PubMed] [Google Scholar]
- 60.Blossom SJ, Melnyk SB, Li M, Wessinger WD, Cooney CA: Inflammatory and oxidative stress-related effects associated with neurotoxicity are maintained after exclusively prenatal trichloroethylene exposure. Neurotoxicology 2017, 59: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbert KM, Woodruff W, Blossom SJ: Differential immunotoxicity induced by two different windows of developmental trichloroethylene exposure. Autoimmune Dis 2014, 2014: 982073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.• Gilbert KM, Bai S, Barnette D, Blossom SJ: Exposure cessation during adulthood did not prevent immunotoxicity caused by developmental exposure to low-level trichloroethylene in drinking water. Toxicol Sci 2017, 157(2):429–437.With10,000 fold lower concentration of TCE than that shown to be effective for triggering autoimmue hepatitis in adult mice, exposure to TCE beginning at gestation triggered CD4+ T cell proinflammatory cytokine production and autoimmune hepatitis, as well as antibodies directed towards liver proteins. These findings are also significant in that removal of the TCE from the drinking water for several weeks did not prevent disease or immunotoxicity.
- 63.Gilbert KM, Blossom SJ, Erickson SW, Broadfoot B, West K, Bai S, Li J, Cooney CA: Chronic exposure to trichloroethylene increases DNA methylation of the Ifng promoter in CD4+ T cells. Toxicol Lett 2016, 260:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.• Gilbert KM, Blossom SJ, Reisfeld B, Erickson SW, Vyas K, Maher M, Broadfoot B, West K, Bai S, Cooney CA, Bhattacharyya S: Trichloroethylene-induced alterations in DNA methylation were enriched in polycomb protein binding sites in effector/memory CD4+ T cells. Environ Epigenetics 2017, 3(3). dvx013–dvx013.In a genome-wide DNA methylation study employing reduced representation bisufite sequencing, it was reported that chronic TCE exposure in vivo differentially methylated CpGs in regions that bind polycomb group proteins. One such protein included EZH2 which is important in differentiation of effector cell subsets and T effector cell expansion. The differential methylation of these binding sites may represent a new mechansim by which TCE could alter the function of effector CD4+ T cells.
- 65.Yang XP, Jiang K, Hirahara K, Vahedi G, Afzali B, Sciume G, Bonelli M, Sun HW, Jankovic D, Kanno Y, Sartorelli V, et al. : EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Sci Rep 2015, 5:10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.• Gilbert KM, Blossom SJ, Erickson SW, Reisfeld B, Zurlinden TJ, Broadfoot B, West K, Bai S, Cooney CA: Chronic exposure to water pollutant trichloroethylene increased epigenetic drift in CD4(+) T cells. Epigenomics 2016, 8(5):633–649.This study used naïve and effector/memory CD4+ T cells isolated from autoimmune-prone female mice exposed to TCE for 40 weeks to examine whether TCE altered gene-specific DNA methylation using bisulfite next-generation DNA sequencing. These findings of increased epiegenetic drift of specific CpG sites in CD4+ T cells are significant as they demonstrate epigenetic modifications associated with immune function.
- 67.Mustafa A, Holladay S, Witonsky S, Zimmerman K, Manari A, Countermarsh S, Karpuzoglu E, Gogal R: Prenatal TCDD causes persistent modulation of the postnatal immune response, and exacerbates inflammatory disease, in 36-week-old lupus-like autoimmune SNF1 mice. Birth Defects Res B Dev Reprod Toxicol 2011, 92(1):82–94. [DOI] [PubMed] [Google Scholar]
- 68.Mustafa A, Holladay SD, Goff M, Witonsky SG, Kerr R, Reilly CM, Sponenberg DP, Gogal RM Jr: An enhanced postnatal auto-immune profile in 24 week-old C57BL/6 mice developmentally exposed to TCDD. Toxicol Appl Pharmacol 2008, 232(1): 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishimaru N, Takagi A, Kohashi M, Yamada A, Arakaki R, Kanno J, Hayashi Y: Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol 2009, 182(10): 6576–6586. [DOI] [PubMed] [Google Scholar]
- 70.• Boule LA, Burke CG, Fenton BM, Thevenet-Morrison K, Jusko TA, Lawrence BP: Developmental activation of the AHR increases effector CD4+ T cells and exacerbates symptoms in autoimmune disease-prone Gnaq+/− mice. Toxicol Sci 2015, 148(2):555–566.Investigators studied the effects of developmental exposure to AHR on CD4+T cells and disease in Gnaq (+/−) offspring prone to develop lupus- and rheumatoid arthritis-like type disease. This exposure increased cellular changes and disease onset which was more evident in female mice as compared to males. This is a relevant autoimmune model to study gene–environment interactions in autoimmunity with many classes of toxicants.
- 71.• Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS: Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 2011, 6(8):15.These studies demonstrate for the first time that activation of the AHR promotes epigenetic regulation and differentiation of Tregs and Th17 cells to circumvent inflammation.
- 72.• Winans B, Nagari A, Chae M, Post CM, Ko CI, Puga A, Kraus WL, Lawrence BP: Linking the aryl hydrocarbon receptor with altered DNA methylation patterns and developmentally induced aberrant antiviral CD8+ T cell responses. J Immunol 2015, 194(9):4446–4457.This study reveals a novel role for AHR in the developing immune system in the context of CD8 T cell response to influenza infection through alterations in DNA methylation and gene expression patterns.
- 73.Ogden CL, Carroll MD, Fryar CD, Flegal KM: Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief 2015, 219:1–8. [PubMed] [Google Scholar]
- 74.Dinger K, Kasper P, Hucklenbruch-Rother E, Vohlen C, Jobst E, Janoschek R, Bae-Gartz I, van Koningsbruggen-Rietschel S, Plank C, Dotsch J, Alejandre Alcazar MA: Early-onset obesity dysregulates pulmonary adipocytokine/insulin signaling and induces asthma-like disease in mice. Sci Rep 2016, 6:24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xue Y, Wang H, Du M, Zhu MJ: Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J Nutr Biochem 2014, 25(7):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I: Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatr Allergy Immunol 2015, 26(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forno E, Young OM, Kumar R, Simhan H, Celedon JC: Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014, 134(2):e535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]