Abstract
Coronary artery disease remains an important cause of morbidity and mortality. Previous work, including ours, has focused on the role of intraplaque hemorrhage, particularly from immature microvessel angiogenesis, as an important contributor to plaque progression via increases in vascular permeability leading to further intraplaque hemorrhage, which increases red cell membrane-derived free cholesterol in plaque content and inflammatory cell recruitment. Evans Blue Dye (EBD) assay is widely used as a standard assay for vasculature permeability. However, the method has not been established in fresh human coronary artery autopsy samples to evaluate intraplaque microvessel permeability and angiogenesis. In this protocol, we describe a method to evaluate human coronary samples for microvascular permeability, including procedures to perfuse coronary arteries, collection of artery samples for histological analysis and immunostaining as well as the use of appropriate methodology to analyze the images. An optional procedure is also provided for the use of FITC-dextran in mouse model to evaluate vascular permeability. These Evans Blue Dye procedures may be useful in providing functional measure of the endothelium integrity and permeability in both human samples and animal models in various pathological conditions.
Keywords: Coronary artery disease, Atherosclerosis, Microvessel permeability, Evans blue dye, FITC-dextran, Angiogenesis, Intraplaque hemorrhage
Background
Vascular endothelial cells actively regulate the infiltration of plasma constituents and circulating cells, including leukocytes, from blood to sub-endothelial tissues. This mechanism is generally considered to be a critical step of initiation and progression of atherosclerosis ( Mundi et al., 2018 ). The regulation of vascular permeability is achieved through the coordinated opening and closure of endothelial cell-cell junctions. In several diseased conditions, endogenous agents such as histamine, thrombin, and vascular endothelial growth factors (VEGF) dramatically, but reversibly, alter the function and organization of cell-cell junctional complexes in diverse ways, resulting in various degrees of increase in permeability ( Dejana et al., 2008 ). We recently demonstrated a unique mechanism, by which CD163-positive alternative macrophages [M(Hb)] engulf hemoglobin-haptoglobin complexes at the sites of intra-plaque hemorrhage (IPH), promoting the release of vascular endothelial growth factor (VEGF), which further causes internalization of vascular endothelial cadherin (VE-cad) from inter-cellular adherens junctions, thus increasing vascular permeability and leading to atherosclerotic plaque progression ( Guo et al., 2018 ). In the aforementioned study, we conducted a novel technique to assess vascular permeability of human coronary arteries by using Evans Blue Dye (EBD) solution. In the same study, FITC-dextran was used as an alternative indicator in one-year-old apoE mouse model to demonstrate the intraplaque hemorrhage in brachiocephalic artery, due to the small size of the mouse arteries and dye sensitivity required for confocal imaging.
Among the vast range of blue dyes that were created during the 20th century, EBD has been the one with the longest biological history since its first application by Herbert McLean Evans in 1914 (Evans and Schulemann, 1914). EBD is an alkaline synthetic bis-azo (benzidine group) with a molecular weight of 961 Da., and high water solubility, allowing the dye to quickly diffuse throughout the blood stream. Most importantly, when the dye is injected intravenously, it has a high affinity for plasma albumin, giving the dye the ability to remain stable and remain distributed throughout the body for a longer time as a result of a slow excretion rate. All the features of EBD allow it to be an extraordinary agent with multiple potential applications in biomedicine as recently reviewed by Linpeng Yao ( Yao et al., 2018 ). These include but are not limited to estimation of plasma volume, identification of tumors and lymph nodes and as a potential marker of vessel permeability by the use of fluorescence when exposed to green light ( Hamer et al., 2002 ). The principles behind the use of EBD in assays to determine vascular permeability lies in the fact that in normal tissues with normal vascular integrity, albumin is not able to migrate out into the interstitium through the vessel wall endothelial layer. This means that in cases of Albumin-EBD complex, the dye would be limited only to circulation. EBD is relatively non-toxic when used in appropriate concentrations. In vivo experiments using both mice and human subjects have demonstrated that when used in excess, albumin reaches its maximum percent of saturation causing vascular leakage and resulting in a rapid bluish discoloration of tissues (Miles and Miles, 1952). In normal conditions, an adequate permeability barrier is maintained through tight cell-to-cell adherens junctions that are strictly controlled by growth factors, cytokines and other molecules (Radu and Chernoff, 2013). However, in pathological conditions where the integrity of the endothelial layer is affected, plasma proteins including albumin, are able to leak out the vessels as may occur in various disease states. The most common pathophysiological event leading to an increase vascular permeability is acute inflammation which may occur when the vascular wall including the endothelial layer is injured. Vasodilatation, increase in blood flow, disruption of endothelial cell junctions and infiltration of leukocytes are the key players in this process. The EBD-Albumin complex can be seen microscopically as basophilic color in interstitial tissues and indicated increased vascular permeability. In our recent study on endothelial barrier dysfunction after drug-eluting stents implantation, EBD perfusion was performed in rabbit iliac artery stenting model to demonstrate the arteries permeability is associated with poor endothelial VE-cadherin/P120 junctions and higher macrophage infiltration ( Harari et al., 2018 ). Given the advantages of EBD, we decided to use the technique to study the human coronary arteries permeability.
Materials and Reagents
Pipette tips
250 ml Stericup filter unit (Merck, catalog number: SCGPU02RE)
Coverslips (Fisher Scientific, catalog number: 12-543D)
Kimwipes (KCWW, Kimberly-Clark, catalog number: 34155)
Ultra-fine Syringe (BD, catalog number: 324911)
Human coronary artery samples selected from freshly collected autopsy specimens (from the CVPath Registry)
[Optional] One-year-old apoE knockout (KO) mouse (THE JACKSON LABORATORY, catalog number: 002052)
EcoMount (Biocare Medical, catalog number: EM897L)
CoverMount for non-EBD staining, Xylene based (Avantik, catalog number: SL6012-A)
Evans blue dye (Sigma-Aldrich, catalog number: E2129-50G)
FITC-dextran (Sigma-Aldrich, catalog number: 46945)
5% BSA (Fisher, catalog number: BP1600-100)
PBS (1x, Ultra Pure Grade, VWR, catalog number: 97063-658)
Neutral buffered formalin (NBF) (Sigma-Aldrich, catalog number: HT501128)
Tissue-Tek® O.C.T. Compound (Sakura Finetek, Miles, catalog number: 4583)
Hydrogen peroxide H2O2 3% (VWR, catalog number: BDH7540-2)
Dako protein block (Agilent Technologies, DAKO, catalog number: X0909)
CD163 antibody (Santa Cruz Biotechnology, catalog number: sc-20066, clone GHI/61)
CD68 antibody (Dako, clone Kp1)
Von Willebrand factor (vWF) antibody (SDIX, Strategic BioSolutions, catalog number: S4003GND1)
Hypoxia induced factor 1α (HIF1α) antibody (Novus Biologicals, catalog number: NB100-105)
Vascular endothelial growth factor-A (VEGF-A) antibody (BioGenex, catalog number: PU483-UP)
VE-cadherin antibody (R&D Systems, catalog number: AF1002, dilution 1:100, and BD Biosciences, catalog number: 555661)
Vascular cell adhesion protein (VCAM) antibody (Abcam, catalog number: ab134047)
CD3 antibody (Roche Diagnostics, catalog number: 790-4341, prediluted)
Biotinylated goat anti-rabbit, horse anti-mouse, and rabbit anti-goat (Vector Laboratories, catalog numbers: BA-1000, BA-2000, BA-5000, respectively)
Alexa Flour 488 and 555 streptavidin (Thermo Fisher Scientific, InvitrogenTM, catalog numbers: S32354 and S32355, respectively)
DAPI (Thermo Fisher Scientific, InvitrogenTM, catalog number: D3571)
2-methylbutane (Spectrum Chemical Manufacturing, catalog number: M1246)
Liquid nitrogen
20% paraformaldehyde (Electron Microscopy Sciences, catalog number: 15713-S)
Acetone (Fisher Scientific, catalog number: A929-1)
Liquid blocker (Ted Pella, catalog number: 22309)
Glacial acetic acid (Fisher Scientific, catalog number: A38-212)
-
Hematoxylin and Eosin
Xylene, Reagent grade/ACS (Avantik, catalog number: RS4050)
Mounting media/Permount (Fisher Scientific, catalog number: SP15-500)
Deionized water from laboratory
Mayer’s Hematoxylin Solution (Astral Diagnostics, catalog number: 7020)
Gill 3 (Sigma, catalog number: GHS3128)
Eosin-phloxine stain (Astral Diagnostics, catalog number: 7010)
100% reagent alcohol (Avantik, catalog number: RS4029)
95% reagent grade alcohol (Avantik, catalog number: RS4031)
Ammonium hydroxide, ACS grade (Sigma-Aldrich, catalog number: A6899)
Evans blue dye solution (see Recipes)
Equipment
Pipettes
Belly button shaker (IBI Scientific)
Axio Scan.Z1 digital slide scanner (Carl Zeiss, catalog number: Axio Scan.Z1)
Bright OTF 5000 microtome cryostat (Hacker Instruments, Hacker, catalog number: OTF 5000) using sectioning blades (Thermo Fisher Scientific, catalog number: 3152735)
CryoJane tape transfer system (Leica Biosystems, catalog number: 39475205)
LSM 800 confocal laser scanning microscope (Carl Zeiss, catalog number: LSM 800)
Olympus BX51 microscope (Olympus, model: BX51)
RNAscope Probe-Hs-CD163-C2 (Advanced Cell Diagnostics, catalog number: 417061-C2)
RNAscope Probe-Hs-VEGFA (Advanced Cell Diagnostics, catalog number: 423161)
TLE series ultra-low freezer (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: TLE60086A)
Stemi DV4 Stereo dissecting microscope (ZEISS, model: Stemi DV4)
Software
HALO Image Analysis Platform (Indica Labs) version 2.0
Zen Blue 2012 (Carl Zeiss) version 2.0
Zen Black 2012 (Carl Zeiss) version 2.0
Procedure
-
Preparing and freezing tissues
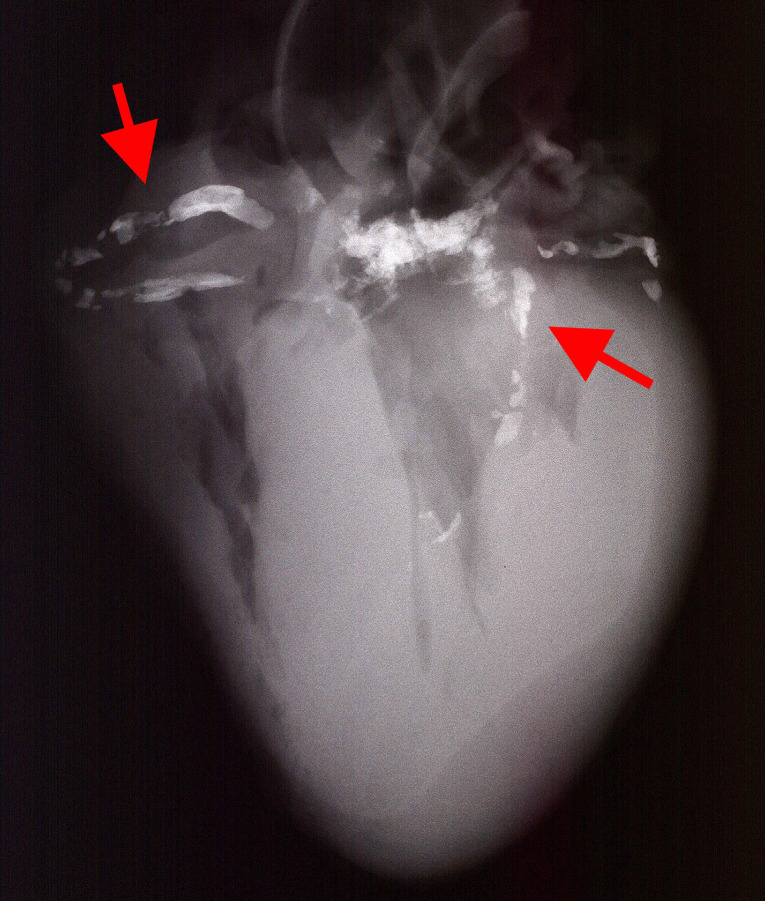
Select fresh collected autopsy human heart with coronary artery disease. Samples must have a post-mortem interval (PMI) cut-off of less than 12 h as well as evidence of advanced atherosclerosis based on x-ray images of the heart (which would allow for detection of vascular calcification). (Figure 1)
Prepare a saline solution containing 5% BSA.
Prepare a 0.5% solution of Evans Blue Dye by dissolving Evans Blue powder in the BSA-containing saline.
Filter the Evans Blue Dye solution using 250 ml Stericup filter unit.
Perfuse the filtered Evans Blue into both right and left coronary arteries for 15 min at 37 °C through a mean pressure of approximately 60 mmHg by gravity flow.
Wash the Evans Blue-perfused samples with 500 ml PBS for 20 min using the same perfusion method by gravity flow.
Next, fix the samples by 500 ml neutral buffered formalin for 20 min.
Harvest the formalin-fixed coronary artery tissue samples and place in a PBS solution containing 50 ml 15% sucrose overnight at 4 °C in a Falcon tube.
Remove tissues from the sucrose solution and dry with Kimwipes.
Cut the harvested samples in 2-3 mm segment intervals.
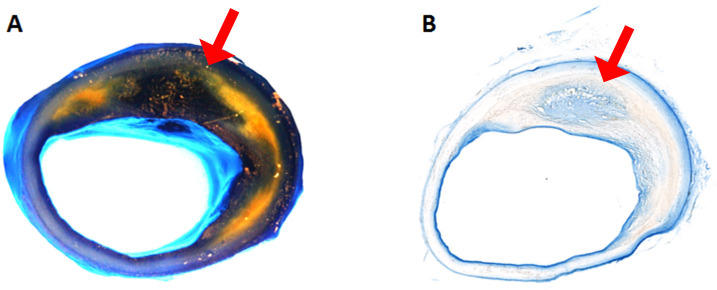
Under the dissecting scope, examine each coronary artery segments for visible intraplaque Evan Blue stained areas (Figure 2A).
Freeze samples by dipping tissues in O.C.T. embedding medium. Then place tissues in labeled cryomolds and top off the molds with more O.C.T.
In a chemical hood, and using appropriate PPE, cool a bottle of methylbutane by submerging it in a container with liquid nitrogen. Ensure that the liquid nitrogen reaches the meniscus of the methylbutane. Allow it to cool for 1-2 min.
In a chemical hood, arrange the O.C.T.-filled cryomolds in a foil-lined container and using forceps or cryogloves, remove the methylbutane from the liquid nitrogen and pour enough methylbutane into the foil-lined container to surround the molds. Ensure that no methylbutane goes over the top of the cryomolds. Cover the container with an insulated lid and allow samples to freeze 2-5 min, or until O.C.T is fully frozen.
Remove the cryomolds from the methylbutane and store in individually-labeled small plastic bags. Store the frozen blocks in -80 °C conditions.
Using a Microtome Cryostat, cut 10 micron-thick sections from each frozen block and place on slides. Cut enough for 3 methods of evaluation: staining, histology, and immunofluorescence analysis. Air-dry slides to be imaged for Evans Blue, and coverslip using Eco-mount (Figure 2B). Store unstained slides at -80 °C in the dark until ready for immunofluorescence analysis using confocal microscopy.
[Optional Procedure] Using Evan Blue dye or FITC-dextran in mouse study- One-year-old apoE knockout (KO) mouse that developed advanced atherosclerotic plaque can be used in intraplaque permeability study by administrated Evan Blue dye or FITC-dextran solution.
- Prepare FITC-dextran solution by dissolving FITC-dextran in PBS (10 mg/ml).
- FITC-dextran is injected via i.v. administration for 50 μg/g body weight under isoflurane anesthetic condition using ultra-fine syringe.
- Sacrifice the mouse 1 h after FITC-dextran is injected.
- Optional: Blood samples can be collected for measurement of serum FITC-dextran levels using a fluorescence plate reader.
- Perfuse the mouse through left ventricle using a syringe pump or by gravity with 20 ml PBS solution and perfusion fixed with 20 ml 4% paraformaldehyde, followed by 20 ml PBS rinses. The perfusion procedures are described previously ( Gage et al., 2012 ).
- Carefully dissect the heart and aorta with brachiocephalic artery under dissecting scope.
- Embed the aortic root and brachiocephalic artery in O.C.T. and section as described above.
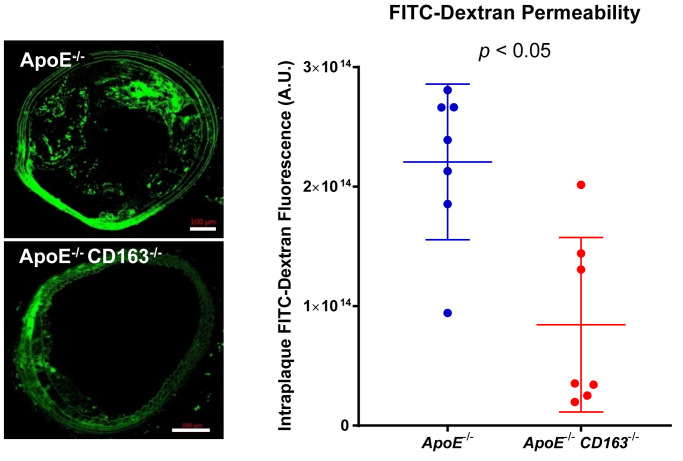
- FITC-dextran in the section can be imaged by confocal microscopy as described below (Figure 3).
-
Dual immunofluorescence staining
Warm frozen slides to room temperature for 10-20 min.
Fix the frozen sections by placing the slides in cold (approximately -20 °C) acetone for 10 min. Allow sections to air-dry for 10 min at room temperature, then outline sections with Liquid Blocker, and air-dry for 10-20 min at room temperature.
Expose slides to 0.15% H2O2 for 20 min.
Next, treat all sections with Dako protein block for 10 min on a Belly Button shaker set to medium speed before incubation with primary antibodies.
Prepare a solution of 1% BSA in PBS. Prepare enough of this solution to dilute all primary antibodies.
-
Perform immunofluorescence primary antibody dual staining for CD163/VE-cadherin and VE-cadherin/VCAM (plus negative controls for both pairs) on the cryosections (apply enough volume of antibody or blocking solutions to cover the tissue sections):
-
Dilute primary antibodies as follows in the PBS with 1% BSA solution:
Make a 1:200 dilution of CD163 clone GHI/61.
Make either a 1:100 or 1:400 dilution of VE-cadherin.
Make a 1:100 dilution of VCAM.
Remove protein block from slides and perform dual staining, pairing CD163 with VE-cadherin, as well as VE-cadherin with VCAM.
-
Add the first primary antibody to the test and positive control slides and incubate on shaker as detailed below. In between primary antibody incubations, remove the first primary antibody, rinse slides 3 times with PBS for 5 min on the shaker, remove PBS, then add the second primary antibody and incubate on the shaker as detailed below. Treat the negative control simultaneously by incubating in PBS instead of the primary antibody, for 1 h at room temperature or overnight at 4 °C. Incubate test slides with primary antibodies in the following conditions:
Incubate in CD163 clone GHI/61 diluted 1:200, overnight at 4 °C.
Incubate in VE-cadherin, diluted 1:100 or 1:400, overnight at 4 °C.
Incubate in VCAM diluted 1:100, 1 h at room temperature.
Remove the second primary antibody, rinse slides 3 times with PBS for 5 min on the shaker, and remove PBS.
-
Perform primary antibody detection by incubating test slides and positive control slides with biotinylated anti-mouse secondary antibody, diluted 1:200 in PBS for 30 min on the shaker, at room temperature. Keep dilution covered until ready to use, and keep slides covered during incubation to protect secondary antibody from light.
Remove the secondary antibody, rinse slides 3 times with PBS each for 5 min on the shaker (covered), and remove PBS.
Incubate all slides (including positive and negative controls) in Alexa Flour 488 and 555 streptavidin, diluted 1:100 in PBS, for 30 min on the shaker, at room temperature. Keep covered.
Remove the streptavidin and rinse slides with PBS 3 times for 5 min, covered, at room temperature on the shaker.
Counterstain all slides with DAPI. Dilute DAPI in PBS (the dilution should be determined via titer assay and may vary by lot, around 1:1,000 dilution is a good start). Add diluted DAPI to test and control slides, and incubate at room temperature on a shaker for the time determined via titer. Keep covered during incubation.
Remove DAPI and rinse slides with PBS 3 times each for 5 min, covered, at room temperature on the shaker.
Place all slides in 10% neutral buffered formalin for 10 min (covered). Remove with a pipette.
Rinse slides with PBS 3 times each for 5 min, covered, at room temperature on the shaker.
Remove PBS and apply coverslips using fluorescence compatible mounting medium. Store slides at 2-8 °C in the dark until ready for immunofluorescence analysis using confocal microscope.
-
Hematoxylin and Eosin Staining
Warm slides to room temperature, 10-20 min.
Stain slides in Mayer’s Hematoxylin Solution (filtered before use) for 10-20 min. Stain in Gill’s 3 solution for 1-6 min if Mayer’s Hematoxylin Solution is not available.
Wash slides in warm to hot tap water to remove hematoxylin until slides are clear and nuclei are blue.
If the tissue is dense and not staining properly, place slides in 1% Glacial Acetic Acid for 3-10 sec, then rinse in warm tap water.
If slides are not blue, place them in ammonia water (5-10 drops of concentrated Ammonium Hydroxide in a large staining dish with water) for 3 sec or longer.
Wash slides in tap water for 4-5 water changes.
Place slides in 80% Reagent Alcohol for 1 min or 5-10 dips.
Stain slides in Eosin-Phloxine Stain (Eosin Y/Phloxine B Working Solution) (filtered before use) for 1-3 min.
Dehydrate slides by placing them through graded alcohols, followed by xylene: 95% alcohol, 1-2 dips; 100% alcohol, 1-2 dips; 100% alcohol, 20 sec-1 min (repeat a total of 3 times, using fresh alcohol each time); xylene 1-3 min (repeat a total of 3 times, using fresh xylene each time).
Check slides for proper staining and restain in Hematoxylin if needed. Nuclear chromatin should appear blue and cytoplasm should appear pink to red.
Mount coverslips onto slides with Permount.
-
Evans blue brightfield imaging
Acquire bright field images of Evans Blue slides using the Axio Scan.Z1 slide scanner and the Carl-Zeiss Microscopy Zen software Blue edition version 2.0.
Load slides into the Axio Scanner.
Select “Prescan” and the appropriate “Prescan Setting” after checking the appropriate trays holding the slides to be scanned.
Review pre-scanned images and areas to be scanned by opening the “Tissue detection wizard”. If necessary, make changes to the automatic detection by using the “Polygonal outline tool”. Once review is complete, select “Finished”.
Use “number of points” setting for coarse focus map, and “onion skin” setting for fine focus map.
Name slides, choose a location in which to save, and select “Scan”.
-
Fluorescence imaging
Using a Zeiss LSM 800 or 880 Confocal Microscope, and Carl-Zeiss Microscopy Zen software (Blue edition version 2.0 and Black edition version 2.0, respectively) to capture Z-stack images for the test and control slides, using different channels to co-detect CD163, VE-cadherin, and VCAM as needed, based on dual staining performed.
Evans Blue dye and FITC-dextran can also be imaged using confocal microscope. Use lambda mode and spectral unmixing in acquisition to reduce other noise signals. Representative images can be found in Figure 5J in the original article ( Guo et al., 2018 ).
Figure 1. Digital X-ray image of the heart.
Highlighted bright spots are calcification in the coronary arteries (red arrows). Calcification in aortic valves can be seen in the center between coronary arteries.
Figure 2. Evans Blue Dye perfused human coronary artery.
A. Gross image of the coronary artery after perfusion. The yellow area is lipid-rich plaque. The dark blue to black are Evans Blue stained areas, see corresponding cryo-sections for blue stain in B (the light blue area is the reflection of the light from luminal surface in A). B. Cryo-sectioned slide from the same artery.
Figure 3. Representative immunofluorescence confocal microscopic images of intraplaque FITC-dextran (green) as a marker for permeability.
Scale bars: 100 μm. Total intraplaque FITC fluorescence was quantified from confocal images of BCA plaques perfused with FITC-dextran to determine permeability. (Adapted from Figure 5J in Guo et al., 2018 )
Data analysis
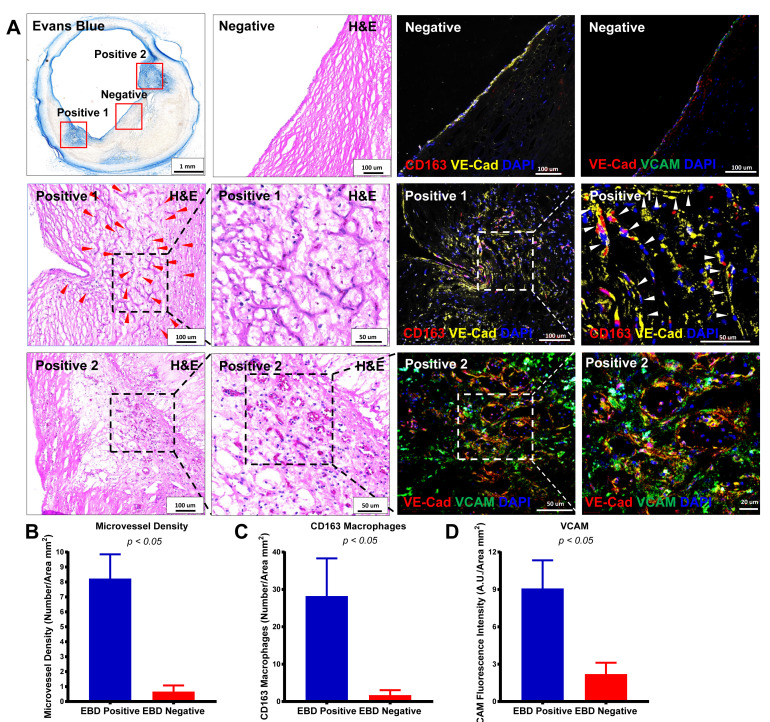
Evans Blue negative and positive areas are quantified using cross-sectional images of EBD-perfused human coronary arteries. The images in Figure 4A show that areas of heavy EBD staining have intraplaque microvessels surrounded by CD163+ macrophages. Quantitation of microvessels, CD163+ macrophages, and VCAM in EBD-positive versus EBD-negative areas showed significantly higher microvessel density, more CD163+ macrophages, and upregulated VCAM expression in EBD-positive versus EBD-negative areas in the plaque. Comparisons between two groups were achieved using a two-sided Student’s t-test. Further details of these analyses can be found in the original research article ( Guo et al., 2018 ).
Figure 4. Human coronary artery microvessel permeability was assessed by EBD perfusion.
A. Representative images of EBD-perfused human coronary arteries, H&E-stained images, and confocal immunofluorescence images of CD163 (red) and VE-cadherin (yellow) or VE-cadherin (red) and VCAM (green) in an EBD-negative area (top row), EBD-positive area 1 (middle row), and EBD-positive area 2 (bottom row). Positive areas 1 and 2 are shown in progressively higher-magnification H&E-stained images from left to right in the second and third rows. Red and white arrowheads point to microvessels. Confocal images of the EBD-negative areas for CD163/VE-cadherin and VE-cadherin/VCAM are shown in the top row of columns 3 and 4, respectively, while the positive area 1 is shown for CD163/VE-cadherin in the middle rows of columns 3 and 4 (higher-magnification image on the right), and positive area 2 is shown for VE-cadherin/VCAM in the bottom of row of columns 3 and 4 (higher-magnification image on the right). B-D. Quantification of microvessel density, CD163+ macrophages, and VCAM in an EBD-positive area versus an EBD-negative area. (Adapted from Figure 7 in Guo et al., 2018 )
Notes
There is certain variability in the degree of intraplaque angiogenesis and permeability in human coronary artery disease. One-year-old apoE knockout mouse can be an animal model to study the intraplaque hemorrhage and permeability. However, the intraplaque angiogenesis is more difficult to determine in mouse atherosclerotic lesions than in human lesions, due to the same size of the mouse artery.
Recipes
-
0.5% Evans blue dye solution (100 ml)
Evans Blue Dye 0.5 g
Bovine Serum Albumin 5.0 g
0.9% NaCl Saline 100 ml
Store at 4 °C
Acknowledgments
This protocol is adapted from Guo et al. (2018). The study was funded by CVPath Institute, a non-profit research Institute dedicated to the study of cardiovascular diseases and their treatment.
Competing interests
The authors do not have any potential conflicts of interest to declare.
Ethics
The study involving the use of deidentified human pathological or autopsy specimens were approved for exempt review by the IRB of the CVPath Institute. The IACUC of the MedStar Health Research Institute approved all animal protocols. All animal experiments were conducted according to the NIH’s Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Dejana E., Orsenigo F. and Lampugnani M. G.(2008). The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 13): 2115-2122. [DOI] [PubMed] [Google Scholar]
- 2. Evans H. M. and Schulemann W.(1914). The action of vital stains belonging to the benzidine group. Science 39(1004): 443-454. [DOI] [PubMed] [Google Scholar]
- 3. Gage G. J., Kipke D. R. and Shain W.(2012). Whole animal perfusion fixation for rodents. Jove(65). DOI: 10.3791/3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo L., Akahori H., Harari E., Smith S. L., Polavarapu R., Karmali V., Otsuka F., Gannon R. L., Braumann R. E., Dickinson M. H., Gupta A., Jenkins A. L., Lipinski M. J., Kim J., Chhour P., de Vries P. S., Jinnouchi H., Kutys R., Mori H., Kutyna M. D., Torii S., Sakamoto A., Choi C. U., Cheng Q., Grove M. L., Sawan M. A., Zhang Y., Cao Y., Kolodgie F. D., Cormode D. P., Arking D. E., Boerwinkle E., Morrison A. C., Erdmann J., Sotoodehnia N., Virmani R. and Finn A. V.(2018). CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis . J Clin Invest 128(3): 1106-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamer P. W., McGeachie J. M., Davies M. J. and Grounds M. D.(2002). Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability . J Anat 1): 69-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harari E., Guo L., Smith S. L., Paek K. H., Fernandez R., Sakamoto A., Mori H., Kutyna M. D., Habib A., Torii S., Cornelissen A., Jinnouchi H., Gupta A., Kolodgie F. D., Virmani R. and Finn A. V.(2018). Direct targeting of the mTOR(mammalian target of rapamycin) kinase improves endothelial permeability in drug-eluting stents. Arterioscler Thromb Vasc Biol. 38: 2217-2224. [DOI] [PubMed] [Google Scholar]
- 7. Miles A. A. and Miles E. M.(1952). Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol 118(2): 228-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mundi S., Massaro M., Scoditti E., Carluccio M. A., van Hinsbergh V. W. M., Iruela-Arispe M. L. and De Caterina R.(2018). Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res 114(1): 35-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radu M. and Chernoff J.(2013). An in vivo assay to test blood vessel permeability . J Vis Exp(73): e50062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao L., Xue X., Yu P., Ni Y. and Chen F.(2018). Evans Blue Dye: A revisit of its applications in biomedicine. Contrast Media Mol Imaging 2018: 7628037. [DOI] [PMC free article] [PubMed] [Google Scholar]