Abstract
Stress increases the likelihood of cocaine relapse in humans and animals, even following a prolonged extinction/abstinence period. Exercise has previously been shown to reduce stress and decrease the likelihood of drug dependence, while also reducing cravings in humans and inhibiting relapse behaviors due to other risk factors in rodents. The present study evaluated the efficacy of exercise to reduce stress-induced relapse to cocaine in a rodent model. Young adult female Sprague Dawley rats were tested for cocaine conditioned place preference (CPP), then split into sedentary or exercise (six weeks of one-hour daily treadmill running, five days per week) groups. Following cocaine CPP, rats were tested for extinction behavior, and then tested for stress-primed reinstatement (15 minute immobilization) following the six-week intervention period. Exercise inhibited stress-induced reinstatement of cocaine CPP despite increasing serum corticosterone levels following 15 minutes of immobilization, suggesting that chronic aerobic exercise intervention may result in adaptations of stress pathways. These findings suggest that exercise may help prevent stress-induced drug relapse, adding to a growing body of evidence supporting the utility of exercise to combat substance abuse.
Keywords: Stress, exercise, psychostimulant, cocaine, addiction, relapse
1. Introduction
Recent estimates found that nearly one million Americans met the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for dependence or abuse of cocaine within the last year [1 ]. Cocaine addiction is a disorder often characterized by cycles of recovery (abstinence) and relapse. Although many cocaine-dependent individuals seek out treatment, dropout rates for these treatment programs often range from 10% to 30% or higher [2, 3]. Despite attempts to remain abstinent, one- to two- thirds of individuals who complete treatment programs report relapsing even within just a few months after completion [2, 4]. Stress and negative affect, including that which is associated with withdrawal, are major factors leading to relapse [5–8] and are linked in general to reward deficiency syndrome [9, 10]. It has been shown that cocaine-dependent individuals exhibit altered neural, behavioral, and physiological responses to stressful stimuli [11–14], and that the magnitude of neural, physiological, and behavioral reactivity to stressors are correlated with rehabilitation program dropout rates, stress-induced drug cravings, and relapse behaviors [3, 4, 13, 15–18]. Comparable findings are seen in the animal literature, such that drug pre-exposed rats exhibit the reinstatement of drug-seeking behaviors in response to acute physical, psychological, and pharmacological stressors, even after extinction and several weeks of abstinence [19–25]. These findings suggest that treatments for substance abuse that are capable of attenuating physiological and/or behavioral reactivity to stressors could be effective in preventing relapse.
While cognitive behavioral therapy and stress management have begun to be used in substance abuse rehabilitation programs [26, 27], it would presumably be beneficial to treat patients with therapy that directly targets neural systems to counteract enhanced stress and blunted reward reactivity that is consequent from chronic drug use [14, 28–32]. While pharmacological agents such as glucocorticoid and corticotropin releasing factor (CRF) antagonists, as well as alpha2-adrenergic agonists, have been shown to curb drug cravings and block stress-induced relapse [18, 33–36], possible side effects of such treatment must be considered. An alternative to pharmacological treatment could be aerobic exercise, which is free of such side effects, has a myriad of other brain and general physical and psychological health benefits, and is also more cost-effective [37–39].
Clinical research has found that acute and chronic exercise reduces negative mood, withdrawal symptoms, and substance cravings during abstinence, and increases the likelihood of cessation of substance use [40–44]. Behavioral modifications have been shown to be accompanied by exercise-induced reductions in neural reactivity to drug cues in regions involved in reward, motivation, and visuospatial attention [43]. Previous rodent studies have confirmed exercise’s ability to reduce the acquisition and escalation of drug preference and self-administration [45–50], increase the breakpoint for operant responding for psychostimulants [51], and attenuate other types of primed reinstatement (drug-primed and cue-induced) [52–54]. These exercise-induced changes in drug-seeking behavior may be linked to alterations in the mesolimbic dopamine pathway [55, 56], which mediates the rewarding/reinforcing properties of drugs of abuse [57, 58]. Very few studies, however, have investigated the ability of exercise to prevent stress-induced relapse specifically. Exercise has been shown to be effective in reducing the reinstatement of cocaine-seeking behavior precipitated by administration of a pharmacological stressor (yohimbine) [50]. Additionally, exercise reduced foot-shock induced reinstatement of cocaine-seeking by about 25% when exercise began following extinction; however, this difference was not statistically significant [59].
Exercise has been shown to reduce neuroendocrine stress hormones [60] and elevate mood (to an even greater degree in regular exercisers) [61], while also having anxiolytic and anti-depressant effects in both humans and rodents [62–64]. These benefits may help alleviate anxiety and negative affect associated with withdrawal. Chronic exercise has also been shown to reduce behavioral reactivity (e.g. freezing behavior, social avoidance) to acute stressors in rodents [65, 66]. These behavioral modifications are thought to be attributable to altered physiological reactivity to stressors. Exercise’s ability to dampen the response of the hypothalamic-pituitary-adrenal (HPA) axis has been documented, as chronic exercise attenuates adrenocorticotropic hormone (ACTH) responses to novel stressors [67–69] and facilitates the habituation of corticosterone increases in response to repeated stress [70].Modifications to the sympathomedullary pathway are also suggested, as exercise blunts foot shock-induced increases in brain norepinephrine levels [71] and prevents stress-induced hypertension in rats [72].
It remains to be explored how exercise-mediated differences in physiological and behavioral responses to stressors will affect behaviors such as propensity for drug relapse. Therefore, the primary goal of this study is to evaluate the efficacy of exercise for attenuating the stress-induced reinstatement of cocaine-seeking behavior, and to determine whether these effects may be associated with exercise-induced changes in physiological and behavioral stress reactivity.
2. Materials and Methods
2.1. Animals
Female (n=24) Sprague Dawley rats were obtained at eight weeks of age (Taconic Laboratories, Hudson, NY). The estrous cycle of female rats were not monitored, similar to recent related studies [46, 52]. Food (standard Purina rat chow) and water were provided ad libitum, and subjects were individually housed under standard laboratory conditions [22.0°C± 2oC and on a 12h reverse light/dark cycle (lights off at 0800)]. Rats were allowed three days of habituation to their new environment before experimental procedures began. All experiments were conducted during the dark cycle and in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and approved by the University at Buffalo Institutional Animal Care and Use Committee.
2.2. Drugs
Cocaine (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline to make a solution to be injected at 25 mg/kg.
2.3. Procedures
2.3.1. Conditioned Place Preference (CPP), Extinction, and Reinstatement
The CPP apparatus (Coulbourn Instruments, Allentown, PA, USA) consisted of two compartments (30.5 × 26.5 × 37cm) that were connected by a central corridor (12.75 × 23 × 15.25cm).The left compartment had black walls and a perforated stainless-steel floor with round holes on staggered centers. The central corridor was transparent with a smooth Plexiglas floor, and the right compartment had white walls with a square stainless-steel grated floor. Access between boxes and the central corridor was controlled by guillotine-style doors. Experimental variables and data were controlled and recorded with Graphic State v2.0 software. The procedures used for cocaine CPP were similar to those previously described [46]. Briefly, CPP consisted of the sessions listed below.
2.3.1.1. CPP Pretest
Rats were removed from their home cages and placed in the central corridor of the CPP apparatus. Free access to the entire apparatus lasted 15 minutes, after which animals were returned to their home cage. Time spent in each compartment was recorded and was used to determine initial compartment preference for each rat before cocaine conditioning using the biased design.
2.3.1.2. Conditioning
Conditioning occurred on eight consecutive days (four days of cocaine pairings alternating with four days of saline pairings). Each rat was administered cocaine (25 mg/kg, i.p.) before being placed in the initially non-preferred chamber (cocaine-paired chamber) and saline before being confined to the initially preferred chamber (control chamber) for 30 minutes, after which rats were returned to their home cage. During each conditioning session, locomotor activity was recorded to assess cocaine-induced changes in locomotor activity.
2.3.1.3. CPP Test
On the day immediately following the eight days of conditioning, all rats were tested for cocaine conditioned place preference. Rats were placed in the central corridor of the CPP apparatus and allowed free access to all three compartments for 15 minutes. Time spent in each compartment was recorded and CPP for cocaine was determined by comparing time spent in the cocaine-paired chamber before (pretest phase) and after (test phase) conditioning.
2.3.1.1. CPP Extinction
Extinction of preference for the cocaine-paired chamber was achieved by repeating CPP tests (15 minutes of free exploration of all compartments with no cocaine administered) daily until no place preference was present. Extinction sessions coincided with the beginning of the exercise intervention period, and these sessions were performed prior to daily treadmill running. All rats were run for at least four extinction sessions, and criterion for extinction was met when an animal spent less than 55% of the time in the cocaine-paired chamber for two consecutive days [73]. No animal received more than 14 extinction trials. Extinction of preference for cocaine was considered to be met by two criteria: (1) there was no significant difference in time spent in the cocaine paired chamber in the extinction and pretest sessions, and (2) rats spent significantly less time in the cocaine-paired chamber during the extinction sessions compared to the test session.
2.3.1.5. Cue-induced CPP Reinstatement
On the day following the end of the six-week exercise regimen (or sedentary period), rats were tested in the CPP boxes for cue-induced reinstatement (in the presence of cues, the CPP chambers that were previously paired with cocaine). This reinstatement test lasted for 15 minutes and was identical to the test and extinction test sessions (no cocaine administered). Time spent in each box was recorded and preference was assessed as previously for pretest, test, and extinction sessions. Theoretically, a reinstatement of preference should not have been observed due to previous extinction of preference. Previous studies have shown that re-exposure to the CPP apparatus following extinction (in the absence of the drug) is not sufficient to reinstate preference for the drug-paired chamber [74]. This session was performed, however, in order to better dissociate any reinstating effects of contextual cues from stress-induced reinstatement tested in the subsequent session.
2.3.1.6. Stress-induced CPP Reinstatement
Immobilization stress is a commonly used form of physical stress that has been shown to produce a physiological stress response in rodents [69], as well as reinstate cocaine-seeking behavior in the CPP paradigm to a similar degree as a cocaine-priming injection [22]. On the day following the cue-induced reinstatement test session, rats were tested for stress-induced reinstatement. Rats were immobilized for 15 minutes by being placed in a Decapicone (Braintree Scientific, Inc., Braintree, MA). Following immobilization, rats were tested for reinstatement of cocaine preference in the cocaine CPP boxes (15 minutes of free exploration of all compartments). Time spent in each compartment was recorded and preference evaluated.
Both cue and stress reinstatement were defined by three criteria: (1) there was greater time spent in the cocaine-paired chamber during the respective reinstatement session compared to the pretest, (2) there was greater time spent in the cocaine-paired chamber during the respective reinstatement session compared to the extinction sessions, and (3) there was no difference in time spent in the cocaine-paired chamber during the respective reinstatement session compared to the test session.
2.3.2. Exercise
Following the cocaine CPP test phase, rats were assigned to exercise (n=12) or sedentary (n=12) groups. A treadmill was used to conduct forced exercise, which was located in a separate room from housing and CPP testing. Exercise was conducted between 10:00h and 14:00h. Treadmill running was held at a steady rate of 10 meters/min on a motor-driven treadmill with no incline, as previously described [46, 54]. Rats were habituated to treadmill running, such that animals ran for 10 minutes on the first day, with 10-minute increments added each subsequent day until the maximum time of 60 minutes per day was reached. Rats therefore ran ~0.6 km/day. The exercise-treated rats were maintained on this daily exercise regimen for six weeks following CPP testing (starting on the first day of extinction sessions). During the extinction period, rats were run on the treadmill daily, after which exercise was performed five days per week. Sedentary rats remained in their home cages, with their only physical activity being normal unrestricted cage ambulation. Sedentary rats were handled for 30 seconds each day on days that exercise rats were placed on the treadmill to control for any effects of daily handling.
2.3.3. Stress Reactivity Testing
2.3.3.1. Physiological Stress Reactivity (Serum Corticosterone)
Physiological stress reactivity was assessed by assaying serum corticosterone levels at baseline and after a 15-minute immobilization period on the subsequent day, during the week following reinstatement testing. During this week between CPP reinstatement testing and stress reactivity testing, exercise was performed as was done prior to CPP reinstatement testing. All blood samples were taken via tail vein catheterization while rats were under light gas anesthesia (~1.5% isoflourane). All blood samples were collected in two minutes or less from the time that anesthesia was first administered. Brief isoflourane anesthesia has been shown to have no significant effect on glucocorticoid levels in humans and rats [75, 76]. Blood was allowed to clot for 30 minutes, after which samples were spun for 10 minutes at 4°C and 3000 rpm in a microcentrifuge. Serum was drawn off the top and stored at −80°C until being assayed in duplicate for corticosterone using an ELISA (IBL International, Toronto, Canada) according to the manufacturer’s instructions.
2.3.3.2. Behavioral Stress Reactivity (Open Field)
To assess behavioral reactivity to stress, following each blood sampling, rats were placed in an open field arena (dimensions 40.64 cm x 40.64 cm x 40.64 cm) fitted with a photobeam activity monitoring system (TruScan, Coulbourn Instruments, Allentown, PA). Rats were allowed ~5 minutes to recover from isoflurane anesthesia prior to open field testing. Open field measures of distance traveled and center time were recorded.
2.4. Statistical Analysis
Unpaired t-tests were used to assess differences between sedentary and exercise rats in mean daily food intake and percent change in body weight from the beginning to the end of the experiment.
Two way repeated measures ANOVAs (between-subjects factor: exercise; within-subjects factors: session) were used to analyze locomotor activity during CPP conditioning sessions; as well as analyze cocaine CPP, extinction, and reinstatement behavior, as measured by time spent in the cocaine-paired chamber. Serum corticosterone levels and open field activity were also each analyzed with two way repeated measures ANOVAs (between-subjects factor: exercise; within-subjects factor: stress). ANOVAs were followed by multiple pairwise comparisons (Tukey method), and statistical significance for all tests was set at α=0.05.
3. Results
3.1. Food Intake and Body Weight
Unpaired t-tests found that there was no difference between sedentary and exercise rats for mean daily food intake [t(22)=0.894, p=0.381; ηp2 = 0.035] or percent change in body weight from the beginning to the end of the experiment [t(22)=1.470, p=0.156; ηp2 = 0.089] (Figure 1).
Figure 1.
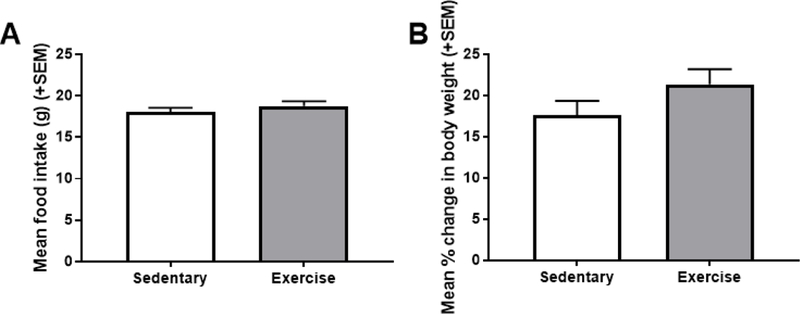
Mean (+SEM) daily food intake (A) and percent change in body weight from the beginning to the end of the experiment (B). Exercise did not have any significant effect on either measure.
3.2. Cocaine Conditioned Place Preference (CPP)
A two way repeated measures ANOVA (between-subjects factor: exercise; within-subjects factor: session) was performed to compare time spent in the cocaine-paired chamber by sedentary and exercise rats across sessions to assess formation, extinction, and reinstatement of cocaine preference (Figure 2). The main effect of session was significant [F(4,88)=7.541, p<0.001; ηp2 = 0.255], while the main effect of exercise [F(1,22)=0.586, p=0.452; ηp2 = 0.026] and the exercise x session interaction [F(4,88)=1.271, p=0.288; ηp2 = 0.055] were not significant. Multiple pairwise comparisons (Tukey method) were then performed, as described below.
Figure 2.
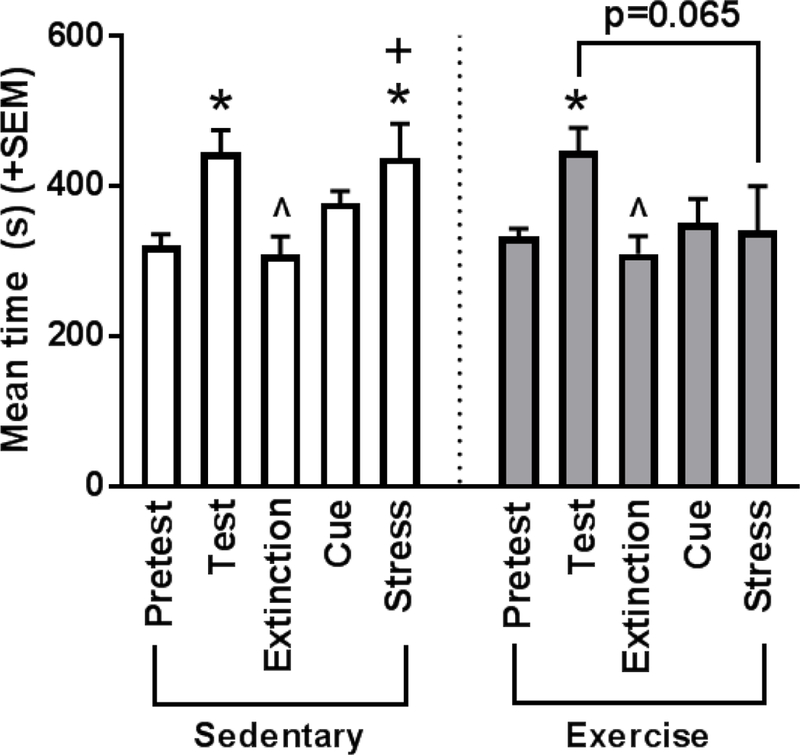
Mean (+SEM) time spent in the cocaine-paired chamber during conditioned place preference testing by session for sedentary and exercise rats. Exercise and sedentary rats formed a significant preference for cocaine, as measured by an increase in time spent in the paired chamber from pretest to test (*p<0.05). Both treatment groups also showed extinction of this preference, as measured by a decrease in preference from test day (^p<0.05) and no change from pretest day (p>0.05). Sedentary rats exhibited stress-induced reinstatement of preference, as measured by an increase in time spent in the paired chamber from pretest (*p<0.05) and extinction (+p<0.05) to the stress reinstatement session, and no difference between test and stress reinstatement (p>0.05). Exercise inhibited stress-induced reinstatement, such that there was no difference between pretest or extinction and the stress reinstatement session (p>0.05), and there was a decrease in preference from test day (p=0.067). *p<0.05 vs. pretest, +p<0.05 vs. extinction, ^p<0.05 vs. test
3.2.1. Cocaine CPP
Both sedentary and exercise rats formed a conditioned place preference for cocaine, defined as rats spending more time in the drug-paired chamber during the test compared to the pretest session (p<0.05 for both; Figure 2).
3.2.2. Cocaine CPP Extinction
For both sedentary and exercise groups, rats spent less time in the cocaine-paired chamber during extinction than during the test session (sedentary p=0.018; exercise p=0.037) and similar time in the cocaine-paired chamber during extinction and pretest sessions (sedentary p=0.998; exercise p=0.979). Taken together, these results, shown in Figure 2, suggest that both groups extinguished their preference for the cocaine-paired chamber. Additionally, the average number of days for rats in each group to reach full extinction criteria was computed. A t-test found that the difference between sedentary (M=6.8 ± SEM=1.6 days) and exercise (M=6.4 ± SEM=1.6 days) rats was not significant (p>0.05; ηp2 =0.001), suggesting that exercise had no effect on the rate of extinction of cocaine-seeking behavior.
3.2.3. Cue Reinstatement of Cocaine CPP
For both sedentary and exercise rats, there was no difference in time spent in the cocaine-paired chamber during the cue reinstatement session compared to the pretest (sedentary p=0.599; exercise p=0.990) or extinction (sedentary p=0.407; exercise p=0.836) sessions, suggesting a lack of cue-induced reinstatement following extinction and a prolonged abstinence period (Figure 2).
3.2.4. Stress Reinstatement of Cocaine CPP
Sedentary rats spent significantly more time in the cocaine-paired chamber during the stress reinstatement session compared to the pretest (p=0.030) and extinction sessions (p=0.013). Sedentary rats also showed no difference in preference for the cocaine-paired chamber between test and stress reinstatement sessions (p=0.999). Additionally, sedentary rats spent more time in the cocaine-paired during the stress reinstatement session compared to the cue reinstatement session, though this comparison did not reach significance. Taken together, these results, shown in Figure 2, suggest that acute (immobilization) stress resulted in the reinstatement of preference for the cocaine-paired chamber in sedentary rats.
Exercise rats, however, failed to exhibit stress-induced reinstatement of cocaine CPP. Exercise rats did not spend significantly more time in the cocaine-paired chamber during the stress reinstatement session compared to the pretest (p=0.999), extinction (p=0.934), or cue reinstatement (p=0.999) sessions. Additionally, exercise rats still spent less time in the drug-paired chamber during the stress reinstatement session compared to the test session, though this comparison only approached significance (p=0.065). Taken together, these results, shown in Figure 2, suggest that exercise inhibits the reinstatement of cocaine-seeking behavior following acute stress (immobilization).
3.3. Locomotor Activity during CPP Conditioning
A two way repeated measures ANOVA (between subjects factor: exercise, within-subjects factors: session) was performed to assess locomotor activity during conditioning sessions with saline and cocaine (Figure 3). The main effect of session was significant [F(7,154)=4.541, p<0.001; ηp2 = 0.171], such that rats were hyperactive in response to cocaine administration compared to saline during the first two pairing sessions (p<0.05 for all) but not the last two pairing sessions (p>0.05 for all). The main effect of exercise [F(1,22)=0.488, p=0.492; ηp2 = 0.022], and the exercise x session interaction [F(7,154)=0.239, p=0.975; ηp2 = 0.011], were not significant. Additionally, within each session, sedentary and exercise rats were similarly active (p>0.05 for all). Taken together, these findings suggest that sedentary and exercise rats had similar locomotor responses to cocaine prior to exercise intervention.
Figure 3.
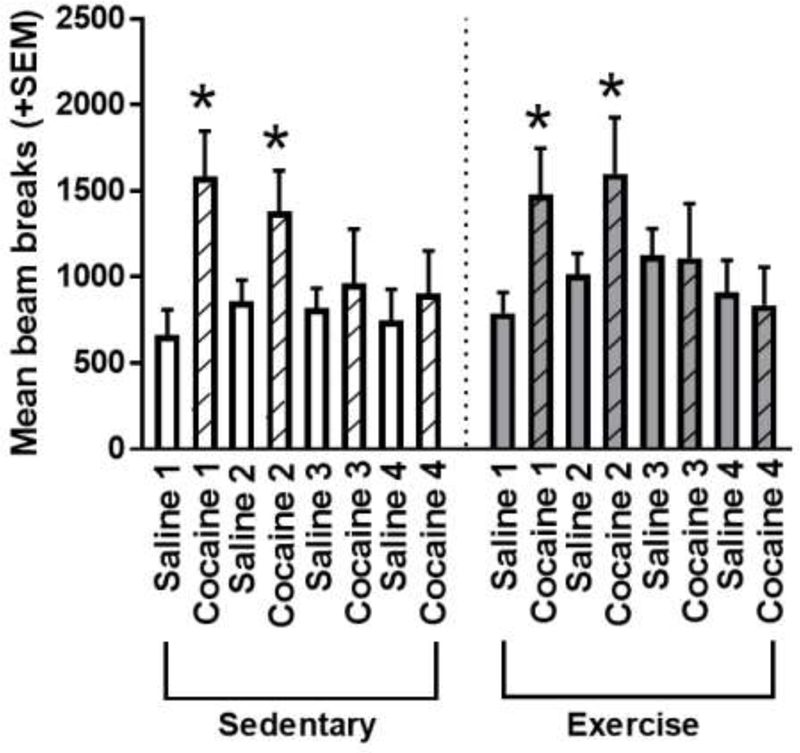
Locomotor activity exhibited by all rats during saline (plain bars) and cocaine (hashed bars) conditioning sessions, as measured by the mean number of beam breaks during each 30 minute conditioning session for sedentary and exercise rats. Rats exhibited significantly greater activity in response to cocaine compared to saline during the first two sessions of cocaine conditioning (*p<0.05). Sedentary and exercise rats did not exhibit differences in activity during any of the conditioning sessions (p>0.05 for all).
3.4. Behavioral Responses to Stress (Open Field Activity)
During the week following cocaine reinstatement testing, rats were tested in an open field arena for 30 minutes at baseline and following an acute stressor (15 minutes immobilization). Measures of general locomotor activity (distance traveled) and anxiety-like behavior (center time) were assessed.Data was analyzed for the first 5 minutes (for immediate stress-evoked behavioral responses) and entire 30 minute sessions (Figure 4).
Figure 4.
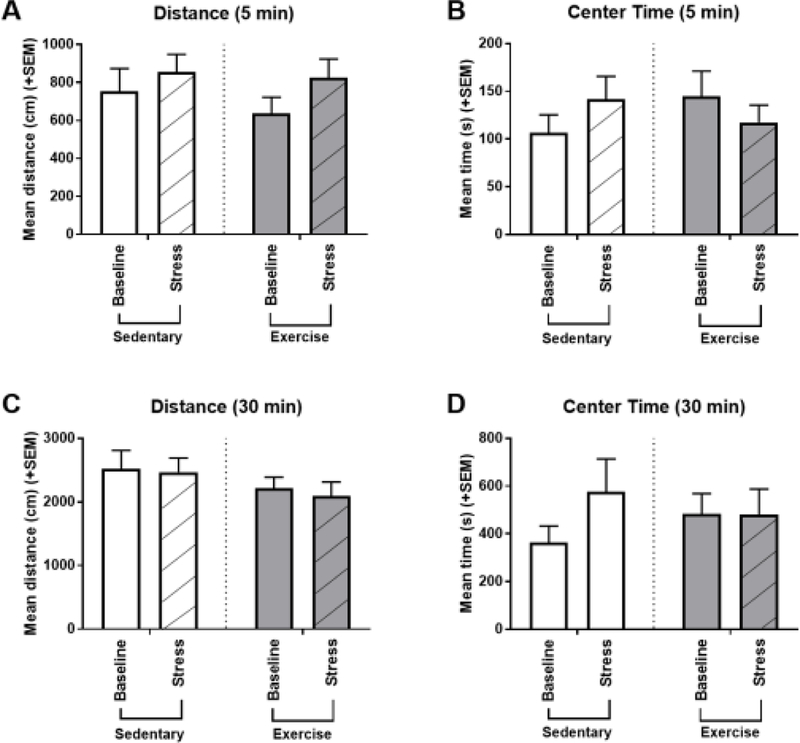
Mean (+SEM) behavioral responses at baseline (plain bars) and following 15 minutes of immobilization stress (hashed bars) as measured by distance traveled (A, C) and center time (B, D) in the open field during the week following the CPP reinstatement test. Activity was assessed during the first 5 minutes (A, B) and entire 30 minute (C, D) test. (A) For the first 5 minute data, there was no significant difference in distance traveled from baseline to stress testing for either treatment group. (B) During the first 5 minutes, sedentary exhibited a trend of rats spending more time in the center following stress compared to baseline, and the opposite trend was seen in exercise rats. Neither of these trends, however, reached significance. (C) For the entire 30 minute session data, there were no differences between baseline and stress-induced activity for either group. (D) For the entire 30 minute session data, there was still a trend of sedentary rats spending more time in the center of the arena following stress compared to baseline, while there was no difference between baseline and stress in exercise rats.
3.4.1. Distance Traveled
The main effects of exercise (5 min: [F(1,22)=0.430, p=0.519; ηp2 = 0.019]; 30 min: [F(1,22)=1.618, p=0.217; ηp2 = 0.069]), stress (5 min: [F(1,22)=2.80, p=0.10; ηp2 = 0.113]; 30 min: [F(1,22)=0.235, p=0.632; ηp2 = 0.011]), and the exercise x stress interaction (5 min: [F(1,22)=0.270, p=0.609; ηp2 = 0.012]; 30 min: [F(1,22)=0.044, p=0.837; ηp2 = 0.002]) were not significant for distance traveled in the first 5 minutes or the entire 30 minute open field test.
3.4.2. Center Time
For the first 5 minutes, the exercise x stress interaction approached significance for center time [F(1,22)=3.655, p=0.069; ηp2 = 0.142]. For sedentary rats, there was a trend of rats spending more time in the center following stress compared to baseline, and the opposite trend was seen in exercise rats; however, neither results were significant. For the entire 30 minute session data, the main effects of exercise [F(1,22)=0.011, p=0.917; ηp2 <0.001] and stress [F(1,22)=1.824, p=0.191]; ηp2 = 0.077, and the exercise x stress interaction [F(1,22)=1.954, p=0.176; ηp2 = 0.082] were not significant for center time.
3.5. Physiological Responses to Stress (Serum Corticosterone ELISA)
During the week following cocaine reinstatement testing, blood was collected at baseline and following an acute stressor (15 minutes immobilization). Serum corticosterone levels were assessed using ELISA to determine whether exercise affected physiological reactivity to stress (Figure 5). A two way repeated measures ANOVA (between-subjects factor: exercise, within-subjects factor: stress) found no significant main effect of exercise treatment on corticosterone levels [F(1,22)=2.568, p=0.123; np2 = 0.105]. There was a significant main effect of stress [F(1,22)=122.568, p<0.001; ηp2 = 0.848], such that corticosterone was higher following stress compared to baseline (p<0.001). The exercise x stress interaction was also significant [F(1,22)=13.418, p<0.001; ηp2 = 0.379]. There was no difference in baseline corticosterone levels between sedentary and exercise rats (p>0.05). While both exercise and sedentary rats displayed a significant increase in corticosterone levels from baseline to stress (p<0.001 for both), corticosterone levels were higher following stress for exercise rats compared to sedentary rats (p<0.01).
Figure 5.
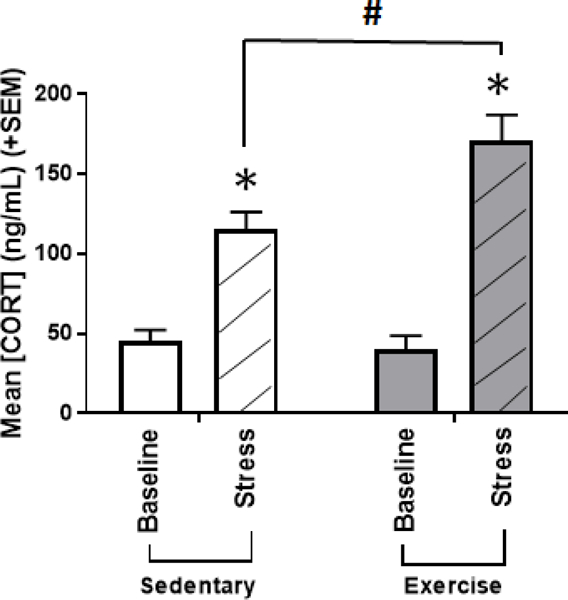
Mean (+SEM) physiological responses to stress at baseline (plain bars) and following 15 minutes of immobilization stress (hashed bars), as measured by serum corticosterone levels (ng/dL) during the week following the CPP reinstatement test. Both groups of rats exhibited an increase in serum corticosterone in response to stress (*p<0.001 for both). Following CPP, while there were no differences in baseline corticosterone levels between sedentary and exercise rats, exercise rats exhibited significantly greater stress-induced serum concentration of corticosterone compared to sedentary controls (^p<0.01). *Significant difference from baseline, #significant difference between treatment groups within the same session.
4. Discussion
This study examined the effects of chronic forced exercise (six weeks treadmill running, 1hour/day, 5days/week) on the extinction and reinstatement of cocaine conditioned place preference (CPP) in female rats. The major finding of this study is that regular aerobic exercise inhibited the stress-induced reinstatement of cocaine-seeking behavior that was demonstrated by sedentary rats.Differences in reinstatement cannot be attributed to initial preference differences between treatment groups, as cocaine preference formation was similar (36% increase in time spent in the cocaine-paired chamber for sedentary versus 37% for exercise rats), nor differences in extinction. Exercise also altered behavioral and physiological responses to stress assessed following cocaine CPP reinstatement testing. These results suggest that aerobic exercise may be a useful therapeutic strategy as part of a comprehensive treatment approach for relapse prevention of recovering cocaine abusers.
Exercise failed to have a robust effect on extinction, which is in contrast to previous studies that found that aerobic exercise facilitates the extinction of cocaine conditioned place preference [77] and also attenuates cocaine-seeking during extinction in a self-administration paradigm [52, 78]; however, these studies utilized voluntary exercise (wheel running). This key methodological difference may explain the discrepancies in results, as voluntary and forced exercise have been shown to produce different effects on the brain and behavior [79]. There are also some differences in the timing and duration of exercise between our study and those done previously. Another explanation is that the first few days of exercise intervention during extinction were not strong enough to produce a significant change in extinguishing cocaine preference. On the first six days of extinction testing/exercise, rats were slowly habituated to running on the treadmill by increasing running time by 10 minutes per day. With rats from both groups extinguishing within about six days on average, exercise treatment had just reached its full length and may not have had the chance to exert a substantial effect on the brain and behavior.
Acute stressors and the administration of pharmacological stressors (e.g. glucocorticoids, CRF agonists, and drugs that increase the transmission of norepinephrine) produce the reinstatement of drug-seeking behavior, even after a prolonged abstinence period [19, 21–23, 80, 81]. The major finding from this study is that six weeks of forced treadmill running inhibited stress-induced reinstatement of cocaine conditioned place preference. This is in agreement with a recent study that found that exercise reduces reinstatement precipitated by a pharmacological stressor (yohimbine) [50], and another study that found that exercise attenuated foot shock-induced reinstatement, albeit not to a significant degree [59]. The latter study differed methodologically from ours in a number of ways, including sex and strain of rat, as well as type and timing of exercise. These factors have been shown to significantly affect the efficacy of exercise in several studies [82–84]. Additionally, foot shock and immobilization have been shown to result in different behavioral and physiological reactivity to stress [85–88].
While others have shown that exercise has protective effects against reinstatement due to other factors (i.e. cue-induced and drug-primed reinstatement) [52–54, 78], ours is one of the first to demonstrate that exercise may be beneficial in preventing relapse triggered by stress. It has been previously hypothesized that different priming stimuli (e.g. cue, drug, and stress) have distinct subcircuits driving reinstatement. All three circuits, however, share a final common pathway from the anterior cingulate/prelimbic region to the core of the nucleus accumbens [89]. Results from this and previous studies suggest that exercise may affect one or both of these brain regions that is/are common to the variety of relapse pathways, since exercise has been shown to alter reinstatement due to each of these priming stimuli [52–54, 78]. Future research should examine the possible mechanisms involved in driving these behavioral changes, including alterations to the mesolimbic dopamine pathway seen following exercise [55, 56]. Alternatively, it is also possible that exercise facilitated the forgetting of the cocaine-paired chamber, possibly due to memory clearance/degradation resulting from exercise-induced increases hippocampal neurogenesis [90, 91]. Indeed, others have suggested that exercise can facilitate the forgetting of contextual information [92].
It is of importance to note that exercise inhibited stress-induced relapse to cocaine-seeking behavior, despite increasing physiological responses to stress. Running did not affect baseline levels of corticosterone or open field behavior, in contrast to previous findings that exercise can increase both baseline corticosterone levels and behavioral measures of anxiety [93–95]. While both exercise and sedentary animals exhibited an increase in serum corticosterone levels following stress, corticosterone levels were significantly higher in exercise rats. Additionally, there were trends of sedentary and exercise rats exhibiting different behavioral reactivity, as measured by time spent in the open field arena, in response to stress. It appeared that sedentary rats increased time in the center of the open field following stress, generally considered to be an anxiolytic response. Since these findings did not reach significance, and behavior was measured following a brief recovery from isoflurane anesthesia for blood draws, conclusions regarding these results cannot be made confidently. It is also possible that isoflurane could have impacted locomotor or anxiety-like behavior in sedentary and exercise rats differently, thereby introducing a confound for the behavioral findings; future studies exploring the effects of exercise on behavioral reactivity to stress should eliminate its use. Corticosterone results in particular were in contrast to the expected result of exercise attenuating reactivity to stress, especially considering exercise inhibited stress-induced relapse. Exercise has generally been shown to reduce both physiological and behavioral responses to stressors in humans and animals [65, 66, 70, 96]; however, differences have been noted due to exercise type, controllability, and duration/pattern of exercise. Most strikingly, voluntary wheel running appears to have consistent benefits in rodent models of stress-induced anxiety [92,97–100]; however, findings on the effects of forced treadmill running on anxiety-like behavior are more variable [63, 101–103]. Reasons for the choice of exercise regimen used in the current study are discussed below.
It is possible that our exercise regimen resulted in sensitization of the hypothalamic-pituitary-adrenal (HPA) axis response. Another explanation for our unexpected findings is the single timepoint used for blood sampling post-immobilization stress. Hare and colleagues found that exercised rodents had a faster peak of corticosterone following restraint compared to the sedentary condition, as well as a more rapid return to baseline. In that study, peak corticosterone levels for exercise animals were seen at 20 minutes into restraint, and at this timepoint, corticosterone levels were significantly higher in the exercise group compared to the sedentary group. The same trend was seen at 10 minutes into restraint, though the difference between groups did not reach significance [104]. Since blood was taken at 15 minutes into restraint in the current study, it is possible that we would have seen similar trends of an altered time course of corticosterone response in exercise versus sedentary rats had more time points been sampled. In addition to determining how exercise-induced changes in the responsivity of the HPA axis may contribute to attenuated drug-seeking, exploration of the effects of exercise on the sympatho-adreno-medullary responses and how this may contribute to altered drug-seeking is also necessary, as both stress pathways have been shown to be significantly involved in stress-induced drug craving and relapse [12].
Lastly, we chose to use forced exercise (treadmill running) rather than voluntary wheel running, despite evidence that forced exercise is a stressor [105, 106]. The exercise regimen chosen in this study has been previously shown to attenuate other drug-seeking behaviors [46, 54], warranting investigation of its use for stress-induced relapse. It is arguable that forced treadmill running better models the clinical scenario of a drug-dependent person exercising as part of a rehabilitation program. In contrast, voluntary wheel running, generally yielding much greater volumes of exercise, may better model trained endurance athletes (as recovering cocaine addicts would not be presumed to be) [79]. While our exercise regimen resulted in running volumes of 0.6 km/day, adult rats given daily free access to a running wheel run up to 10 km/day [107, 108]. Additionally, rodents given unlimited access to a running wheel will use the wheel for approximately 5 hours per day [109], and, as argued by others [105], most humans do not have such large amounts of leisure time to devote to physical activity, even if motivated to do so.
Conclusions
In conclusion, the current study found that exercise inhibited stress-induced reinstatement of cocaine CPP. While demonstrating promise for regular aerobic exercise to be preventative against stress-induced relapse to drug seeking behavior, this study only utilized female rats, and the estrous cycle was not monitored to allow the generalizability of results across the estrous cycle. Future studies should be aimed at determining the efficacy of exercise for reducing stress-induced reinstatement across sexes and throughout the estrous cycle in females, as a recent study found that exercise is more effective at attenuating cue-induced reinstatement in male rats than in females, and in non-estrous compared to estrous females [82]. While sex has been demonstrated to play a role in the efficacy of exercise in attenuating drug-seeking behavior [46, 82, 110], it has been shown in a clinical population that neural responses to different relapse triggers (i.e. stress and cues) produce different neural responses in males and females [111]. Therefore, assessing exercise-induced changes in neural reactivity to relapse triggers, in addition to changes in behavioral and physiological reactivity measured in the current study, would also be of great interest.
Highlights:
Acute immobilization stress resulted in reinstatement of cocaine CPP in sedentary control rats
Exercise inhibited stress-induced reinstatement of cocaine CPP
Exercise did not affect the rate of extinction of cocaine CPP
Exercise increased serum corticosterone levels following 15 minutes of immobilization stress
Acknowledgments
Funding: This research was supported by the NY Research Foundation (Q9040).
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMSHA, Behavioral health trends in the United States: Results from the 2016 National Survery on Drug Use and Health. 2017.
- 2.Simpson D, Joe GW, and Broome KM, A national 5-year follow-up of treatment outcomes for cocaine dependence. Archives of General Psychiatry, 2002. 59(6): p. 538–544. [DOI] [PubMed] [Google Scholar]
- 3.Daughters SB, et al. , HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: a prospective study. Drug and alcohol dependence, 2009. 105(3): p. 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha R, et al. , Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry, 2006. 63(3): p. 324–31. [DOI] [PubMed] [Google Scholar]
- 5.Kreek MJ and Koob GF, Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend, 1998. 51(1–2): p. 23–47. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl), 2001. 158(4): p. 343–59. [DOI] [PubMed] [Google Scholar]
- 7.Shalev U, Grimm JW, and Shaham Y, Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev, 2002. 54(1): p. 1–42. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, et al. , Addiction as a stress surfeit disorder. Neuropharmacology, 2014. 76 Pt B: p. 370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum K, et al. , Substance use disorder a bio-directional subset of reward deficiency syndrome. Frontiers in bioscience (Landmark edition), 2017. 22: p. 1534. [DOI] [PubMed] [Google Scholar]
- 10.Blum K, et al. , The Food and Drug Addiction Epidemic: Targeting Dopamine Homeostasis. Current pharmaceutical design, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R, Catapano D, and O’Malley S, Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl), 1999. 142(4): p. 343–51. [DOI] [PubMed] [Google Scholar]
- 12.Sinha R, et al. , Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl), 2003. 170(1): p. 62–72. [DOI] [PubMed] [Google Scholar]
- 13.Sinha R, et al. , Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology, 2005. 183(2): p. 171–180. [DOI] [PubMed] [Google Scholar]
- 14.Fox HC, et al. , Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology, 2005. 30(9): p. 880–891. [DOI] [PubMed] [Google Scholar]
- 15.Brown RA, et al. , Distress tolerance and duration of past smoking cessation attempts. J Abnorm Psychol, 2002. 111(1): p. 180–5. [PubMed] [Google Scholar]
- 16.Daughters SB, et al. , Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. J Abnorm Psychol, 2005. 114(4): p. 729. [DOI] [PubMed] [Google Scholar]
- 17.Daughters SB, et al. , Psychological distress tolerance and duration of most recent abstinence attempt among residential treatment-seeking substance abusers. Psychology of Addictive Behaviors, 2005. 19(2): p. 208. [DOI] [PubMed] [Google Scholar]
- 18.Sinha R, et al. , Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology, 2007. 190(4): p. 569–574. [DOI] [PubMed] [Google Scholar]
- 19.Erb S, Shaham Y, and Stewart J, Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology, 1996. 128(4): p. 408–412. [DOI] [PubMed] [Google Scholar]
- 20.Shaham Y, Erb S, and Stewart J, Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews, 2000. 33(1): p. 13–33. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez CJ and Sorg BA, Conditioned fear stimuli reinstate cocaine-induced conditioned place preference. Brain Res, 2001. 908(1): p. 86–92. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez C, et al. , Manipulation of dopamine d1-like receptor activation in the rat medial prefrontal cortex alters stress-and cocaine-induced reinstatement of conditioned place preference behavior. Neuroscience, 2003. 119(2): p. 497–505. [DOI] [PubMed] [Google Scholar]
- 23.Le A, et al. , Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology, 2005. 179(2): p. 366–373. [DOI] [PubMed] [Google Scholar]
- 24.Epstein DH, et al. , Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl), 2006. 189(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do Couto BR, et al. , Social stress is as effective as physical stress in reinstating morphine- induced place preference in mice. Psychopharmacology, 2006. 185(4): p. 459–470. [DOI] [PubMed] [Google Scholar]
- 26.Davis J, et al. , A pilot study on mindfulness based stress reduction for smokers. BMC Complementary and Alternative Medicine, 2007. 7(1): p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowen S, et al. , Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors, 2006. 20(3): p. 343–347. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, et al. , Decreased striatal dopaminergic responsiveness in detoxified cocaine- dependent subjects. Nature, 1997. 386(6627): p. 830–3. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein R, et al. , Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? American Journal of Psychiatry, 2007. 164(1): p. 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkow ND, et al. , Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays, 2010. 32(9): p. 748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koob GF and Le Moal M, Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 2001. 24(2): p. 97–129. [DOI] [PubMed] [Google Scholar]
- 32.Blum K, et al. , Clinically Combating Reward Deficiency Syndrome (RDS) with Dopamine Agonist Therapy as a Paradigm Shift: Dopamine for Dinner? Molecular neurobiology, 2015: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glassman AH, et al. , Cigarette craving, smoking withdrawal, and clonidine. Science, 1984. 226(4676): p. 864–6. [DOI] [PubMed] [Google Scholar]
- 34.Shaham Y, et al. , The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology, 2003. 168(1–2): p. 3–20. [DOI] [PubMed] [Google Scholar]
- 35.Erb S, Shaham Y, and Stewart J, The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci, 1998. 18(14): p. 5529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goeders NE, The HPA axis and cocaine reinforcement. Psychoneuroendocrinology, 2002. 27(1–2): p. 13–33. [DOI] [PubMed] [Google Scholar]
- 37.Morgan WP and Goldston SE, Exercise and mental health. 2013: Taylor & Francis. [Google Scholar]
- 38.Reiner M, et al. , Long-term health benefits of physical activity - a systematic review of longitudinal studies. BMC Public Health, 2013. 13(1): p. 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagberg J, et al. , Effect of exercise training on the blood pressure and hemodynamic features of hypertensive adolescents. Am J Cardiol, 1983. 52(7): p. 763–768. [DOI] [PubMed] [Google Scholar]
- 40.Marcus BH, et al. , The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res, 2005. 7(6): p. 871–80. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AH, Ussher MH, and Faulkner G, The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction, 2007. 102(4): p. 534–543. [DOI] [PubMed] [Google Scholar]
- 42.Bock BC, et al. , Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors, 1999. 24(3): p. 399–410. [DOI] [PubMed] [Google Scholar]
- 43.Van Rensburg KJ, et al. , Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology, 2009. 203(3): p. 589–598. [DOI] [PubMed] [Google Scholar]
- 44.Buchowski MS, et al. , Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PloS one, 2011. 6(3): p. e17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zlebnik NE, Anker JJ, and Carroll ME, Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl), 2012. 224(3): p. 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanos PK, et al. , Chronic forced exercise during adolescence decreases cocaine conditioned place preference in Lewis rats. Behav Brain Res, 2010. 215(1): p. 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MA and Pitts EG, Wheel running decreases the positive reinforcing effects of heroin. Pharmacol Rep, 2012. 64(4): p. 960–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith MA and Pitts EG, Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav, 2011. 100(2): p. 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacy RT, et al. , Exercise decreases speedball self-administration. Life Sciences, (0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlebnik NE, Saykao AT, and Carroll ME, Effects of combined exercise and progesterone treatments on cocaine seeking in male and female rats. Psychopharmacology, 2014. 231(18): p. 3787–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith MA, et al. , Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend, 2008. 98(1–2): p. 129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlebnik NE, et al. , Reduction of extinction and reinstatement of cocaine seeking by wheel running in female rats. Psychopharmacology (Berl), 2010. 209(1): p. 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith MA, et al. , Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend, 2012. 121(1–2): p. 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thanos PK, et al. , Daily treadmill exercise attenuates cocaine cue-induced reinstatement and cocaine induced locomotor response but increases cocaine-primed reinstatement. Behavioural brain research, 2013. 239: p. 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenwood BN, et al. , Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural brain research, 2011. 217(2): p. 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robison LS, et al. , Exercise reduces dopamine D1R and increases D2R in rats: implications for addiction . Submitted. [DOI] [PubMed] [Google Scholar]
- 57.Koob GF and Volkow ND, Neurocircuitry of addiction. Neuropsychopharmacology, 2010. 35(1): p. 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Chiara G and Imperato A, Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, 1988. 85(14): p. 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogbonmwan YE, et al. , The effects of post-extinction exercise on cocaine-primed and stress- induced reinstatement of cocaine seeking in rats. Psychopharmacology, 2014: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabkasorn C, et al. , Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. The European Journal of Public Health, 2006. 16(2): p. 179–184. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman MD and Hoffman DR, Exercisers achieve greater acute exercise-induced mood enhancement than nonexercisers. Arch Phys Med Rehabil, 2008. 89(2): p. 358–63. [DOI] [PubMed] [Google Scholar]
- 62.Salam JN, et al. , Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res, 2009. 197(1): p. 31–40. [DOI] [PubMed] [Google Scholar]
- 63.Dishman RK, et al. , Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav, 1996. 60(3): p. 699–705. [DOI] [PubMed] [Google Scholar]
- 64.Salmon P, Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical psychology review, 2001. 21(1): p. 33–61. [DOI] [PubMed] [Google Scholar]
- 65.Greenwood BN, et al. , Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci, 2007. 121(5): p. 992. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood BN, et al. , The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behavioural brain research, 2012. 233(2): p. 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dishman RK, et al. , Activity wheel running blunts increased plasma adrenocorticotrophin (ACTH) after footshock and cage-switch stress. Physiol Behav, 1998. 63(5): p. 911–7. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe T, et al. , Running training attenuates the ACTH responses in rats to swimming and cage-switch stress. J Appl Physiol, 1992. 73(6): p. 2452–6. [DOI] [PubMed] [Google Scholar]
- 69.Fediuc S, Campbell JE, and Riddell MC, Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol, 2006. 100(6): p. 1867–75. [DOI] [PubMed] [Google Scholar]
- 70.Sasse SK, et al. , Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats: Original Research Report. Stress: The International Journal on the Biology of Stress, 2008. 11(6): p. 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soares J, et al. , Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci, 1999. 113(3): p. 558–66. [DOI] [PubMed] [Google Scholar]
- 72.Cox RH, et al. , Exercise training attenuates stress-induced hypertension in the rat. Hypertension, 1985. 7(5): p. 747–51. [DOI] [PubMed] [Google Scholar]
- 73.Shoblock JR, Wichmann J, and Maidment NT, The effect of a systemically active ORL-1 agonist, Ro 64–6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology, 2005. 49(4): p. 439–46. [DOI] [PubMed] [Google Scholar]
- 74.Parker LA and McDonald RV, Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacol Biochem Behav, 2000. 66(3): p. 559–61. [DOI] [PubMed] [Google Scholar]
- 75.Oyama T, Latto P, and Holaday D, Effect of isoflurane anaesthesia and surgery on carbohydrate metabolism and plasma cortisol levels in man. Canadian Anaesthetists’ Society Journal, 1975. 22(6): p. 696–702. [DOI] [PubMed] [Google Scholar]
- 76.Zardooz H, et al. , Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res, 2010. 59(6): p. 973–8. [DOI] [PubMed] [Google Scholar]
- 77.Mustroph ML, et al. , Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. European Journal of Neuroscience, 2011. 34(7): p. 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lynch WJ, et al. , Aerobic exercise attenuates reinstatement of cocaine-seeking behavior and associated neuroadaptations in the prefrontal cortex. Biol Psychiatry, 2010. 68(8): p. 774–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leasure JL and Jones M, Forced and voluntary exercise differentially affect brain and behavior. Neuroscience, 2008. 156(3): p. 456–65. [DOI] [PubMed] [Google Scholar]
- 80.Deroche V, et al. , Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther, 1997. 281(3): p. 1401–7. [PubMed] [Google Scholar]
- 81.Shepard JD, et al. , The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry, 2004. 55(11): p. 1082–9. [DOI] [PubMed] [Google Scholar]
- 82.Peterson AB, Hivick DP, and Lynch WJ, Dose-dependent effectiveness of wheel running to attenuate cocaine-seeking: impact of sex and estrous cycle in rats. Psychopharmacology (Berl), 2014. [DOI] [PubMed] [Google Scholar]
- 83.Lynch WJ, et al. , Exercise as a novel treatment for drug addiction: a neurobiological and stage- dependent hypothesis. Neuroscience & Biobehavioral Reviews, 2013. 37(8): p. 1622–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Y, Zhou C, and Li R, Sex differences in exercise and drug addiction: A mini review of animal studies. Journal of Sport and Health Science, 2014. 3(3): p. 163–169. [Google Scholar]
- 85.Tsukada F, et al. , Evaluation of the effects of restraint and footshock stress on small intestinal motility by an improved method using a radionuclide, 51Cr, in the rat. Biological & pharmaceutical bulletin, 2001. 24(5): p. 488–490. [DOI] [PubMed] [Google Scholar]
- 86.Tsukada F, et al. , Effect of restraint and footshock stress and norepinephrine treatment on gastric emptying in rats. Biological and Pharmaceutical Bulletin, 2003. 26(3): p. 368–370. [DOI] [PubMed] [Google Scholar]
- 87.Daviu N, et al. , Not all stressors are equal: behavioral and endocrine evidence for development of contextual fear conditioning after a single session of footshocks but not of immobilization. Frontiers in behavioral neuroscience, 2012. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ong L, et al. , The effects of footshock and immobilization stress on tyrosine hydroxylase phosphorylation in the rat locus coeruleus and adrenal gland. Neuroscience, 2011. 192: p. 20–27. [DOI] [PubMed] [Google Scholar]
- 89.Kalivas PW and McFarland K, Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl), 2003. 168(1–2): p. 44–56. [DOI] [PubMed] [Google Scholar]
- 90.Akers KG, et al. , Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science, 2014. 344(6184): p. 598–602. [DOI] [PubMed] [Google Scholar]
- 91.Van Praag H, et al. , Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience, 2005. 25(38): p. 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greenwood BN, et al. , Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behavioral neuroscience, 2007. 121(5): p. 992. [DOI] [PubMed] [Google Scholar]
- 93.Svensson M, et al. , Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiology of Stress, 2016. 5: p. 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayes K, et al. , Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol, 2008. 115(3): p. 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sciolino NR and Holmes PV, Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev, 2012. 36(9): p. 1965–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wipfli B and Ramirez E, Stress ractivity in humans and animals: Two meta-analyses. International Journal of Exercise Science, 2013. 6(2): p. 144–156. [Google Scholar]
- 97.Greenwood B and Fleshner M, Mechanisms underlying the relationship between physical activity and anxiety: Animal data. 2013. 130–142. [Google Scholar]
- 98.Greenwood BN, et al. , Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci, 2003. 23(7): p. 2889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dishman RK, Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc, 1997. 29(1): p. 63–74. [DOI] [PubMed] [Google Scholar]
- 100.Greenwood BN, et al. , The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav Brain Res, 2012. 233(2): p. 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burghardt PR, et al. , The effects of chronic treadmill and wheel running on behavior in rats. Brain Res, 2004. 1019(1–2): p. 84–96. [DOI] [PubMed] [Google Scholar]
- 102.Fulk LJ, et al. , Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med, 2003. 25(1): p. 78–82 [DOI] [PubMed] [Google Scholar]
- 103.Lalanza JF, et al. , Physiological and behavioural consequences of long-term moderate treadmill exercise. Psychoneuroendocrinology, 2012. 37(11): p. 1745–54. [DOI] [PubMed] [Google Scholar]
- 104.Hare BD, et al. , Exercise-Associated Changes in the Corticosterone Response to Acute Restraint Stress: Evidence for Increased Adrenal Sensitivity and Reduced Corticosterone Response Duration. Neuropsychopharmacology, 2014. 39(5): p. 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uda M, et al. , Effects of chronic treadmill running on neurogenesis in the dentate gyrus of the hippocampus of adult rat. Brain Research, 2006. 1104(1): p. 64–72. [DOI] [PubMed] [Google Scholar]
- 106.Ploughman M, et al. , Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience, 2004. 136(4): p. 991–1001. [DOI] [PubMed] [Google Scholar]
- 107.Fediuc S, Campbell JE, and Riddell MC, Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. Journal of applied physiology, 2006. 100(6): p. 1867–1875. [DOI] [PubMed] [Google Scholar]
- 108.Burghardt PR, et al. , The effects of chronic treadmill and wheel running on behavior in rats. Brain Research, 2004. 1019(1): p. 84–96. [DOI] [PubMed] [Google Scholar]
- 109.Robison LS, Dose-dependent Effects of Voluntary Exercise on Physiology, Behavior, and Pathology in Mouse Models of Healthy Aging and Alzheimer’s Disease. 2017, State University of New York at Stony Brook. [Google Scholar]
- 110.Cosgrove KP, Hunter RG, and Carroll ME, Wheel-running attenuates intravenous cocaine self-administration in rats: Sex differences. Pharmacology Biochemistry and Behavior, 2002. 73(3): p. 663–671. [DOI] [PubMed] [Google Scholar]
- 111.Potenza MN, et al. , Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. American Journal of Psychiatry, 2012. 169(4): p. 406414. [DOI] [PMC free article] [PubMed] [Google Scholar]


