Abstract
Introduction:
The aim of this systematic review is to investigate the effects of perioperative intravenous administration of MgSO4 on postoperative pain, analgesic consumption and adverse effects in patients undergoing orthopedic surgery.
Methods:
Two investigators independently searched for articles on randomized controlled trials (RCTs) from 1998 to 2016 in Pubmed, Web of science and Google scholar. We evaluated clinical outcomes, comparing postoperative pain scores, cumulative analgesic consumption, time to first analgesia, and adverse effects between orthopedic surgery patients with and without the administration of MgSO4.
Results:
After screening 2350 articles, 11 RCTs (with a total sample size of 535 subjects) were included in this systematic review. Perioperative intravenous administered MgSO4 could reduce postoperative pain intensity compared with control in 6 trials (55%), but without significant difference in 5 trials (45%). With MgSO4 treatments, postoperative analgesic consumption was significantly reduced in 8 trials (73%), and without significant difference in 2 trials (18%). Two trials evaluated the time to first request of analgesic after surgery and showed prolong of 2.3 hours and 93 minutes respectively. MgSO4 group had less postoperative nausea (relative risk [RR] = 0.32, 95% confidence interval [CI] = 0.12–0.82, number needed to harm [NNH] = 8.8), vomiting (RR = 0.38, 95% CI = 0.15–0.92, NNH = 9.7), and shivering (RR = 0.31, 95% CI = 0.11–0.88, NNH = 5.2).
Conclusion:
Perioperative intravenous administration of MgSO4 in orthopedic surgery could reduce postoperative analgesic consumption and adverse effects such as vomiting, nausea, and shivering. These trials do not provide convincing evidence of beneficial effects on postoperative pain intensity and the time to first analgesic request. More trials should be conducted for the roles of MgSO4 in pain management for orthopedic surgery. However, intravenous MgSO4 administration should be considered as a strategy to relieve postoperative pain in orthopedic surgery patients.
Keywords: analgesic consumption, MgSO4, orthopedic surgery, postoperative pain
1. Introduction
Magnesium is an important cation for human homeostasis that plays a vital role in analgesic effects in both human and animal models of pain.[1] The analgesic effects of magnesium are based on acting as an antagonist of N-methyl-D-aspartate (NMDA) receptors in central nervous system.[2] For decades, magnesium has been used to reduce postoperative pain. In 1996, the first randomized controlled trial (RCT) used magnesium as an analgesic adjuvant to support the effect of postoperative analgesia.[3] However, several trials reported conflicting results.[4,5] In 2007, a systematic review concluded that perioperative magnesium may not provide favorable effects on postoperative pain intensity and analgesic requirements. Authors suggested the need for further study due to the potential analgesic effect of magnesium.[6]
During 2013 to 2015, 3 subsequent systematic reviews and meta-analyses confirmed the positive postoperative outcomes of perioperative administration of magnesium sulfate.[7–9] Albrecht et al reviewed 25 RCTs including 1461 patients and concluded that perioperative intravenous magnesium can reduce opioid consumption and pain scores in the first 24 hours postoperatively without any reported serious adverse effects.[7] De Oliveira et al analyzed 20 RCTs with 1257 patients also supported that systemic administration of perioperative magnesium reduce postoperative pain and opioid consumption.[8] In 2015, Guo et al performed a meta-analysis of 27 RCTs involving 1504 patients also concluded that systemic magnesium administration during surgery significantly reduced postoperative pain scores without increasing adverse effects.[9] However, the authors noted that since there were 18 ongoing trials without published data, it was still premature to conclude the postoperative analgesic effects of magnesium.
The analgesic effects of magnesium in orthopedic surgery have also been widely investigated. In 1997, Koinig et al conducted the first RCT investigating the analgesic effects of magnesium sulfate for the knee arthroscopic surgery.[10] Since then, RCTs investigating the analgesic effects of magnesium in orthopedic surgeries have been reported, including lumbar disc surgery,[11] total knee or hip replacement,[12–15] osteotomy,[16] posterior spinal fusion surgery[17] and so on. Up to the present time, the systematic review and meta-analyses[7–9] reported the perioperative magnesium administration for postoperative analgesia included a variety of surgeries. However, a systematic review that emphasizes the analgesic effects of systematic administrated magnesium in orthopedic surgery is still limited. The adverse effects of intravenous administration of MgSO4 were investigated in previous meta-analyses. Two studies[7,8] concluded that perioperative intravenous magnesium was effective in analgesia, and with no prominent adverse effects. None of the studies reported on clinical manifestations of toxicity related to high serum levels of magnesium.
The purpose of this systematic review is to evaluate the effects of perioperative intravenous administrated magnesium sulfate on postoperative outcomes in orthopedic surgeries, including pain scores, cumulative analgesic consumption and adverse effects.
2. Material and methods
We performed this systemic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA)[18] and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.[19] IRB approval is unnecessary because this is a review of previously published RCTs, and does not involve any processing of individual patient data.
2.1. Systematic search for trials
We searched the RCTs evaluating the effects of perioperative administrated magnesium sulfate on postoperative pain, analgesic consumption and adverse effects in orthopedic surgery from National Library of Medicine's PubMed database, Web of Science, and Google Scholar. MeSH terms and keywords of magnesium, orthopedic surgery, analgesia, postoperative, perioperative, and pain were used in various combinations. We initially searched for studies published between 1998 and 2018, but the latest RCT that met our inclusion criteria was published in 2016. Thus, the included RCTs in our review were published between April 7, 1998 and November 14, 2016. Reference lists of the studies were reviewed to search for additional studies.
2.2. Inclusion criteria
Two authors (Yu-Ning Peng and Mei-Li Huang) independently evaluated titles and abstracts of the articles searched initially, and the full articles were examined independently to determine whether they met our inclusion criteria. We limited our search to RCTs published in English and performed on humans. Studies were selected based on the following inclusion criteria:
-
(1)
RCTs that compared perioperative intravenous MgSO4 administration with control group (normal saline) in patients undergoing orthopedic surgery;
-
(2)
RCTs that evaluated the patients postoperatively at least with pain scores, analgesic consumptions or adverse effects.
Exclusion criteria were:
-
(1)
RCTs without a control group;
-
(2)
RCTs that did not use intravenous MgSO4 perioperatively.
2.3. Risk of bias assessment
Two authors (Peng and Huang) independently evaluated each trial according to the Cochrane risk of bias assessment scale.[19] Discrepancies in scoring were discussed among the authors. Seven categories of bias were evaluated: random selection, allocation concealment, blinding of participants and outcome assessment, outcome data, reporting bias, and other study bias. Three levels (low risk, unclear risk, high risk) were summarized in each category.
2.4. Data extraction
Two authors (Peng and Huang) independently reviewed and extracted the following information from each study: roles of authors; country of origin; publication year; patient characteristics (American Society of Anesthesiologists [ASA] physical status classification, age, sex); sample sizes in treatment and control groups; types of surgery; types of anesthesia; route and dosage of MgSO4 administration; cumulative analgesic consumption; pain scores; time to first analgesic request; and adverse effects. We extracted the data from tables or texts. For data presented only in figures and authors failed to respond to our requests for their original data, we extracted the data from available figures.
2.5. Data analysis
We initially intended to combine data from the 11 RCTs to perform meta-analyses. However, the RCTs reported patients undergoing different kinds of orthopedic surgery and used various kinds of postoperative analgesia. Due to the high heterogeneity of data, therefore, we conducted a systematic review of the RCTs, comparing MgSO4 in regarding to the effect of control on intensity of postoperative pain, cumulative analgesic consumption, and adverse effects. Moreover, in order to provide subgroups that might be meaningful in clinical conditions, we aimed to group studies by types of surgery (arthroscopic versus open surgery). For each subgroup, we reported the intensity of postoperative pain, cumulative analgesic consumption, and adverse effects.
3. Results
3.1. Study selection
We retrieved 19 potentially relevant RCTs. We excluded 8 RCTs[12–14,20–24] due to MgSO4 was administered by an unclear route in 1 study, administered intra-articularly in 2 studies,[13,24] administered intrathecally in 2 studies,[14,21] administered by various routes in 1 study,[12] and administered under regional anesthesia in 2 studies.[20,22,23] Eleven RCTs were eligible for inclusion in this study, with a total number of 535 patients met the inclusion criteria, including 267 received MgSO4 and 268 received normal saline as placebo (Fig. 1). These studies were published from 1998 to 2016. The characteristics of these trials are listed in Table 1.
Figure 1.
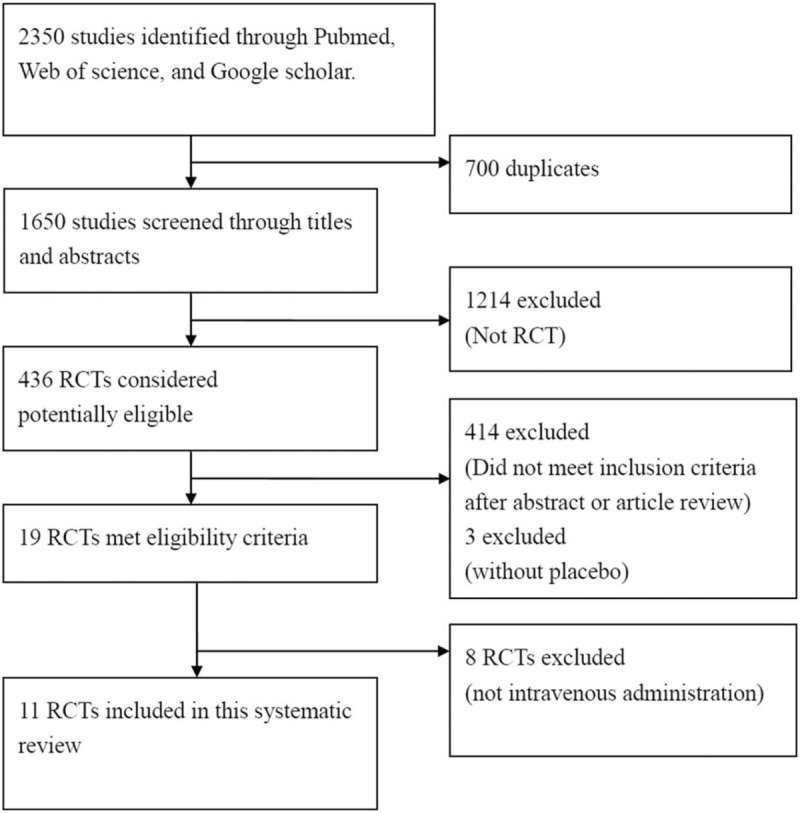
PRISMA flow chart showing literature search and selection process.
Table 1.
Characteristics of included trials.
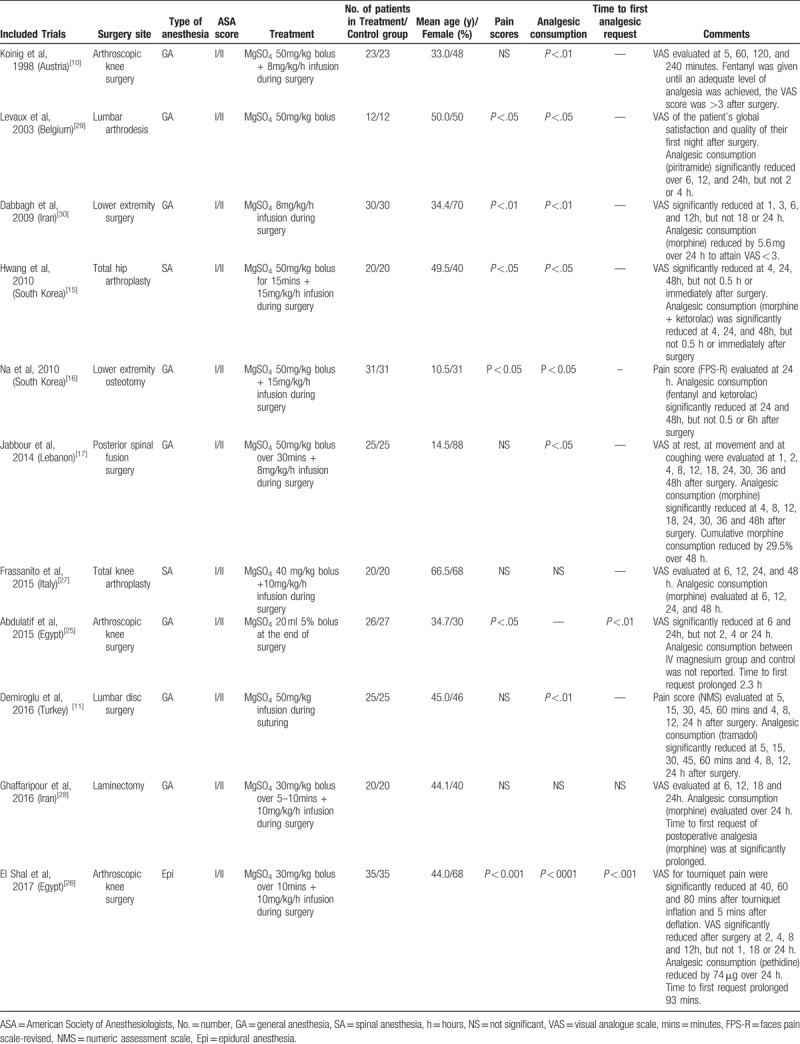
Among these studies, types of orthopedic surgeries included arthroscopic knee surgery (3 trials[10,25,26]), total knee arthroplasty (1 trial[27]), total hip arthroplasty (1 trial[15]), lower extremity osteotomy (1 trial[16]), posterior spinal fusion surgery (1 trial[17]), lumbar disc surgery (1 trial[11]), laminectomy (1 trial[28]), lumbar arthrodesis (1 trial[29]), and lower extremity surgery (1 trial[30]). General anesthesia (8 trials) was used in most of the trials,[10,11,16,17,25,28–30] spinal anesthesia was used in 2 trials,[15,27] and epidural anesthesia was used in 1 trial.[26] MgSO4 was administered intravenously in all the trials, as a single bolus in 2 trials,[25,29] an infusion in 2 trials,[11,30] and as a bolus followed by an infusion in 7 trials.[10,15–17,26–28] Adverse effects were reported in 6 trials,[11,17,25,26,29,30] including vomiting, nausea, shivering, burning sensations, and flushing.
3.2. Quality of the studies
Figures 2 and 3 show the risk of all types bias. Ten studies described the details of randomization process, and 6 studies reported allocation concealment. All of the 11 studies were double-blinded, and 9 studies reported blinding of outcome assessors.
Figure 2.
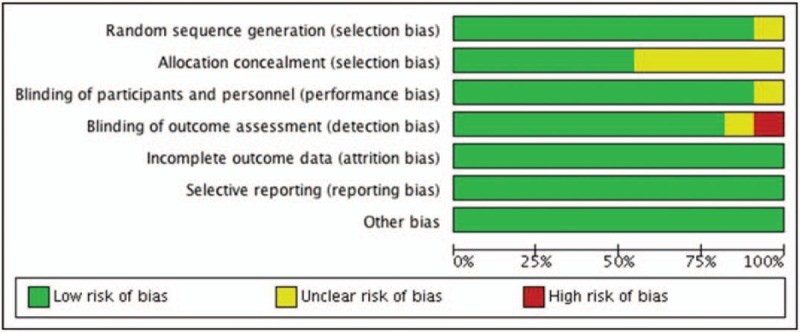
Risk of bias graph.
Figure 3.
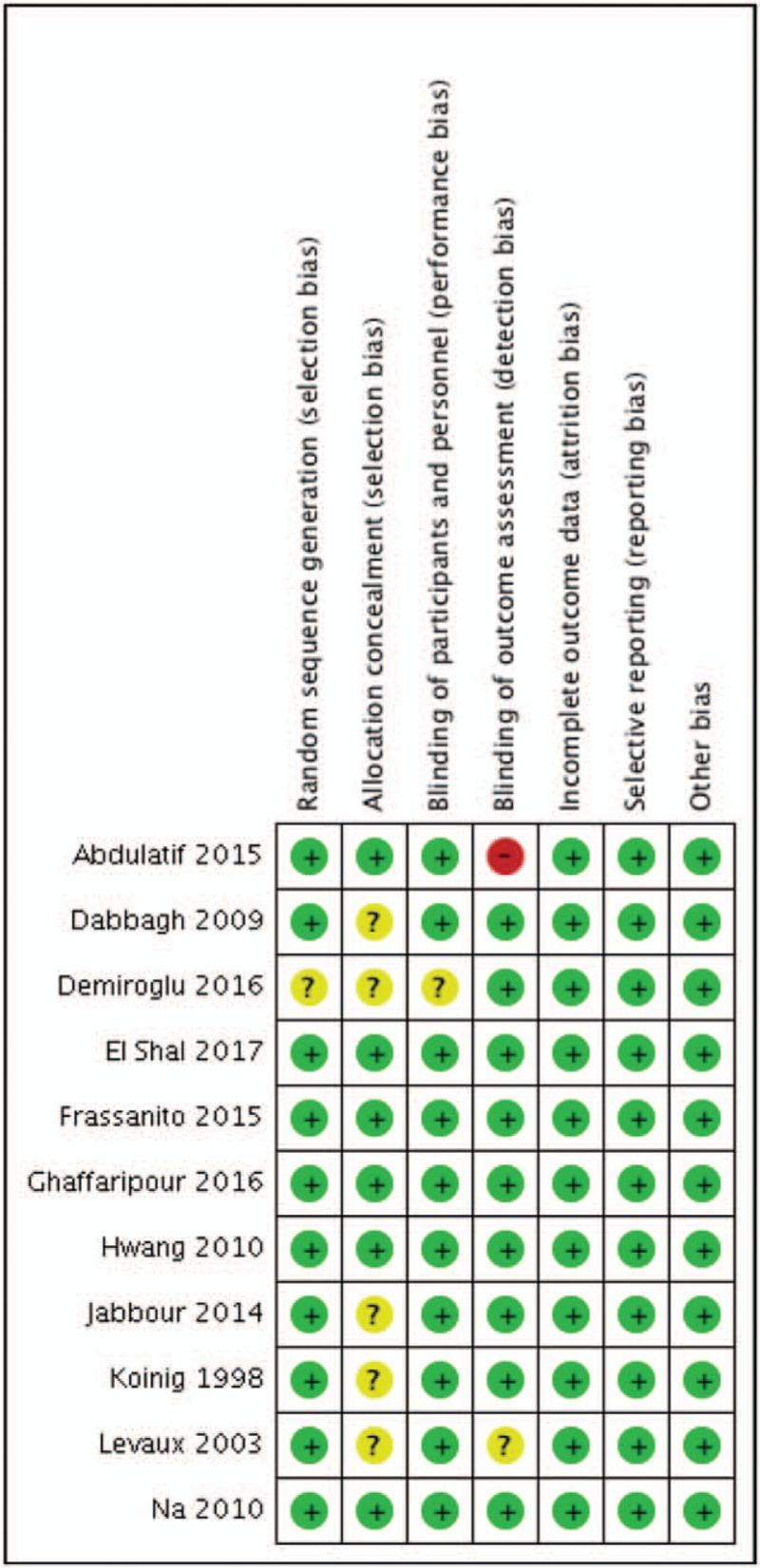
Risk of bias summary.
3.3. Postoperative pain scores
To measure the pain intensity, most of the trials (9 trials) used VAS scores,[10,15,17,25–30] 1 trial used numeric assessment scale (NMS),[11] and 1 trial used faces pain scale-revised (FPS-R).[16] The observation time ranged between immediately after surgery to 48 hours postoperatively. Within all the 1s trials, 6 trials (55%)[15,16,25,26,29,30] revealed a significant decrease (P <.05) in postoperative pain in patients treated with MgSO4 compared with control group (Table 1). The mean pain intensity scores decreased by 53% (median, 47%) in 24 hours in 3 studies,[15,25,26] by 55%,[15,26] 41%,[25,30] and 45%[26,30] in 4, 6 and 12 hours, respectively. In 2 studies,[26,30] pain intensity scores were decreased by 56%, 52%, 52%, and 45% in 1, 2, 3, and 8 hours, respectively. In 1 study,[29] the analgesic effect of MgSO4 could reduce VAS scores by 56% and 70%, respectively, measured by the patient's global satisfaction and quality of their first night after surgery.
Five of the 6 studies used general anesthesia during surgeries[11,16,17,25,29,30]; 1 study used spinal anesthesia,[15] and 1 study used epidural anesthesia.[26] MgSO4 was administered as a single bolus followed by infusion in 3 of the 6 studies that revealed the beneficial effect of MgSO4,[15,16,26] administered as a single bolus before surgery in 1 study,[29] administered as a bolus at the end of surgery in 1 study,[25] and administered by infusion during the whole surgery in 1 study.[30] There was no obvious relationship between pain intensity outcomes and types of anesthesia or routes of administration.
3.4. Arthroscopic surgery
Three studies[10,25,26] were all of patients undergoing knee arthroscopic surgery, and all used VAS scores to measure patient's pain intensity. Two studies (67%)[25,26] revealed a significant decrease (P < .05) in postoperative pain in patients treated with MgSO4 compared with control group (Table 1). Abdulatif et al [25] revealed that postoperative pain was significantly reduced in 6 and 24 hours postoperatively. El shal et al[26] showed that postoperative pain was significantly reduced in 2, 4, 8 and 12 hours after surgery. This study also measured postoperative tourniquet pain, and results showed that VAS scores were decreased by 50%, 66%, 33%, and 50%, respectively, in 40, 60, and 80 minutes after tourniquet inflation and 5 minutes after deflation.
3.5. Open surgery
Eight studies measured postoperative pain in patients undergoing open surgery. Types of surgery included knee or hip arthroplasty,[15,27] lower extremity surgery,[30] lower extremity osteotomy,[16] posterior spinal fusion surgery,[17] lumbar disc surgery,[11] laminectomy,[28] and lumbar arthrodesis.[29]
Only 4 studies (50%)[15,16,29,30] revealed a significant decrease (P <.05) in postoperative pain in patients treated with MgSO4 compared with control group (Table 1). Three of the 4 studies (75%) included patients undergoing lower extremity surgery,[15,16,30] and 1 study (25%) included patients undergoing spine surgery[29].
Among the 4 trials[11,17,28,29] that included patients undergoing spine surgery, only 1 trial (25%)[29] showed significant effect of MgSO4 in postoperative pain. Among the 4 trials[15,16,27,30] that included patients undergoing lower limb surgery, 3 trials (75%)[15,16,30] showed significant effect of MgSO4 in postoperative pain.
3.6. Cumulative analgesic consumption
Among 11 trials, 8 (73%) trials[10,11,15–17,26,29,30] revealed a significantly decreased (P < .05) dose in cumulative analgesic in patients treated with MgSO4 compared with control group (Table 1). The effect of MgSO4 varied by medication of postoperative pain management. The cumulative morphine consumptions were reduced by 38%[16] and 57%[30] in 24 hours in 2 studies. The cumulative tramadol consumptions were decreased by 17% and 16% in 12 and 24 hours after surgery, respectively, in 1 study.[11] The cumulative pethidine consumption was decreased by 33% 24 hours post operation, in 1 study.[26] The cumulative piritramide consumptions were reduced by 37% and 38% in 12 and 24 hours after surgery, respectively, in 1 study.[29] The cumulative fentanyl consumption was reduced by 85% when an adequate level of analgesia was achieved for patients with VAS >3 in 1 study.[10] One study[15] used PCA (containing morphine and ketorolac) as postoperative pain management, and the cumulative PCA consumptions at 24 and 48 hours were reduced by 46% and 39%, respectively.
Of these 8 trials, 6 trials used general anesthesia during surgeries,[10,11,16,17,29,30] 1 study used spinal anesthesia,[15] and 1 study used epidural anesthesia.[26] MgSO4 was administered as a single bolus followed by infusion in 5 of the 8 studies that revealed the beneficial effect of MgSO4.[10,15–17,26] One study administered as a single bolus before surgery,[29] and 2 studies administered by infusion during the whole surgery.[11,30] There was no correlation between the cumulative analgesic consumption and the type of anesthesia or the route of administration.
3.7. Arthroscopic surgery
Among the 3 trails[10,25,26] of patients undergoing knee arthroscopic surgery, 2 trials (67%)[10,26] revealed a significantly decreased (P < 0.05) dose in cumulative analgesic in patients treated with MgSO4. El shal et al[26] reported that analgesic consumption (pethidine) reduced by 50% over 24 hours postoperatively. Koinig et al[10] revealed that analgesic requirement (fentanyl) reduced by 35% intraoperatively, and 91% postoperatively.
3.8. Open surgery
Among the 8 studies of patients undergoing open surgery, 6 trials (75%)[11,15–17,29,30] revealed a significantly decreased (P <.05) dose in cumulative analgesic in patients treated with MgSO4 compared to control group. Three (50%)[15,16,30] of the 6 studies included patients undergoing lower extremity surgery, and the other 3 studies (50%)[11,17,29] included patients undergoing spine surgery.
Of the 4 trials[11,17,28,29] that included patients undergoing spine surgery, 3 trials (75%)[11,17,29] showed a significantly decreased (P <.05) dose in analgesic consumption in MgSO4 group. The result was also found in 3 (75%)[15,16,30] of the 4 trials[15,16,27,30] that included patients undergoing lower limb surgery.
3.9. Time to first analgesic request
Only 2 (18%) trials compared the time required to administer the first analgesic after surgery between MgSO4 group and control.[25,26] A total of 123 patients underwent arthroscopic knee surgery in these 2 studies. Subgroup analysis could not be conducted due to the 2 trials both included patients undergoing arthroscopic surgery. One study[25] revealed that the average time to first request of postoperative analgesia, diclofenac, was 2.3 hours longer for the MgSO4 group than for the control group. The other study[26] used pethidine for pain management and showed that the time to first request of postoperative analgesia was 93 minutes longer for the MgSO4 group than for the control group in average.
3.10. Adverse effects
Table 2 compares the postoperative adverse effects between the MgSO4 group and controls. The MgSO4 group experienced significantly less adverse effects than controls, including vomiting (5.1% vs 16.5% [number needed to harm [NNH] = 8.8], with a relative risk (RR) of 0.32 [95% confidence interval [CI] 0.12–0.82]), nausea (6.2% vs 16.5% [NNH = 9.7], with a RR of 0.38 [95% CI 0.15–0.92]) and shivering (8.5% vs 27.7% [NNH = 5.2], with a RR of 0.31 [95% CI 0.11–0.88]). Two trials[25,30] reported increased incidence of adverse effects in MgSO4 group: 10% patients complained about burning or heat sensations[30] and 58% patients reported flushing, compared to none in controls. However, no major complication was reported in both trials.
Table 2.
Adverse effects.

4. Discussion
The main finding from these 11 trials is that perioperative systemic MgSO4 administration could lead to at least 2 beneficial effects, including reduced cumulative analgesic consumption, and longer time to first request of analgesic in some trials. However, the effect of systemic MgSO4 administration on postoperative pain intensity still remains controversial. The intravenous administration of MgSO4 could also reduce certain postoperative adverse effects, such as nausea, vomiting, and shivering.
Comparing to previous meta-analyses,[7–9] our study emphasizes the perioperative “intravenous” administration of MgSO4 for patients who underwent “orthopedic surgery”. To our knowledge, this is the first systematic review investigating effects of MgSO4 on orthopedic surgery, including some latest RCTs[11,25–28] that have not been included in the previous studies.[7–9] The administration of MgSO4 could obviously minimize postoperative pain in some RCTs, but it failed to show beneficial effects in others trials. However, no patients showed deterioration in pain postoperatively.
The most obvious beneficial effect is the decrease of analgesic consumption after surgery. Eight of the 11 trials revealed a significant decrease in analgesic use in patients who had been treated with MgSO4. Arthroscopic surgery patients were included in 2 trials. Spine surgery and lower extremity surgery patients were included in 3 trials, respectively. The analgesics that have been used among studies included morphine, tramadol, pethidine, ketorolac, piritramide, and diclofenac. The doses of postoperative 24-hour cumulative consumption of analgesic were reduced by 16% to 57% (median: 38%) in 6 trials. General anesthesia was used in most of RCTs, but there was no correlation between the type of anesthesia and cumulative analgesic consumption or pain intensity. As for postoperative pain intensity, 6 trials reported significant reduction in pain scores in MgSO4—treated patients. Among these trials, patients in 3 trials experienced a 53% reduced 24-hour postoperative pain intensity. One of the trials evaluated the patient's global satisfaction and quality of their first night after surgery, and results showed that the VAS scores reduced by 56% and 70%, respectively. Overall, nearly half of trials failed to provide evidence that systemic MgSO4 administration may have a beneficial effect on pain intensity. One potential explanation of this controversy between our findings with previous studies is types of surgery. Most RCTs with patients undergoing arthroscopic or lower extremity revealed a positive analgesic effect of intravenous MgSO4 administration, however, only 1 trial (25%) involving patients undergoing spine surgery showed the positive effect. We noticed a potential correlation between types of surgery and the effects postoperative analgesia of MgSO4, but still, need further investigation in future study. Another potential explanation is patients’ own perception of pain because it can be influenced by a variety of factors such as gender, psychological, genetic and personality. [31–33] Some ethnicity was reported significantly more pain tolerance, and gender was reported as a significant factor, with men reporting less pain than women.[33]
The dose of MgSO4 that used for orthopedic surgery patients varied among studies. Most trials used infusion during operation; while some studies tested a single bolus before or at the end of surgery. An initial MgSO4 bolus dose of 30 mg/kg or 50 mg/kg was used in the trials and followed by an infusion of 8 mg/kg/h or 10 mg/kg/h during surgery. Ghaffaripour et al[28] tested the largest amount of magnesium by using a 30 mg/kg bolus and following with an infusion of 10 mg/kg/h during surgery, but beneficial effects on pain intensity and analgesic consumption were not reported. On the other hand, Levaux et al[29] found that the injection of a bolus dose of 50 mg/kg MgSO4 resulted significant postoperative analgesic effect. However, it remains unclear whether the amount of magnesium is correlated with the analgesic efficacy. Further study should be investigated.
As for the postoperative adverse effects, the intravenous MgSO4 administration could benefit patients with reduced vomiting, nausea, and shivering. To our surprise is that a higher incidence of burning sensations and flushing in the MgSO4 group was also reported in some trials. Two meta-analyses[7,8] included studies that reported on the incidence of postoperative dizziness,[4,5,34] headache,[4,5,34] nausea or vomiting,[4,5,34,35] sedation,[36,37] and hypotension[4,5,37,38] in patients receiving magnesium administration during operation. However, aggregated effect did not suggest a significant effect of intravenous magnesium on adverse perioperative outcomes. Three studies[3,29,34] included in the meta-analyses reported on postoperative shivering, and combined effects showed a reduction on the incidence of postoperative shivering in the intravenous magnesium group compared with control. However, it might be too early to draw a conclusion on these adverse events because of limited trials. More studies are needed to confirm.
There are some limitations to our study. First, these RCTs varied in types of anesthesia, types of orthopedic surgery, MgSO4 dosages and characteristics of patients (e.g. age, sex, race). Second, most RCTs were conducted with small sample sizes. The largest trial[26] included only 70 patients and the smallest trial[29] with 24 patients. Beneficial effects of treatment may be mistakenly detected in small sample trials.[39] Third, all of the included RCTs used intravenous administration of MgSO4, because the number of RCTs which other routes of administration were limited. However, we did notice the potential clinical effects of other administration routes based on related literature.[12,13,20,21,23,24,40] Future study should include RCTs that use other routes of magnesium administration, and further investigate the combined effects. Fourth, the time to deliver and to end magnesium administration differed among RCTs, and the total magnesium dosages varied. Based on the current evidence, we still could not stratify the effect of dosages and time, and whether dosages of magnesium administration may result in different analgesic and adverse effects. Future research and more RCTs should design to assess the efficacy and safety of different magnesium dosages, delivery plans, and routes of administration.
In conclusion, most RCTs reported beneficial effects of magnesium sulfate for orthopedic surgery patients, especially for arthroscopic surgery and lower extremity surgery patients. The perioperative intravenous administration of MgSO4 could reduce postoperative analgesic consumption, and reduce postoperative vomiting, nausea and shivering. However, limited studies suggested that MgSO4 treatment favors postoperative pain intensity and the time to first analgesic request. Since MgSO4 is a cost-effective, harmless and easily accessible medication, the role of its analgesic effects in orthopedic surgery should be further investigated. Further trials using larger samples would reduce random variations. In consideration of potential analgesic effect and rare adverse events of MgSO4, we recommend intravenous MgSO4 administration during anesthesia for orthopedic surgery, but should be used under safe dosage, and follow the delivery plans that have been surveyed in previous studies.
Author contributions
Analyze the data: Yu-Ning Peng, Fung-Chang Sung, Mei-Li Huang, Cheng-Li Lin, Chia-Hung Kao
Conceptualization: Yu-Ning Peng, Mei-Li Huang.
Data curation: Yu-Ning Peng, Fung-Chang Sung, Mei-Li Huang, Cheng-Li Lin, Chia-Hung Kao.
Design and conduct: Yu-Ning Peng, Mei-Li Huang
Final approval of manuscript: All authors
Formal analysis: Yu-Ning Peng, Cheng-Li Lin.
Funding acquisition: Chia-Hung Kao.
Investigation: Yu-Ning Peng, Fung-Chang Sung, Chia-Hung Kao.
Methodology: Yu-Ning Peng, Cheng-Li Lin.
Modify the manuscript: Fung-Chang Sung, Chia-Hung Kao
Project administration: Chia-Hung Kao.
Resources: Chia-Hung Kao.
Software: Cheng-Li Lin.
Supervision: Fung-Chang Sung, Chia-Hung Kao.
Validation: Yu-Ning Peng, Fung-Chang Sung, Mei-Li Huang, Cheng-Li Lin.
Visualization: Yu-Ning Peng.
Write the manuscript: Yu-Ning Peng, Cheng-Li Lin, Chia-Hung Kao
Writing – original draft: Yu-Ning Peng, Mei-Li Huang, Cheng-Li Lin.
Writing – review & editing: Yu-Ning Peng, Fung-Chang Sung, Chia-Hung Kao.
Footnotes
Abbreviations: CI = confidence interval, NNH: number needed to harm, RCT = randomized controlled trial, RR = relative risk.
This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212–123004), China Medical University Hospital (DMR-107-192, CMU107-ASIA-19); Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
References
- [1].McCarthy RJ, Kroin JS, Tuman KJ, et al. Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats. Anesth Analg 1998;8:830–6. [DOI] [PubMed] [Google Scholar]
- [2].PhilippeAscher L. Electrophysiological studies of NMDA receptors. Trends Neurosci 1987;10:284–8. [Google Scholar]
- [3].Tramèr MR, Schneider J, Marti RA, et al. Role of magnesium sulfate in postoperative analgesia. Anesthesiology 1996;84:340–7. [DOI] [PubMed] [Google Scholar]
- [4].Tramèr MR, Glynn CJ. An evaluation of a single dose of magnesium to supplement analgesia after ambulatory surgery: randomized controlled trial. Anesth Analg 2007;104:1374–9. [DOI] [PubMed] [Google Scholar]
- [5].Zarauza R, Saez-Fernandez AN, Iribarren MJ, et al. A comparative study with oral nifedipine, intravenous nimodipine, and magnesium sulfate in postoperative analgesia. Anesth Analg 2000;91:938–43. [DOI] [PubMed] [Google Scholar]
- [6].Lysakowski C, Dumont L, Czarnetzki C, et al. Magnesium as an adjuvant to postoperative analgesia: a systematic review of randomized trials. Anesth Analg 2007;104:1532–9. [DOI] [PubMed] [Google Scholar]
- [7].Albrecht E, Kirkham KR, Liu SS, et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia 2013;68:79–90. [DOI] [PubMed] [Google Scholar]
- [8].De Oliveira GS, Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178–90. [DOI] [PubMed] [Google Scholar]
- [9].Guo BL, Lin Y, Hu W, et al. Effects of systemic magnesium on post-operative analgesia: is the current evidence strong enough. Pain Physician 2015;18:405–18. [PubMed] [Google Scholar]
- [10].Koinig H, Wallner T, Marhofer P, et al. Magnesium sulfate reduces intra- and postoperative analgesic requirements. Anesth Analg 1998;87:206–10. [DOI] [PubMed] [Google Scholar]
- [11].Demiroglu M, Ün C, Ornek DH, et al. The effect of systemic and regional use of magnesium sulfate on postoperative tramadol consumption in lumbar disc surgery. Biomed Res Int 2016;2016: 3216246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arcioni R, Palmisani S, Tigano S, et al. Combined intrathecal and epidural magnesium sulfate supplementation of spinal anesthesia to reduce post-operative analgesic requirements: a prospective, randomized, double-blind, controlled trial in patients undergoing major orthopedic surgery. Acta Anaesthesiol Scand 2007;51:482–9. [DOI] [PubMed] [Google Scholar]
- [13].Chen Y, Zhang Y, Zhu YL, et al. Efficacy and safety of an intra-operative intra-articular magnesium/ropivacaine injection for pain control following total knee arthroplasty. J Int Med Res 2009;37:1733–41. [DOI] [PubMed] [Google Scholar]
- [14].Dayioğlu H, Baykara ZN, Salbes A, et al. Effects of adding magnesium to bupivacaine and fentanyl for spinal anesthesia in knee arthroscopy. J Anesth 2009;23:19–25. [DOI] [PubMed] [Google Scholar]
- [15].Hwang JY, Na HS, Jeon YT, et al. I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth 2010;104:89–93. [DOI] [PubMed] [Google Scholar]
- [16].Na HS, Lee JH, Hwang JY, et al. Effects of magnesium sulphate on intraoperative neuromuscular blocking agent requirements and postoperative analgesia in children with cerebral palsy. Br J Anaesth 2010;104:344–50. [DOI] [PubMed] [Google Scholar]
- [17].Jabbour HJ, Naccache NM, Jawish RJ, et al. Ketamine and magnesium association reduces morphine consumption after scoliosis surgery: prospective randomised double-blind study. Acta Anaesthesiol Scand 2014;58:572–9. [DOI] [PubMed] [Google Scholar]
- [18].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Green S. Cochrane Collaboration Cochrane Handbook for Systematic Reviews of Interventions. Chichester, West Sussex; Hoboken NJ: John Wiley & Sons; 2008. [Google Scholar]
- [20].Choi IG, Choi YS, Kim YH, et al. The effects of postoperative brachial plexus block using MgSO4 on the postoperative pain after upper extremity surgery. Korean J Pain 2011;24:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khalili G, Janghorbani M, Sajedi P, et al. Effects of adjunct intrathecal magnesium sulfate to bupivacaine for spinal anesthesia: a randomized, double-blind trial in patients undergoing lower extremity surgery. J Anesth 2011;25:892–7. [DOI] [PubMed] [Google Scholar]
- [22].Mukherjee K, Das A, Basunia SR, et al. Evaluation of magnesium as an adjuvant in ropivacaine-induced supraclavicular brachial plexus block: a prospective, double-blinded randomized controlled study. J Res Pharm Pract 2014;3:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haghighi M, Soleymanha M, Sedighinejad A, et al. The effect of magnesium sulfate on motor and sensory axillary plexus blockade. Anesth Pain Med 2015;5:e21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sadoni H, Toloueghamari B, Teymourei K, et al. Effects of intra-articular magnesium sulfate injection in post-operative pain in knee arthroscopy: a prospective comparative study. Asian J Pharm 2017;11.:1–4. [Google Scholar]
- [25].Abdulatif M, Amin SMM, Abou-Ela A, et al. Intra-articular versus intravenous magnesium sulfate as adjuvant to femoral nerve block in arthroscopic knee surgery under general anesthesia: a randomized controlled trial. Egypt J Anaesth 2015;31:239–46. [Google Scholar]
- [26].El Shal SM, Lotfy E. Evaluation of effect of intravenous magnesium sulfate infusion on tourniquet induced hypertension and pain in arthroscopic knee surgery patients under epidural anesthesia. Egypt J Anaesth 2017;33:73–82. [Google Scholar]
- [27].Frassanito L, Vergari A, Messina A, et al. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva Anestesiol 2015;81:1184–91. [PubMed] [Google Scholar]
- [28].Ghaffaripour S, Mahmoudi H, Eghbal H, et al. The effect of intravenous magnesium sulfate on post-operative analgesia during laminectomy. Cureus 2016;8:e626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Levaux C, Bonhomme V, Dewandre PY, et al. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anaesthesia 2003;58:131–5. [DOI] [PubMed] [Google Scholar]
- [30].Dabbagh A, Bastanifar E, Foroughi M, et al. The effect of intravenous magnesium sulfate on serum levels of N-terminal pro-brain natriuretic peptide (NT pro-BNP) in elective CABG with cardiopulmonary bypass. J Anesth 2013;27:693–8. [DOI] [PubMed] [Google Scholar]
- [31].Kim H, Neubert JK, San Miguel A, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 2004;109:488–96. [DOI] [PubMed] [Google Scholar]
- [32].Al-Hashimi M, Scott S, Griffin-Teall N, et al. Influence of ethnicity on the perception and treatment of early post-operative pain. Br J Pain 2015;9:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Faucett J, Gordon N, Levine J. Differences in postoperative pain severity among four ethnic groups. J Pain Symptom Manag 1994;9:383–9. [DOI] [PubMed] [Google Scholar]
- [34].Song JW, Lee YW, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyper- algesia in patients undergoing thyroidectomy. Anesth Analg 2011;113:390–7. [DOI] [PubMed] [Google Scholar]
- [35].Benhaj AM, Barakette M, Dhahri S, et al. Effect of intra and postoperative magnesium sulphate infusion on postoperative pain. Tunis Med 2008;86:550–5. [PubMed] [Google Scholar]
- [36].Kiran S, Gupta R, Verma D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postopera- tive pain after inguinal surgery. Indian J Anaesth 2011;55:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kaya S, Kararmaz A, Gedik R, et al. Magnesium sulfate reduces postoperative morphine requirement after remifentanil-based anesthesia. Med Sci Monit 2009;15:I5–9. [PubMed] [Google Scholar]
- [38].Jaoua H, Zghidi SM, Wissem L. Effectiveness of intravenous magnesium on postoperative pain after abdominal surgery versus placebo: double blind randomized controlled trial. Tunis Med 2010;88:317–23. [PubMed] [Google Scholar]
- [39].Moore RA, Gavaghan D, Tramèr MR, et al. Size is everything—the impact of event rate variation on clinical trials and meta- analysis. Pain 1998;78:208–16. [Google Scholar]
- [40].Silvasti M, Svartling N, Pitkanen M, et al. Comparison of intravenous patient-controlled analgesia with tramadol versus morphine after microvascular breast reconstruction. Eur J Anaesthesiol 2000;17:448–55. [DOI] [PubMed] [Google Scholar]


