Abstract
Tropisetron is an adjuvant for dezocine used in intravenous patient-controlled analgesia (PCA) and has been reported to provide superior pain control. It is efficacious in reducing the institutional incidence of postoperative nausea and vomiting (PONV), which decreases resource utilization and cost. However, no scientific evidence has been reported in the literature demonstrating analytical confirmation of the compatibility and stability of the combination of dezocine and tropisetron. Thus, the present study aimed to investigate the stability of dezocine with tropisetron in 0.9% sodium chloride injection form for PCA administration.
Commercial solutions of dezocine and tropisetron were combined and examined for compatibility and stability when diluted with 0.9% sodium chloride injection in polyolefin bags and glass bottles stored at 4°C or 25°C for up to 14 days. The initial concentrations were 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron. For all samples, the compatibility parameters (including precipitation, cloudiness, discoloration, and pH values) were evaluated. Chemical stability was also determined using high-performance liquid chromatographic (HPLC) analysis.
After a 14-day period of storage at 4°C or 25°C, the initial concentrations of dezocine and tropisetron were maintained at at least 98%. All of the mixtures remained clear and colorless throughout the observation period, and no color change or precipitation was observed.
These results indicated that admixtures of 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron in 0.9% sodium chloride injection were stable for at least 14 days when stored in polyolefin bags or glass bottles at 4°C or 25°C and protected from light.
Keywords: dezocine, drug stability, patient-controlled analgesia, tropisetron
1. Introduction
Opioids are known to be the most commonly used drugs for postoperative pain management.[1] However, severe surgical patients are unable to take oral medication and are instead administered intravenous analgesics.[2] Patient-controlled techniques allow a patient to administer small boluses of analgesic to manage their own pain relief, effectively controlling the titration and providing a better response to analgesic demands.[3,4] However, one potential problem is that the mixing of 2 or more injections together in infusion solutions may induce physical changes and chemical incompatibility such as chemical degradation of ingredients, potentially causing precipitation or crystallization and therefore reduced efficacy.[5]
Dizocine (Fig. 1A), (5R, 11S, 13S)-13-amino-5, 6, 7, 8, 9, 10, 11,12-octahydro-5-methyl-5,11-methanobenzocyclodecen-3-ol, is a new synthetic opioid analgesic with strong κ-receptor agonist and μ-opioid antagonist activity, as well as structural similarity to pentazocine.[6] Due to its unique analgesic ability, dizocine has been commonly used for the management of postoperative, visceral and cancerous pain.[7] However, it is nearly always associated with adverse reactions (ADR) such as nausea and vomiting.[8] Postoperative nausea and vomiting (PONV), like postoperative pain, can result in delayed recovery, prolonged hospital stays, and economic losses for patients.[9] Tropisetron hydrochloride (Fig. 1B), 3-indolecarboxylic acid (endo-8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl)ester hydrochloride, is a selective 5-hydroxytryptamine-3(5-HT3) receptor antagonist that has been widely administered for the prevention and treatment of PONV and analgesic-induced PONV during PCA over several decades.[10]
Figure 1.
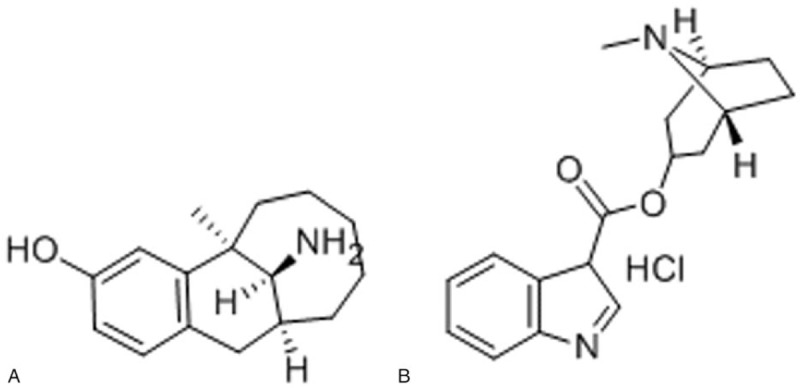
Structures of (A) dezocine and (B) tropisetron hydrochloride.
Previous studies have demonstrated that adding tropisetron hydrochloride to PCA dezocine for postoperative pain provided favorable postoperative analgesia and decreased the incidence of PONV.[11,12] However, there is a lack of documented experimental chemical stability and compatibility data regarding the dezocine–tropisetron mixture for PCA. Thus, the present study aims to evaluate the compatibility and stability of the binary admixture of dezocine and tropisetron hydrochloride in 0.9% sodium chloride injection form in polyolefin bags and glass bottles over a period of 14 days at 4°C or 25°C, as well as to provide background information on the storage of dezocine–tropisetron mixtures.
2. Methods
2.1. Materials and reagents
Dezocine (lot number 121350, chemical purity 99.7%) and tropisetron (lot number 121432, chemical purity 99.8%) reference standards were purchased from Beijing Guangyuan Hengxin Technology Development Co. Ltd. (Beijing, People's Republic of China). Commercially available ampoules of dezocine injection (5 mg/mL, lot number 17092621) were purchased from Liaoning Haisi Pharmaceutical Co., Ltd. (Liaoning, People's Republic of China). Tropisetron hydrochloride injection (5 mg/5 mL, lot number 20170402) was obtained from Comm Scope Pharmaceutical Co., Ltd. (Hunan, People's Republic of China). A total of 0.9 mg/mL of sodium chloride injection, which was used to prepare the sample mixtures, was supplied by Kelun Pharmaceutical Co., Ltd. (Sichuan, People's Republic of China, lot number A150826). This study was approved by the Institutional Review Board and performed at the Renmin Hospital of Wuhan University.
2.2. Instrumentation
Each sample was analyzed by high-performance liquid chromatography (HPLC) to quantify the components. The HPLC system (Ultimate 3000, Dionex, Germering, Germany) consisted of a quaternary liquid gradient system, WPS-3000RS autoinjector, TCC-100 column oven, and DAD-3000RS UV spectrophotometer. Chromatographic data were acquired using Chromeleon software version 6.80. The pH values for the samples at each designated time interval were measured with a pH meter (Model pHS-3C, Leici Instrument Co., Shanghai, China).
2.3. Chromatographic conditions
Chromatographic separations were processed on a 1.9 μm Hypersil Gold C18 column (100 mm × 2.1 mm) (Thermo Fisher Scientific, Madison). The column temperature was 25°C, and the gradient operated at a flow rate of 0.5 mL/min with 10 μL of injection volume for each sample. The mobile phase was composed of 0.1% formic acid in KH2PO4 in water (A) and 0.1% formic acid in acetonitrile (B) at a ratio of 70:30 (v/v) throughout the analysis.[13] The selected detection wavelengths for dezocine and tropisetron were 281 nm and 285 nm, respectively.[14]
2.4. Preparation of stock and standard curve solutions
The analytical reference standards were used for the preparation of stock standard solutions of 4 mg/mL dezocine and 0.5 mg/mL tropisetron hydrochloride in the mobile phase. The stock standard solutions were prepared in deionized water and were stable for 15 days or more at 4°C.[15] The calibration curves were established by injection of a linear plot of the concentrations of dezocine (range, 40.0–400.0 μg/mL) and tropisetron hydrochloride (range, 5.0–100.0 μg/mL).
2.5. Method validation
The validation of the proposed method was performed in terms of range linearity, accuracy, intra- and interday precision and stability indication for the tested drugs. Calibration curves were constructed from a linear plot of peak area versus concentration of the reference standards of dezocine and tropisetron hydrochloride. The accuracy and intra- and interday precisions were calculated from 3 control samples (QCs) of dezocine (80.0, 160.0, and 320 μg/mL) and tropisetron hydrochloride (30.0, 60.0, and 90.0 μg/mL). The recovery value and relative standard deviation (RSD, %), which were calculated from 3 QCs of dezocine and tropisetron hydrochloride with 5 determinations per concentration on the same day, were used for estimation of the accuracy and intraday precision.[16] The interday precision (5 days) was also estimated as the RSD calculated from 5 replicate mixtures of samples prepared in the same way.
2.6. Stability study of the analgesic solutions
Dezocine injection (5 mg/mL) and tropisetron hydrochloride injection (1 mg/mL) were injected into empty 100-mL polyolefin bags or glass bottles. Then, enough 0.9% sodium chloride injection was added to each container to produce a total volume of 100 mL. The solutions, which were prepared using aseptic technique in a laminar airflow hood, were agitated by a rotary shaker after each addition of fluid. The final nominal concentrations of dezocine and tropisetron hydrochloride were 0.4 mg/mL and 0.05 mg/mL, respectively.[17,18] The selection of the concentrations of these drugs in our study was based on those used in daily practice. Three polyolefin bags and 3 glass containers containing the dezocine and tropisetron hydrochloride admixture were stored at 4°C or 25°C. A 2-mL sample was removed from each container immediately after preparation and at 1, 2, 3, 5, 7, 10, and 14 days after mixing. At each timepoint, changes in color, cloudiness, precipitation, and pH values were evaluated. All samples were frozen at −20°C and used for further analysis.[19] On the day of analysis, samples were allowed to reach room temperature and were diluted 1:4 in water before injection into an HPLC system. Each sample was analyzed in triplicate (total n = 3).[20]
2.7. Stability Indication
Degraded samples of dezocine and tropisetron hydrochloride were assessed by chromatographic methods to confirm the separation of the parent molecule from its degradation products. The mixed solutions of dezocine with tropisetron hydrochloride in 0.9% sodium chloride injection form were degraded by heating at 60°C for 5 hours in 0.1 mol/L hydrochloric acid, 0.1 mol/L sodium hydroxide, and 3% hydrogen peroxide. After the degraded preparations were completed, they were assessed by HPLC assay.[21]
2.8. Analysis of data
The results of the study are expressed as the mean ± standard deviation (SD). The initial concentrations of dezocine and tropisetron hydrochloride were defined as 100%, and all subsequent concentrations were expressed as a percentage of the initial concentration. The drugs were considered to be stable if they retained 90% of the initial concentrations. The linear regression analysis was used for analysis of changes in drug concentrations in solution over time. The statistical analyses were performed using the SPSS version 15.0 statistical software package (SPSS Inc.). P values <.05 or .1 were considered statistically significant.
3. Results
3.1. Validation of the HPLC method
A reversed phase HPLC method was developed and validated for the simultaneous determination of dezocine and tropisetron hydrochloride in PCA solution. In the case of dezocine, there was a linear response between the peak area and concentration with a correlation coefficient (r) better than 0.9997 (y = 107.2x − 0.36). For the linearity of tropisetron hydrochloride, the peak area of the drug concentration yielded a correlation (r) better than 0.9992 (y = 1048x − 2.37). Under extreme conditions (strong acidic, basic, and oxidizing solutions), the degradation study results showed that the 2 drugs were stable with < 3% decomposition of the compounds and baseline separation between all analytes (Fig. 2). The average retention times for dezocine and tropisetron hydrochloride were 6.48 and 11.04 minutes, respectively. The results of the accuracy and the intraday and interday precision of the assay method for the four analytes are shown in Table 1. The data indicated that the proposed HPLC method is accurate and precise for the quality control of dezocine and tropisetron hydrochloride in the admixtures.
Figure 2.
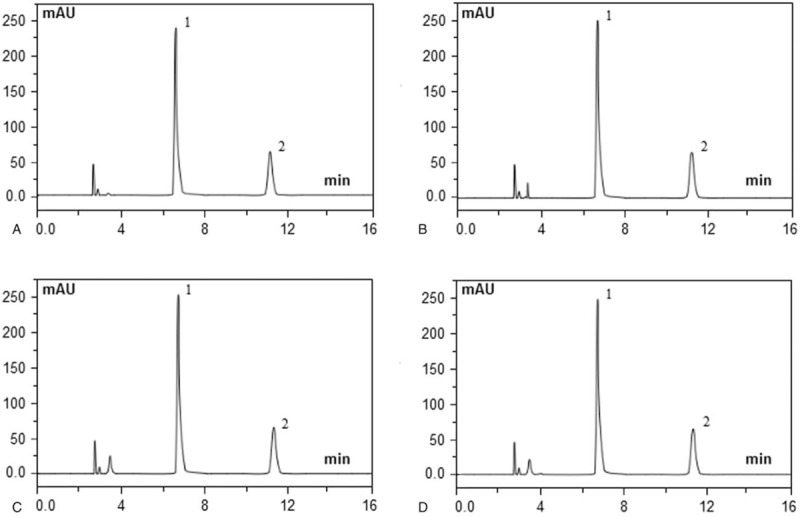
Chromatograms of dezocine 0.4 mg/mL and tropisetron hydrochloride 0.05 mg/mL admixtures that were freshly prepared (A) exposed to 0.1 mol/L hydrochloric acid at 60°C for 5 hours (B) exposed to 0.1 mol/L sodium hydroxide at 60°C for 5 hours (C) and exposed to 3% hydrogen peroxide at 60°C for 5 hours, (D) dezocine elutes at 6.48 minutes (peak 1) and tropisetron hydrochloride at 11.04 minutes (peak 2).
Table 1.
Validation of HPLC method.

3.2. Stability of the analgesic mixtures
The physical compatibility results showed that there was no observation of precipitation, turbidity, color change, opacity, or gas production. Tables 2 and 3 show the percentages of dezocine and tropisetron hydrochloride in normal saline for PCA administration stored at 4°C or 25°C. As indicated in Tables 2 and 3, there were no degradation products of the mixtures over the 14 days, and the concentrations of dezocine and tropisetron hydrochloride remaining in the admixtures were all >98.5%. Chromatography demonstrated that the degradation products found during the accelerated degradation study are not readily apparent in these chromatograms and did not increase in quantity during the study period (Fig. 3). Additionally, the average pH values of the binary mixtures ranged from 4.8 to 5.2 during the 14-day experiment.
Table 2.
Amount of initial concentration of dezocine (400 mg/L) and tropisetron hydrochloride (50 mg/L) remaining after 14 d of storage at 25°C in polyolefin bags or glass containers (%; mean ± sd; n = 3).

Table 3.
Amount of initial concentration of dezocine (400 mg/L) and tropisetron hydrochloride (50 mg/L) remaining after 14 d of storage at 4°C in polyolefin bags or glass containers (%; mean ± sd; n = 3).

Figure 3.
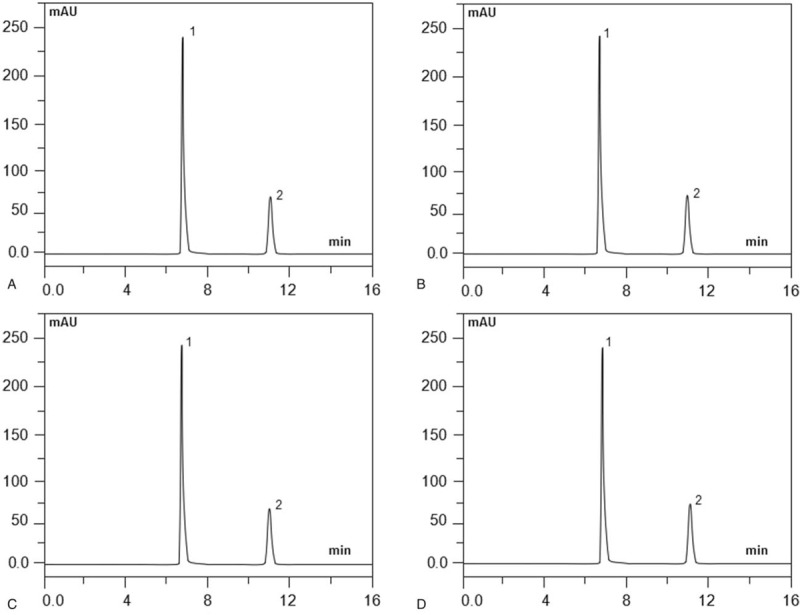
Typical chromatograms of dezocine and tropisetron hydrochloride mixtures on study day 14. Mixtures stored at 25°C in glass containers (A) and polyolefin bags (B) mixtures stored at 4°C in glass containers, (C) and polyolefin bags, (D) dezocine elutes at 6.48 minutes (peak 1) and tropisetron hydrochloride at 11.04 minutes (peak 2). Degradation products found during the accelerated degradation study are not readily apparent in these chromatograms and did not increase in quantity during the study period.
4. Discussion
Combinations of 2 or more drugs are often used in common practice for postoperative pain control. However, little to no information is presently available regarding the physical or chemical changes in analgesic mixtures.[22] Currently, commercially available analgesic mixtures are very scarce, and the drugs are confined to preparation in hospital pharmacies for clinical patients.[23] Thus, there is a great need to confirm that all the main drugs remain stable in admixtures. Furthermore, the benefits of excellent stability and compatibility of drugs will guarantee satisfactory therapeutic outcomes and reduce adverse reactions caused by degradation products.[24] Many clinical studies have evaluated the efficacy of tropisetron hydrochloride as an adjunct to dezocine PCA for providing superior pain relief, and the combination of dezocine and tropisetron hydrochloride has been accepted for the prevention of dezocine-induced PONV.[11,12,25] However, to our knowledge, there are no reports to date regarding the compatibility and stability of such mixtures for PCA. Therefore, the aim of this study was to address this issue.
As mentioned previously, many studies have tested the compatibility and stability of dezocine alone or in combination with other drugs in infusion solutions and suggested that dezocine is a very stable drug.[26] It has been demonstrated that dezocine is stable for at least 1 week in 5% dextrose injection or 0.9% sodium chloride injection at room temperature.[17] No loss of dezocine was found in the previous studies during the testing. The studies also reported that most of the other tested drugs, such as ketorolac tromethamine, droperidol, fentanyl, ibuprofen and dexamethasone, were stable when combined with dezocine.[15,27] However, in the case of lansoprazole, precipitation was observed in the presence of dezocine in 0.9% sodium chloride injection form.[28] This result was most likely because dezocine is a weak acid with a pKa of 8.1. For tropisetron hydrochloride, which is a strong acid-weak base salt with a pKa value of 9.46, the stability showed strong pH dependence with high stability in acidic solution and possible precipitation and crystallization in alkaline solution.[29] However, several studies have reported the instability of analgesic mixtures containing tropisetron hydrochloride with lornoxicam or fosaprepitant, which seemed to precipitate because of pH modification.[30,31]
In the current study, the binary mixtures of dezocine and tropisetron hydrochloride were acidic, with pH values ranging between 4.8 and 5.2. No precipitation was observed, and there was no modification of the chromatographic peaks when dezocine was combined with tropisetron hydrochloride in the infusion solutions. Based on these findings of our study, it was credible that combination use of dezocine and tropisetron hydrochloride in 0.9% sodium chloride injection form was stable for up to 14 days when stored in polyolefin bags and glass bottles under all the storage conditions tested (4°C or 25°C). The satisfactory compatibility and stability of dezocine in combination with tropisetron hydrochloride makes it preferable to authorize licensed central intravenous additive (CIVA) services for preparation of these drugs, which may be more suitable for PCA in hospitals.
Bacterial contamination may occur when the mixtures of drugs are taken from ampoules of sterile solutions. In this study, the physicochemical stability of mixing drugs was researched, but the issue of microbial contamination was ignored.[32] Hence, Chapter 797 of the United States Pharmacopoeia (USP)/national prescription should be respected and enforced in clinical practice.[33] According to these standards, the agents we analyzed were classified as low-risk composite aseptic products.[34] To ensure the stability of the drug, procedures must be within the standards of the USP, which indicate that the preparation could be stored for 48 hours at room temperature and 14 days when refrigerated.[35]
5. Conclusion
According to serial qualitative, pH, and HPLC analyses, solutions of 0.4 mg/mL dezocine admixed with 0.05 mg/mL tropisetron hydrochloride in 0.9% sodium chloride injection, when stored in polyolefin bags or glass bottles at 4°C or 25°C and protected from light, were compatible for at least 14 days. Given that this trial has demonstrated the physical compatibility and stability of these 2 agents, we conclude that this solution can be safely prepared and stored (in the dark) for up to 14 days by CIVA services.
Author contributions
Data curation: Peng Chen.
Formal analysis: Peng Chen.
Funding acquisition: Peng Chen, Fuchao Chen.
Investigation: Peng Chen, Fuchao Chen.
Methodology: Peng Chen.
Resources: Ben-hong Zhou.
Supervision: Peng Chen.
Validation: Peng Chen.
Visualization: Peng Chen.
Writing – original draft: Peng Chen.
Footnotes
Abbreviations: HPLC = high-performance liquid chromatography, PCA = patient-cont-rolled analgesia, QC = quality control, RSD = relative standard derivation, ADR = adverse reactions, PONV = postoperative nausea and vomiting, CIVA services = licensed central intravenous additive services, USP = United States Pharmacopoeia, SD = standard deviation.
PC and FC contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Pasero C, Quinlancolwell A, Rae D, et al. American Society for Pain Management Nursing Position Statement: prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs 2016;17:170–80. [DOI] [PubMed] [Google Scholar]
- [2].Kim DK, Yoon SH, Ji YK, et al. Comparison of the effects of sufentanil and fentanyl intravenous patient controlled analgesia after lumbar fusion. J Korean Neurosurg Soc 2017;60:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Comez M, Celik M, Dostbil A, et al. The effect of pre-emptive intravenous Dexketoprofen + thoracal epidural analgesia on the chronic post-thoracotomy pain. Int J Clin Exp Med 2015;8:8101–7. [PMC free article] [PubMed] [Google Scholar]
- [4].Ren BX, Zong J, Tang JC, et al. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet Mol Res 2015;14:5571–6. [DOI] [PubMed] [Google Scholar]
- [5].Li S, Min S, Wu B, et al. Application of patient-controlled intravenous analgesia of dezocine combined with sufentanil in burn patients after surgery. Zhonghua Shao Shang Za Zhi 2015;31:48–51. [PubMed] [Google Scholar]
- [6].Chen FC, Shi XY, Li P, et al. Stability of butorphanol-tropisetron mixtures in 0.9% sodium chloride injection for patient-controlled analgesia use. Medicine 2015;94:432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amiri S, Aminikhoei H, Hajmirzaian A, et al. Tropisetron attenuated the anxiogenic effects of social isolation by modulating nitrergic system and mitochondrial function. Biochim Biophys Acta 2015;12:2464–75. [DOI] [PubMed] [Google Scholar]
- [8].Shahid M, Manjula BP, Sunil BV. A comparative study of intravenous paracetamol and intravenous tramadol for postoperative analgesia in laparotomies. Anesth Essays Res 2015;9:314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abdolkarimi B, Zareifar S, Golestani ME, et al. Comparison effect of intravenous ketamine with pethidine for analgesia and sedation during bone marrow procedures in oncologic children: a randomized, double-blinded, cross over trial. Int J Hematol Oncol Stem Cell Res 2016;10:206–11. [PMC free article] [PubMed] [Google Scholar]
- [10].Kaufmann MA, Rosow C, Schnieper P, et al. Prophylactic antiemetic therapy with patient-controlled analgesia: a double-blind, placebo-controlled comparison of droperidol, metoclopramide, and tropisetron. Anesth Analg 1994;78:988–94. [DOI] [PubMed] [Google Scholar]
- [11].Shuailong, Anmei Comparison of dezocine and tropisetron for the control of maternal shivering undergoing spinal anesthesia—-a randomized double-blind trail. Rev Global Acad 2014;4:343–5. [Google Scholar]
- [12].Wang C, Li L, Shen B, et al. A multicenter randomized double-blind prospective study of the postoperative patient controlled intravenous analgesia effects of dezocine in elderly patients. Int J Clin Exp Med 2014;7:530–9. [PMC free article] [PubMed] [Google Scholar]
- [13].Chen F, Xiong H, Yang J, et al. Butorphanol and ketamine combined in infusion solutions for patient-controlled analgesia administration: a long-term stability study. Med Sci Monitor 2015;21:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen FC, Zhu J, Li B, et al. Stability of tramadol with three 5-HT3 receptor antagonists in polyolefin bags for patient-controlled delivery systems. Drug Des Dev Ther 2016;10:1869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen FC, Fang BX, Li P, et al. Compatibility of butorphanol and droperidol in 0.9% sodium chloride injection. Am J Health Syst Pharm 2013;70:515–9. [DOI] [PubMed] [Google Scholar]
- [16].Lebitasy M, Hecq JD, Vanbeckbergen D, et al. Long-term stability of tramadol hydrochloride and droperidol mixture in 5% dextrose infusion polyolefin bags at 5+/-3 degrees C. Ann Pharm Fr 2009;67:272–7. [DOI] [PubMed] [Google Scholar]
- [17].Fang BX, Wang LH, Liu HM, et al. Stability study of dezocine in 0.9% sodium chloride solutions for patient-controlled analgesia administration. Medicine 2017;96:e7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Georget S, Vigneron J, Blaise N, et al. Stability of refrigerated and frozen solutions of tropisetron in either polyvinylchloride or polyolefin infusion bags. J Clin Pharm Ther 2010;22:257–60. [DOI] [PubMed] [Google Scholar]
- [19].Brigas F, Sautou-Miranda V, Normand B, et al. Compatibility of tropisetron with glass and plastics. Stability under different storage conditions. J Pharm Pharmacol 2011;50:407–11. [DOI] [PubMed] [Google Scholar]
- [20].Fang BX, Li P, Shi XY, et al. Incompatibilities of lornoxicam with 4 antiemetic medications in polyolefin bags during simulated intravenous administration. Medicine 2016;95:e3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chapalain-Pargade S, Laville I, Paci A, et al. Microbiological and physicochemical stability of fentanyl and sufentanil solutions for patient-controlled delivery systems. J Pain Symptom Manag 2006;32:90–7. [DOI] [PubMed] [Google Scholar]
- [22].Lee DK, Wang DP, Harsono R, et al. Compatibility of fentanyl citrate, ketamine hydrochloride, and droperidol in 0.9% sodium chloride injection stored in polyvinyl chloride bags. Am J Health Syst Pharm 2005;62:1190–2. [DOI] [PubMed] [Google Scholar]
- [23].Chen FC. Compatibility and stability of lornoxicam with morphine, tramadol or fentanyl in infusion solutions. Afr J Pharm Pharmaco 2012;6:2055–60. [Google Scholar]
- [24].Fang B, Wang L, Gu J, et al. Physicochemical stability of ternary admixtures of butorphanol, ketamine, and droperidol in polyolefin bags for patient-controlled analgesia use. Drug Design Devel Ther 2016;10:3873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhou X, Zhang C, Wang M, et al. Dezocine for preventing postoperative pain: a meta-analysis of randomized controlled trials. PLoS One 2015;10:e0136091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu HX, Yao JC, Song HY, et al. Compatible changes of dezocineinjection mixed with ketorolac tromenthamine injection and the analgesic test. Chin Hosp Pharm J 2013;33:2050–3. [Google Scholar]
- [27].Xu JG, Luo AL, Wu XM, et al. Analgesic expert consensus of dezocine. J Clin Anesthesiol 2013;29:921–3. [Google Scholar]
- [28].Dong Y, Geng X, Liu J, et al. Compatible stability of ketorolac tromethamine and dezocine injection. Chin Pharmaco Aff 2016;30:272–5. [Google Scholar]
- [29].Chen FC, Wang LH, Guo J, B X, et al. Simultaneous determination of dexamethasone, ondansetron, granisetron, tropisetron, and azasetron in infusion samples by HPLC with DAD detection. J Anal Methods Chem 2017;2017:6749087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun S, Schaller J, Placek J, et al. Compatibility of intravenous fosaprepitant with intravenous 5-HT3 antagonists and corticosteroids. Cancer Chemo Ther Pharmacol 2013;72:509–13. [DOI] [PubMed] [Google Scholar]
- [31].Fang BX, Zhu J, Chen FC, et al. Stability of butorphanol tartrate injection with lornoxicam injection in patient controlled analgesia pump. Cent South Pharm 2013;11:732–4. [Google Scholar]
- [32].Trissel LA, Saenz CA, Ogundele AB, et al. Physical compatibility of pemetrexed disodium with other drugs during simulated Y-site administration. Am J Health Syst Pharm 2004;61:2289–93. [DOI] [PubMed] [Google Scholar]
- [33].Selbach S, Diederich WE, Fett S, et al. Stability-indicating HPLC assays for the determination of piritramide and droperidol in PCA solution. J Clin Pharm Ther 2011;36:161–5. [DOI] [PubMed] [Google Scholar]
- [34].Knudsen L, Eisend S, Haake N, et al. Physicochemical compatibility of commonly used analgesics and sedatives in the intensive care medicine. Eur J Hosp Pharm 2014;21:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Donnely RF, Willman E, Andolfatto G. Stability of ketamine-propofol mixtures for procedural sedation and analgesia in the emergency depart-ment. Can J Hosp Pharm 2008;61:426–30. [Google Scholar]


