Abstract
The inflammation-based Glasgow Prognostic Score (GPS), which involves C-reactive protein and serum albumin levels, has been reported to be a strong independent predictor of mortality in many cancers. This study aimed to investigate whether the GPS is associated with mortality in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (pPCI).
In this study, 406 consecutive patients with STEMI at our emergency department (ED) who were undergoing pPCI were prospectively enrolled and assigned a GPS of 0, 1, or 2. Kaplan–Meier survival and multivariable Cox regression analyses were used to evaluate the associations between the GPS and long-term mortality.
Twenty-three patients (5.7%) died at the hospital, and 37 (9.7%) died during follow-up (14.4 [9.3–17.6] months). Compared with patients with a lower GPS, those with a higher GPS had significantly higher in-hospital mortality (GPS = 0 vs GPS = 1 vs GPS = 2: 3.3% vs 6.3% vs 28.0%, P < .001), follow-up mortality (4.6% vs 14.3% vs 55.6%, P < .001), and cumulative mortality (9.6% vs 21.1% vs 71.1%, P < .001). Multivariable Cox regression analysis revealed that in patients with a GPS of 1 and 2 (versus 0), the multivariable adjusted hazard ratios (HR) for all-cause mortality were 2.068 (95% CI: 1.082–3.951, P = .028) and 8.305 (95% CI: 4.017–17.171, P < .001), respectively, after controlling for all of the confounding factors. Subgroup analysis showed that a higher GPS was associated with an increased risk of cumulative mortality in the different subgroups.
The GPS on admission may be useful for stratifying the risk of adverse outcomes in patients with STEMI undergoing pPCI in the ED.
Keywords: acute myocardial infarction, biomarkers, Glasgow Prognostic Score, mortality
1. Introduction
Ischemic cardiovascular disease is one of the most common causes of death and is accountable for up to 20% of all deaths.[1] Despite the progress in the techniques of percutaneous coronary intervention (PCI) and seasonable revascularization,[2] the in-hospital mortality rate is still 4% to 12%, and the first-year death rate is about 10% [1] in patients with acute ST-segment elevation myocardial infarction (STEMI). Although traditional risk factors, such as old age, female sex, and diabetes, can be used to predict adverse outcomes of patients with STEMI,[3–6] they are not capable of assessing full nature and severity of the STEMI. Hence, there is great interest in identifying simple and quick bedside biomarkers that could assist in predicting STEMI outcomes on admission so that appropriate treatment can be implemented. Coronary atherosclerosis is the main cause of STEMI, and one of the most important pathophysiological factors is inflammation.[7,8] Patients with more severe inflammation were found to have more vulnerable plaques than those with less severe inflammation.[9] Inflammation also consistently acts on the whole process of cancer, including its occurrence and development.[10,11] Previous studies have demonstrated that more severe inflammation is associated with more advanced cancer and a poorer prognosis.[12] Several common inflammatory markers in acute myocardial infarction (AMI) and different cancers are associated with prognosis, suggesting that these diseases have a similar inflammation-mediated pathophysiological mechanisms.[13–19]
The inflammation-based Glasgow Prognostic Score (GPS), which is composed of C-reactive protein (CRP) and serum albumin (SA), has been reported as a strong independent predictor of long-term mortality in many tumor diseases.[20] CRP and SA can be used to predict the adverse outcome of AMI,[17,21] and examination of these parameters is sensitive, specific, and repeatable at a low cost. However, there is a paucity of relevant research showing that the combination of those 2 parameters or GPS alone has the same or a stronger predictive power in patients with STEMI than does CRP or SA alone. Hence, this study was conducted to confirm whether the GPS is also associated with mortality in patients with acute STEMI undergoing primary percutaneous coronary intervention (pPCI).
2. Methods
This single-center, prospective cohort study was designed to evaluate whether the GPS could predict in-hospital and long-term mortality in patients with STEMI. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Human Ethical Committee of West China Hospital of Sichuan University. All study subjects provided written informed consent.
2.1. Patient selection
On the basis of our pre-experiment, the mortality of STEMI patients with a GPS of 0 was about 50% and that of those with a GPS of 2 was about 5%. To satisfy this difference with 80% power and 5% significance (2-tailed), 15 patients were required. Because STEMI patients with a GPS of 2 account for about 5% of our cohort, we planned to recruit 300 patients with STEMI in this study. The sample size was calculated using MedCalc Statistical Software version 15.2.2 (https://www.medcalc.org). From May 2016 to September 2017, 406 patients who were diagnosed as having spontaneous STEMI (type 1), according to the Third Universal Definition of Myocardial Infarction,[22] and were admitted to the Emergency Department of the West China Hospital were recruited. Inclusion criteria were onset of STEMI less than 12 hours before admission, first admission after the onset of STEMI symptoms, and patients who voluntarily signed the informed consent. Exclusion criteria were a history of neoplasm, cirrhosis, nephrotic syndrome, autoimmune disease, or systemic inflammatory disease; recent infectious disease, eating disorder, or surgery; patients who refused PCI or received coronary artery bypass grafting; and patients diagnosed as having non-spontaneous STEMI after PCI (Fig. 1).
Figure 1.
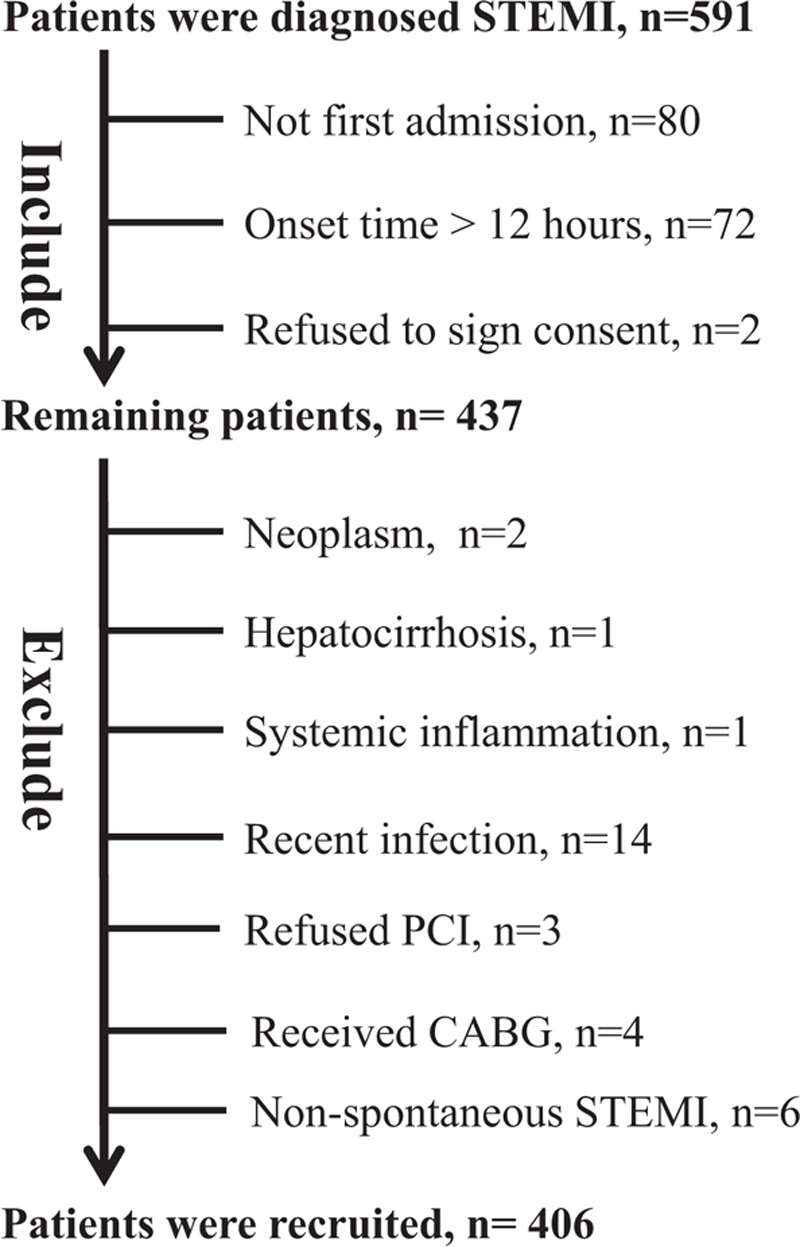
Flow diagram for recruitment of patients.
2.2. Data collection
First, an electrocardiogram was promptly obtained on admission using an electrocardiograph (iMAC1200, Wuhan Zoncare Bio-Medical, Hubei Sheng, China). Second, we recorded patients’ vital signs, medical history, and Killip class. Then, we collected data from laboratory examination, including the hemoglobin level, white blood cell count (WBC), neutrophil count, lymphocyte count, and platelet count, which were analyzed using an automated hematology analysis system (LH750, Beckman Coulter Inc., Brea, CA). The fibrinogen level was measured using a Sysmex CA-7000 analyzer (Siemens Healthcare Diagnostics, Eschborn, Germany); levels of creatinine kinase, albumin, blood urea nitrogen (BUN), creatinine, high-density lipoprotein, triglyceride, and total cholesterol (TC) were analyzed using an Architect c16000 analyzer (Abbott Diagnostics, Dallas, TX); the CRP level was determined using a Cobas S6000 Hitachi analyzer (Roche Diagnostics, Indianapolis, IN); the levels of creatine kinase-myocardial band isoenzyme, cardiac troponin T (cTnT), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were analyzed using an immunology analyzer (Cobas E601, Roche Diagnostics) with the electrochemiluminescence method; and the urine protein level was analyzed using a UF1000 urinalysis analyzer (Sysmex Corporation, Kobe, Japan). The left ventricular ejection fraction (LVEF) was calculated using the biplane Simpson method with the Philips E33 echocardiography machine (Philips Medical Systems, Bothell, WA). Data on coronary artery involvement were obtained after PCI. Finally, The Global Registry of Acute Coronary Events (GRACE),[23] Gensini [24] scores, prognostic nutritional index (PNI),[25] and postoperative atrial fibrillation (AF) were calculated.
2.3. Percutaneous coronary intervention and treatment
All patients with onset of STEMI <12 hours received a loading dose of aspirin (300 mg) and/or clopidogrel (300–600 mg) on admission. All PCI procedures were performed by experienced interventional cardiologists who used a transradial approach and drug-eluting stents. Subsequently, all patients received a daily dose of aspirin (100 mg), clopidogrel (75 mg), and antilipoid (10–20 mg) if there were no contraindications. Other medications, such as a β-blocker, calcium-channel blocker, angiotensin-converting enzyme inhibitor, and angiotensin II receptor blocker, were given on the basis of each patient's condition. Finally, we administered corresponding medication for different diseases, such as diabetes, hypertension, cardiac insufficiency, arrhythmia, and postoperative infection.
2.4. Definition of the Glasgow Prognostic Score
Briefly, patients with an increased CRP level (>10 mg/L) and low SA level (<35 g/L, hypoalbuminemia) were assigned a GPS of 2. Patients with only one of these biochemical abnormalities were allocated a GPS of 1. Patients with neither of these abnormalities were assigned a GPS of 0.[20]
2.5. Follow-up and primary endpoint
The primary endpoint was all-cause death. Data on clinical characteristics and in-hospital outcomes were collected during patient revisits to the hospital. If patients were not readmitted or could not be contacted directly, their relatives were interviewed in person or over the telephone. Finally, we plan to complete a five-year follow-up.
2.6. Statistical analysis
Data were calculated as frequencies and percentages for categorical variables and as mean ± standard deviation or median with interquartile range (25th–75th percentile) for continuous variables. Patient characteristics were compared according to the GPS. Distribution of data was assessed by P–P (probability–probability or percent–percent) plot in SPSS research. If the mean accurately represented the center of distribution, the parametric test was considered. If the median suitably represented the center of distribution, the nonparametric test was considered. Parametric patient characteristics were compared using one-way analysis of variance, whereas nonparametric characteristics were compared using the Kruskal–Wallis test. Categorical data were compared using the chi-square (2) test. The receiver operating characteristic (ROC) curve contrast of the GPS, CRP level, SA level, cTnT level, NT-proBNP level, Gensini score, GRACE score, and PNI was evaluated according to the area under the curve (AUC). Stratified Kaplan–Meier curves were constructed on the basis of the GPS. Cox proportional hazard models were employed to determine whether the GPS was related to time to mortality during the study period. To construct the Cox model, univariate models for each predictive variable were used, with mortality as the outcome variable. Moreover, the variables that were significant (P < .05) in the univariate Cox models were entered into a multivariable Cox model. From the multivariable model, we identified variables that were significant (P < .05) predictors of mortality. In a different subgroup analysis, patients were grouped according to age, heart rate, Killip class, creatinine level, BUN level, LVEF, Gensini score, GRACE score, and cumulative survival of the GPS calculated by Kaplan–Meier survival analysis. Data analysis was performed using SPSS Statistics for Windows, version 22.0 (SPSS, Inc., Chicago, IL).
3. Results
3.1. Baseline patient characteristics
Overall, 406 consecutive patients with STEMI (mean age, 62.63 ± 12.98 years; 308 men) were included in the study. Of these patients, 23 (5.7%) died while hospitalized, and 37 (9.7%) died during the follow-up period (median follow-up time, 14.4 [9.3–17.6] months). Of these, 269 patients (66.2%) had a GPS of 0, 112 (27.6%) had a GPS of 1, and 25 (6.2%) had a GPS of 2 on admission.
Table 1 shows the baseline characteristics according to the GPS. Compared to patients with a lower GPS, those with a higher GPS were older (P < .001) and more likely to have a lower body mass index (P < .021), quicker heart rate (P < .001), higher frequency of a Killip class ≥2 (P < .001), higher WBC count (P < .001), higher neutrophil count (P = .001), lower lymphocyte count (P = .008), lower hemoglobin level (P < .001), higher fibrinogen level (P < .001), higher blood glucose level (P = .009), higher creatinine level (P = .017), higher BUN level (P < .001), higher triglyceride level (P = .002), higher TC level (P < .001), higher NT-proBNP level (P < .001), higher cTnT level (P < .001), lower LVEF (P < .001) on admission, less affected left anterior descending artery (P = .001), less affected left circumflex artery (P = .001), less affected right coronary artery (P < .003), and more affected left main coronary artery (P = .001). Other clinical characteristics did not vary significantly by the GPS (Table 1).
Table 1.
Relationships between clinical characteristics and the Glasgow Prognostic Score (GPS) in patients with ST-segment elevation myocardial infarction.

3.2. GPS and other risk factors
In the ROC curve analyses, the AUC of the GPS (0.846, 95% confidence interval [CI]: 0.759–0.925, P < .001) was similar to that of the GRACE score (0.849, 95% CI: 0.762–0.931, P < .001) but larger than that of the SA level (0.272, 95% CI: 0.201–0.346, P = .001), CRP level (0.811, 95% CI: 0.763–0.941, P < .001), NT-proBNP level (0.822, 95% CI: 0.765–0.931, P < .001), cTnT level (0.715, 95% CI: 0.603–0.814, P = .001), Gensini score (0.746, 95% CI: 0.667–0.826, P < .001), and PNI (0.775, 95% CI: 0.689–0.861, P < .001).
Compared with patients with a lower GPS, those with a higher GPS had higher GRACE and Gensini scores. For those with GPSs of 0, 1, and 2, the respective mean GRACE scores were 152.69 ± 38.89, 171.44 ± 44.06, and 209.48 ± 32.03 (P < .001), and the respective mean Gensini scores were 74.58 ± 51.25, 77.06 ± 52.29, and 124.29 ± 71.45 (P < .001) (Fig. 2A and B). In total, 26 patients (6.4%) developed postoperative AF. For those with GPSs of 0, 1, and 2, the number of patients with AF were 19 (7.1%), 11 (9.8%), and 8 (32.0%), respectively (P < .001) (Fig. 2C).
Figure 2.
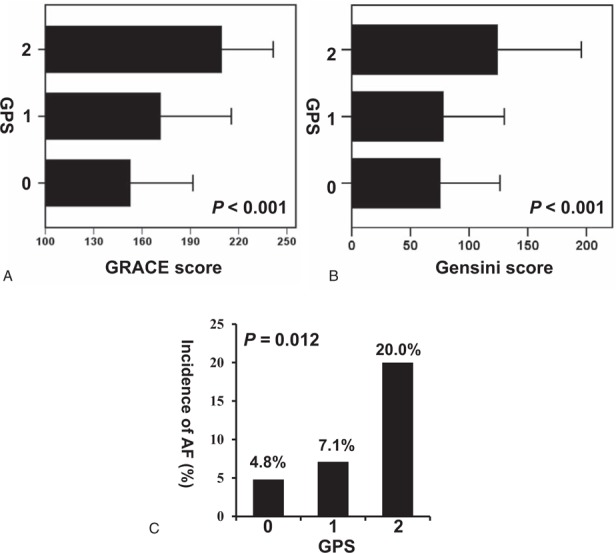
(A) The Global Registry of Acute Coronary Events (GRACE) score, (B) Gensini score, and (C) postoperative incidence of atrial fibrillation (AF) in different Glasgow Prognostic Score (GPS). AF = atrial fibrillation, GRACE = Global Registry of Acute Coronary Events, GPS = Glasgow Prognostic Score.
3.3. GPS and all-cause mortality
The in-hospital mortality (GPS = 0 vs GPS = 1 vs GPS = 2: 3.3% vs 6.3% vs 28.0%, P < .001) (Fig. 3A) and follow-up mortality (4.6% vs 14.3% vs 55.6%, P < .001) (Fig. 3B) of patients gradually increased with an increase in the GPS. The cumulative mortality was consistently more significant (9.6% vs 21.1% vs 71.1%, P < .001) in patients with a higher GPS than in those with a lower GPS (Fig. 4).
Figure 3.
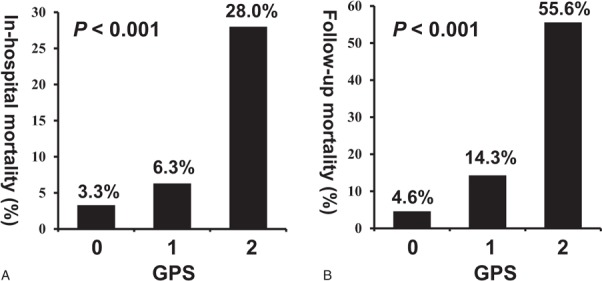
(A) In-hospital and (B) follow-up mortality in different Glasgow Prognostic Score (GPS) patients with ST-segment elevation myocardial infarction. GPS = Glasgow Prognostic Score.
Figure 4.
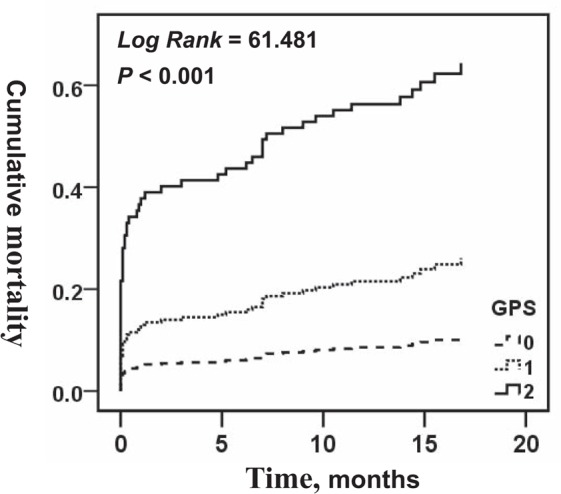
Kaplan–Meier analysis survival curve according to different Glasgow Prognostic Score (GPS) in patients with ST-segment elevation myocardial infarction. GPS = Glasgow Prognostic Score.
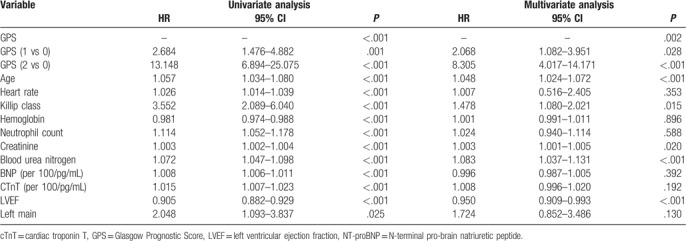
Univariate Cox models indicated that the GPS was positively associated with the hazard of all-cause mortality, and other variables were significant in the univariate Cox models. The multivariable Cox regression analysis revealed that in patients with a GPS of 1 and 2 (versus 0), the multivariable adjusted hazard ratios (HR) for all-cause mortality were 2.068 (95% CI: 1.082–3.951, P = .028) and 8.305 (95% CI: 4.017–17.171, P < .001), respectively, after controlling for all of the confounding factors (Table 2). Other predictors of all-cause mortality in the multivariable Cox model were age, the Killip class, BUN level, creatinine level, and LVEF (Table 2).
Table 2.
Cox regression of all-cause mortality for patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention.
3.4. Subgroup analysis
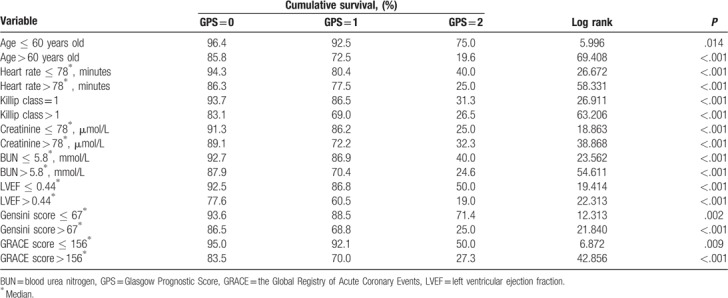
Subgroup analysis of the cumulative survival rate was based on the age, heart rate, Killip class, creatinine level, BUN level, LVEF, Gensini score, and GRACE score of patients with STEMI by the GPS. The GPS remained an independent predictor of mortality on subgroup analysis, and all high-risk subgroups had a higher mortality (Table 3).
Table 3.
Subgroup analysis of cumulative survival in patients with ST-segment elevation myocardial infarction by Glasgow Prognostic Score (GPS).
4. Discussion
Similar to other studies on cancers, our study showed that the GPS successfully predicted adverse outcomes in STEMI patients undergoing pPCI, and this was possibly based on the potential inflammatory mechanism in the whole course of AMI. In our study, patients with a higher GPS had higher in-hospital, follow-up, and cumulative mortalities. After adjusting for confounding factors, the increased GPS was a significant dominant factor of all-cause mortality. As we assumed, the GPS had a more convincing predictive capability than the SA, CRP, NT-proBNP, or cTnT level alone. Current research has revealed that PNI, as a nutritional marker combining SA and lymphocyte, is a prognostic predictor in patients with STEMI[25]; however, GPS seems to be stronger than PNI. Patients with a higher GPS had higher GRACE, Gensini scores, and incidence of postoperative AF, suggesting that the GPS could reflect the complex clinical baseline characteristics of patients, the severity of coronary atherosclerotic stenosis, and the inflammatory state which plays an important role in the myocardial remodeling process leading to AF.[26] In addition, the GPS had a stronger prognostic ability in the high-risk group than in the low-risk group; hence, we conclude that the GPS may be more predictive in critical patients. Despite timely reperfusion treatment, it is difficult for patients with a GPS of 2 to have a good prognosis (their median survival time was 3 months), indicating that traditional treatment is ineffective for such patients.
The formation of many cancers and coronary heart disease is accompanied by inflammation ;[10] subsequently, inflammation contributes to the whole disease progression.[7,11] Expectedly, destruction of the surrounding tissue by the growing tumor and ruptured atherosclerotic plaques lead to microcirculatory disturbance, followed by aggravated systemic inflammation.[7,27] In AMI, the inflammatory response can directly affect the transfer of lipoprotein within the vessel wall, increase the binding of lipoprotein to the endothelium and smooth muscle cells, induce deposition of lipid-laden foam cells in the subendothelial space, and accelerate atherosclerotic formation.[7,8] Inflammation plays a critical role in the instability of atherosclerotic plaque and the adhesion of thrombus to the surface of damaged plaques.[9] Furthermore, inflammation can attenuate myocardial ischemia-reperfusion injury, indicating that very severe inflammation possibly causes a larger infarction area.[28] In previous reports, many inflammation-related markers, such as the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, interleukin-6 level, CRP level, and SA level, were related to AMI [13–17] and certain cancers.[18,19] In our study, similar to those inflammatory markers, GPS, which includes the CRP and SA levels, could also reflect the inflammatory state and predict the survival time in STEMI on the basis of the inflammatory pathophysiological mechanism.
As one of the components of the GPS, SA is the main material that maintains plasma oncotic pressure and carries other massive materials and components in acute and chronic inflammatory reactions.[29,30] Furthermore, SA has antioxidant and anti-inflammatory properties; thus, hypoalbuminemia may result in deteriorating oxidative damage and inflammation.[31–34] SA stabilizes the completeness of the microvasculature and coactions on the endothelium and myocardium by its oncotic nature.[35,36] In addition, SA is the most sensitive indicator of one's nutritional status, which has a significant impact on the prognosis of cancer [37] and AMI.[25] Hypoproteinemia stimulates the synthesis of lipid and coagulation factors, leading to hyperlipidemia and hypercoagulability, which results in the formation of atherosclerotic plaques and thrombosis.[38] Moreover, hypoproteinemia may affect the outcome of reperfusion treatment [39] and anticancer therapy.[35]
As a sensitive and nonspecific biomarker of inflammation, CRP is a very important risk factor for AMI,[21] and an increased CRP level is negatively correlated with survival time, as reported in various cancers.[12] CRP induces peripheral blood monocytes to aggregate neutrophil and product tissue factor, activates the coagulation system, mediates enhanced expression of adhesion molecules, reduces nitric oxide production, and participates in the oxidative stress process, resulting in endothelial dysfunction and thrombus formation.[40,41] Additionally, CRP directly promotes the proliferation and tissue factor expression of endothelial cells and smooth muscle cells and induces the pro-atherothrombotic phenotype of the vascular wall cells.[42] Researchers have shown that adipokines, such as leptin, induce the production of CRP by coronary endothelial cells, increasing the local concentration of CRP, which directly promotes the expression of adhesion molecules and tissue factors and accelerates atherothrombosis in the coronary arteries.[43–45]
These concepts may be the potential common mechanisms of SA and CRP in patients with STEMI that lead to adverse survival (Fig. 5). Although the GPS effectively predicted the prognosis of AMI, it is based on only 2 markers. Recent studies reported that urotensin II and Pentraxin-3 are independent prognostic factors of AMI[46,47]; the pathophysiological mechanism associated with urotensin II is known to induce tissue factor expression and promote atherothrombosis, and to modulate vascular inflammation,[48,49] whereas the mechanism involving Pentraxin-3 may include inflammation and thrombosis.[47] Therefore, urotensin II and Pentraxin-3 combined with the GPS may improve the predictive power to STEMI. Further studies with large samples are needed to prove this theory.
Figure 5.
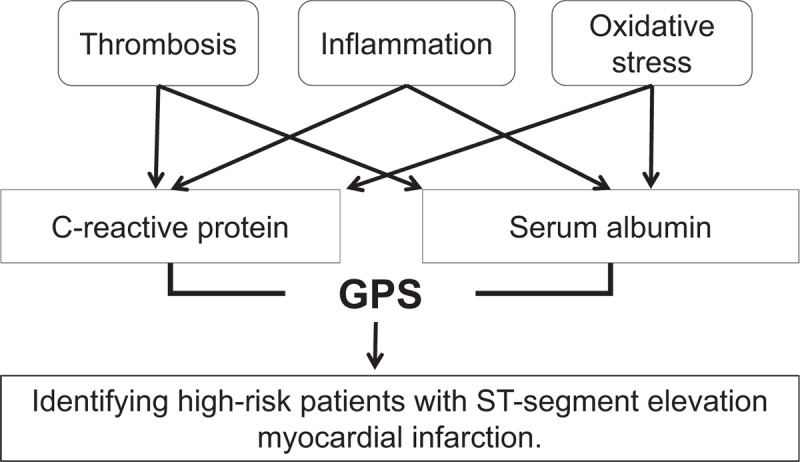
Potential pathophysiological mechanism of Glasgow Prognostic Score (GPS) in patients with ST-segment elevation myocardial infarction. GPS = Glasgow Prognostic Score.
4.1. Study limitations
Some limitations should be noted. First, this was a single-center study with a small sample size. Second, we only measured baseline SA and CRP levels, and the changes in these levels may provide an additional prognostic value. Third, we did not evaluate the complications and cardiovascular death of patients with STEMI. Fourth, the cut-off values of the SA and CRP levels were based on those for cancer; thus, they may not be optimal values for STEMI. Finally, future prospective studies are warranted to clarify whether the GPS is only a biomarker or whether it plays a pathophysiologic role in the course of STEMI.
5. Conclusions
The GPS is useful in reflecting patient's inflammatory status, and it is a powerful predictor of all-cause mortality of patients with STEMI undergoing pPCI. Therefore, the GPS may be used to risk stratify patients with STEMI in the emergency room by short-term and long-term outcomes at an early stage.
Author contributions
Conceptualization: Rui Zeng, Yu Cao, and Zhi Zeng.
Data curation: Yu Jia, Dongze Li, Yisong Cheng, Yongli Gao, Lei Xiao, and Zhi Wan.
Formal analysis: Yu Jia, and Dongze Li.
Methodology: Yu Jia, and Dongze Li.
Project administration: Rui Zeng, Yu Cao, and Zhi Zeng.
Supervision: Rui Zeng, Yu Cao, and Zhi Zeng.
Validation: Rui Zeng.
Writing - original draft: Yu Jia, Dongze Li, and Lin Zhang.
Writing - review & editing: Yu Jia, Dongze Li, and Zhi Wan.
Footnotes
Abbreviations: AF = atrial fibrillation, AMI = acute myocardial infarction, AUC = area under the curve, BUN = blood urea nitrogen, CI = confidence interval, CRP = C-reactive protein, cTnT = cardiac troponin T, ED = emergency department, GPS = Glasgow Prognostic Score, GRACE = Global Registry of Acute Coronary Events, HR = hazard ratios, LVEF = left ventricular ejection fraction, NT-proBNP = N-terminal pro-brain natriuretic peptide, PCI = percutaneous coronary intervention, PNI = prognostic nutritional index, pPCI = primary percutaneous coronary intervention, ROC = receiver operating characteristic, SA = serum albumin, STEMI = ST-segment elevation myocardial infarction, TC = total cholesterol, WBC = white blood cell count.
YJ and DL contributed equally to this work.
This work was supported financially by grants from the Science Foundation of Science and Technology Department of Chengdu (No. 2016-HM02-00099-SF), Science Foundation of Science and Technology Department of Sichuan (No. 2018RZ0139, 2018JY0577 and 2017SZ0190), Ministry of Science and Technology - National Key Projects (2017YFC0908702), and Health and Family Planning Commission of Sichuan Province (No.16PJ305).
The authors declare that there are no competing interests.
The authors have no conflicts of interest to disclose.
References
- [1].Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol 2017;70:1082. [DOI] [PubMed] [Google Scholar]
- [2].Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet (London, England) 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- [3].Pu J, Ding S, Shan P, et al. Comparison of epicardial and myocardial perfusions after primary coronary angioplasty for ST-elevation myocardial infarction in patients under and over 75 years of age. Aging Clin Exp Res 2010;22:295–302. [DOI] [PubMed] [Google Scholar]
- [4].Martino P, Domenico Z, Alessandro C, et al. Role of plasma glucose level on myocardial perfusion in ST-segment elevation myocardial infarction patients. J Diabetes Complications 2018;32:764–9. [DOI] [PubMed] [Google Scholar]
- [5].Kosmidou I, Redfors B, Selker HP, et al. Infarct size, left ventricular function, and prognosis in women compared to men after primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: results from an individual patient-level pooled analysis of 10 randomized trials. Eur Heart J 2017;38:1656–63. [DOI] [PubMed] [Google Scholar]
- [6].Pu J, Shan P, Ding S, et al. Gender differences in epicardial and tissue-level reperfusion in patients undergoing primary angioplasty for acute myocardial infarction. Atherosclerosis 2011;215:203–8. [DOI] [PubMed] [Google Scholar]
- [7].Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 2010;14:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wong BW, Meredith A, Lin D, et al. The biological role of inflammation in atherosclerosis. Can J Cardiol 2012;28:631–41. [DOI] [PubMed] [Google Scholar]
- [9].Zairis MN, Lyras AG, Bibis GP, et al. Association of inflammatory biomarkers and cardiac troponin I with multifocal activation of coronary artery tree in the setting of non-ST-elevation acute myocardial infarction. Atherosclerosis 2005;182:161–7. [DOI] [PubMed] [Google Scholar]
- [10].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [11].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep 2002;4:250–5. [DOI] [PubMed] [Google Scholar]
- [13].Machado GP, Araujo GN, Carpes CK, et al. Comparison of neutrophil-to-lymphocyte ratio and mean platelet volume in the prediction of adverse events after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Atherosclerosis 2018;274:212–7. [DOI] [PubMed] [Google Scholar]
- [14].Prondzinsky R, Unverzagt S, Lemm H, et al. Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol 2012;101:375–84. [DOI] [PubMed] [Google Scholar]
- [15].Sun XP, Li J, Zhu WW, et al. Platelet to lymphocyte ratio predicts contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology 2018;69:71–8. [DOI] [PubMed] [Google Scholar]
- [16].Wang Q, Ma J, Jiang Z, et al. Association of lymphocyte-to-monocyte ratio with in-hospital and long-term major adverse cardiac and cerebrovascular events in patients with ST-elevated myocardial infarction. Medicine 2017;96:e7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oduncu V, Erkol A, Karabay CY, et al. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron Artery Dis 2013;24:88–94. [DOI] [PubMed] [Google Scholar]
- [18].He X, Li JP, Liu XH, et al. Prognostic value of C-reactive protein/albumin ratio in predicting overall survival of Chinese cervical cancer patients overall survival: comparison among various inflammation based factors. J Cancer 2018;9:1877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dupre A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol 2018;44:566–70. [DOI] [PubMed] [Google Scholar]
- [20].Douglas E, McMillan DC. Towards a simple objective framework for the investigation and treatment of cancer cachexia: the Glasgow Prognostic Score. Cancer Treat Rev 2014;40:685–91. [DOI] [PubMed] [Google Scholar]
- [21].Fordjour PA, Wang Y, Shi Y, et al. Possible mechanisms of C-reactive protein mediated acute myocardial infarction. Eur J Pharmacol 2015;760:72–80. [DOI] [PubMed] [Google Scholar]
- [22].Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart 2012;7:275–95. [DOI] [PubMed] [Google Scholar]
- [23].Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163:2345–53. [DOI] [PubMed] [Google Scholar]
- [24].Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- [25].Keskin M, Hayiroglu MI, Keskin T, et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction, the prognostic nutritional index. Nutr Metab Cardiovasc Dis 2017;27:438–46. [DOI] [PubMed] [Google Scholar]
- [26].Bas HA, Aksoy F, Icli A, et al. The association of plasma oxidative status and inflammation with the development of atrial fibrillation in patients presenting with ST elevation myocardial infarction. Scand J Clin Lab Invest 2017;77:77–82. [DOI] [PubMed] [Google Scholar]
- [27].Hwang EC, Hwang IS, Yu HS, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol 2012;42:955–60. [DOI] [PubMed] [Google Scholar]
- [28].Yang M, Chen J, Zhao J, et al. Etanercept attenuates myocardial ischemia/reperfusion injury by decreasing inflammation and oxidative stress. PLoS One 2014;9:e108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39:S143–6. [DOI] [PubMed] [Google Scholar]
- [30].Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432–7. [DOI] [PubMed] [Google Scholar]
- [31].Lindahl B, Toss H, Siegbahn A, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group Fragmin during Instability in Coronary Artery Disease. N Engl J Med 2000;343:1139–47. [DOI] [PubMed] [Google Scholar]
- [32].Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci 2011;22:355–63. [DOI] [PubMed] [Google Scholar]
- [33].Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res 2002;55:820–9. [DOI] [PubMed] [Google Scholar]
- [34].Zoellner H, Hofler M, Beckmann R, et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci 1996;109:2571–80. [DOI] [PubMed] [Google Scholar]
- [35].Arques S, Ambrosi P. Human serum albumin in the clinical syndrome of heart failure. J Card Fail 2011;17:451–8. [DOI] [PubMed] [Google Scholar]
- [36].GK, Taylor AE, Owens LJ, et al. Effect of capillary pressure and plasma protein on development of pulmonary edema. Am J Physiol 1967;213:79–82. [DOI] [PubMed] [Google Scholar]
- [37].Moujaess E, Fakhoury M, Assi T, et al. The Therapeutic use of human albumin in cancer patients’ management. Crit Rev Oncol Hematol 2017;120:203–9. [DOI] [PubMed] [Google Scholar]
- [38].Liebeskind DS. Nephrotic syndrome. Handb Clin Neurol 2014;119:405–15. [DOI] [PubMed] [Google Scholar]
- [39].Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv 2008;72:950–7. [DOI] [PubMed] [Google Scholar]
- [40].Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood 1993;82:513–20. [PubMed] [Google Scholar]
- [41].Fujii H, Li SH, Szmitko PE, et al. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2006;26:2476–82. [DOI] [PubMed] [Google Scholar]
- [42].Cirillo P, Golino P, Calabrò P, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res 2005;68:47–55. [DOI] [PubMed] [Google Scholar]
- [43].De Rosa S, Cirillo P, Pacileo M, et al. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J Vasc Res 2009;46:609–17. [DOI] [PubMed] [Google Scholar]
- [44].Calabrò P, Cirillo P, Limongelli G, et al. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-κB-dependent pathway. J Vasc Res 2010;48:59–66. [DOI] [PubMed] [Google Scholar]
- [45].Cirillo P, Angri V, De Rosa S, et al. Pro-atherothrombotic effects of leptin in human coronary endothelial cells. Thromb Haemost 2010;103:1065–75. [DOI] [PubMed] [Google Scholar]
- [46].Khan SQ, Bhandari SS, Quinn P, et al. Urotensin II is raised in acute myocardial infarction and low levels predict risk of adverse clinical outcome in humans. Int J Cardiol 2007;117:323–8. [DOI] [PubMed] [Google Scholar]
- [47].Altay S, Çakmak HA, Kemaloğlu Öz T, et al. Long-term prognostic significance of pentraxin-3 in patients with acute myocardial infarction: 5-year prospective cohort study. Anatol J Cardiol 2017;17:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cirillo P, De Rosa S, Pacileo M, et al. Human urotensin II induces tissue factor and cellular adhesion molecules expression in human coronary endothelial cells: an emerging role for urotensin II in cardiovascular disease. J Thromb Haemost 2008;6:726–36. [DOI] [PubMed] [Google Scholar]
- [49].Lee JH, Park BK, Oh KS, et al. A urotensin II receptor antagonist, KR36676, decreases vascular remodeling and inflammation in experimental pulmonary hypertension. Int Immunopharmacol 2016;40:196–202. [DOI] [PubMed] [Google Scholar]


