Abstract
Rationale:
Acute kidney injury is common and correctable in patients with a hyperosmolar hyperglycemic state (HHS). Nevertheless, hyperglycemic crisis may also contribute to the development of rhabdomyolysis, which can worsen renal function and lead to high mortality in such patients.
Patient concerns:
Herein, we report a case of hyperosmolar hyperglycemic state-related rhabdomyolysis and acute renal failure with an excellent outcome.
Diagnosis:
A 26-year-old Asian female with underlying paranoid schizophrenia presented with newly diagnosed type 2 diabetes mellitus complicated with HHS. Her renal function deteriorated rapidly in spite of standard management for hyperglycemic crisis. Rhabdomyolysis was subsequently diagnosed according to the high levels of serum creatine kinase (CK) (37,710 U/L, normal range: 20–180 U/L) and myoglobin (5167.7 ng/mL, normal range: 14.3–65.8 ng/mL).
Interventions:
After treatment failure of intravenous hydration plus loop diuretic agent for rhabdomyolysis related acute renal failure, temporary hemodialysis was performed 3 times to relieve oligouria and pulmonary edema.
Outcomes:
Her renal function recovered well after temporary renal replacement therapy.
Lessons:
Rhabdomyolysis is a complication of HHS. Delayed detection can be fatal, and timely renal replacement therapy can result in an excellent prognosis. Therefore, it is crucial for clinicians to detect and treat such patients as early as possible to avoid impairing their renal function.
Keywords: hyperosmolar hyperglycemic state, rhabdomyolysis
1. Introduction
A hyperosmolar hyperglycemic state (HHS) is one of the most serious acute complications of diabetes. The rate of hospital admissions for HHS accounts for less than 1% of all primary diabetic admissions.[1] The mortality rate for patients with HHS is between 10% and 20%, which is approximately 10 times higher than that for diabetic ketoacidosis (DKA).[2]
Rhabdomyolysis, a term used to describe the rapid breakdown of striated muscle, is characterized by rupture and necrosis of muscle fibers. The classic triad of symptoms includes muscle pain, weakness, and dark urine, although more than 50% of patients do not complain of muscle pain or weakness.[3] Rhabdomyolysis is of clinical concern because it can cause acute renal failure, disseminated intravascular coagulation, cardiac arrest and arrhythmias, and significant electrolyte abnormalities, all of which can result in significant morbidity or mortality.[4]
About 20% of patients admitted for HHS have rhabdomyolysis, with an estimated mortality rate of about 35.5%.[5] The clinical features of rhabdomyolysis vary in severity from asymptomatic to acute renal failure requiring hemodialysis, and can be lethal if physicians ignore the possibility.
The clinical course between the 2 conditions is poorly understood. Herein, we report a case of acute renal failure following hyperosmolar hyperglycemic state-related rhabdomyolysis with an excellent outcome after a timely diagnosis and prompt treatment. The experience from this case may help physicians improve treatment and outcomes for such patients.
2. Case report
In December 2015, a patient with newly diagnosed diabetes mellitus and the acute complication of hyperglycemic crisis presented at Chang Gung Memorial Hospital. This patient was a 26-year-old Asian female with underlying paranoid schizophrenia for which she was under routine medical treatment (risperidone, trihexyphenidyl, and clonazepam). She was also obese, with a body mass index of about 31.99 (body weight: 85 kg, body height: 163 cm). A few days before admission, she became irritable, experienced polydipsia, consumed many beverages and subsequently suffered from nausea, vomiting, and loss of appetite. Her status of auditory hallucinations, slurred speech, and bilateral hand tremors had not changed. Finally, she developed profound general weakness and was transferred to our emergency department for treatment. A physical examination revealed no specific abnormalities, except for dry oral mucosa, obese appearance, and mild fever. Hyperglycemia (1408 mg/dl glucose), hyperosmolarity (osmolality = 395 mosm/kgH2O), positive serum ketone body without metabolic acidosis (Table 1) were noted, and HHS with ketosis was diagnosed. Prompt, aggressive fluid replacement, continuous insulin infusion (0.1 IU/kg/h) and potassium correction were started. Because she had a mild fever, empiric ceftriaxone was prescribed to cover bacterial infection and a subsequent switch to levofloxacin was made to cover any possible atypical pathogen infections. No bacterial infection was detected, and she presented with a fairly stable hemodynamic status and only mild fever. Hence, sepsis-related hyperglycemic crisis was not suspected. Unfortunately, her renal function deteriorated rapidly in spite of standard management for hyperglycemic crisis. A series of tests were performed to re-evaluate the unusual clinical course. Thyroid function and atypical infection markers were normal. Sonography revealed a moderately fatty liver and the presence of ascites but no liver abscess or signs of abnormal biliary tract obstruction. Abnormally high levels of serum creatine kinase (CK) (37,710 U/L, normal range: 20–180 U/L) and myoglobin (5167.7 ng/mL, normal range: 14.3–65.8 ng/mL) were noted on hospital day 3; hence, rhabdomyolysis was suspected (Table 1). Her serum creatinine (Cr) level was elevated together with decreasing urine volume and dependent edema; even though her blood sugar had returned to a relatively safe range of 200–300 mg/dl on day 5 (Fig. 1). Gentle intravenous hydration and a loop diuretic medication (furosemide) were started to promote passive diuresis and clearance of heme pigment casts produced by rhabdomyolysis. An osmotic diuretic agent (mannitol) was not used because of the risk of end organ damage induced by increased hyperosmolarity during the hyperglycemic crisis. Her renal function responded poorly to treatment, with persistent oliguria (<1000 mL/day), pitting edema and signs of pulmonary congestion. On day 5, temporary hemodialysis was begun following consultation with a nephrologist. After hemodialysis, her urine volume gradually increased to more than 2500 mL/day. We enhanced volume expansion to avoid secondary kidney injury during the diuretic phase after acute renal failure. Hemodialysis was performed 3 times (on days 5, 7, and 10) and her renal function recovered well after stopping renal replacement therapy (Fig. 1). The intravenous fluid supplementation was stopped on day 15. Her serum CK level decreased to 173 U/L on day 17, and serum blood urea nitrogen (BUN) and Cr levels were normal on day 24 (13.2 mg/dL and 0.83 mg/dL, respectively). Finally, because of suspected type 2 diabetes rather than type 1 according to her obese appearance, family history of type 2 diabetes and absence of ketoacidosis during the hyperglycemic crisis, her diabetes treatment was changed from multiple insulin injections to oral antidiabetic agents (repaglinide and linagliptin), with an average blood sugar level of 120–180 mg/dL. She was then discharged.
Table 1.
Biochemistry.
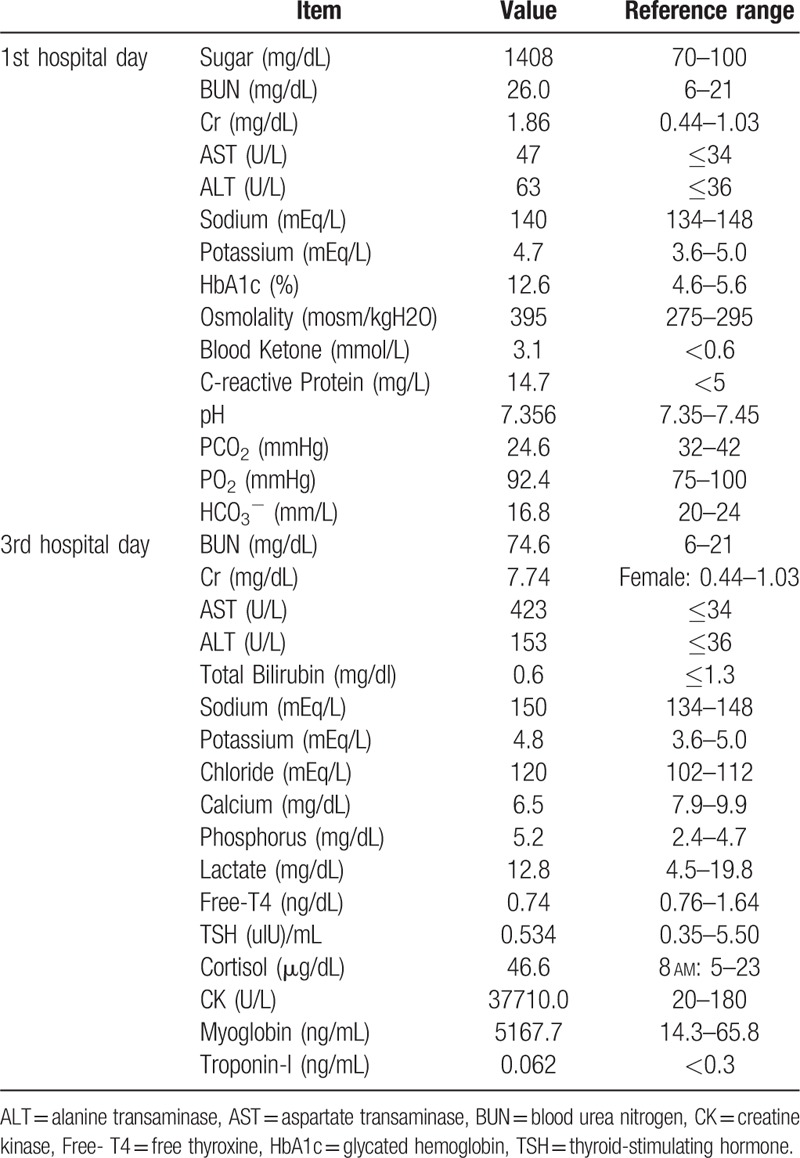
Figure 1.
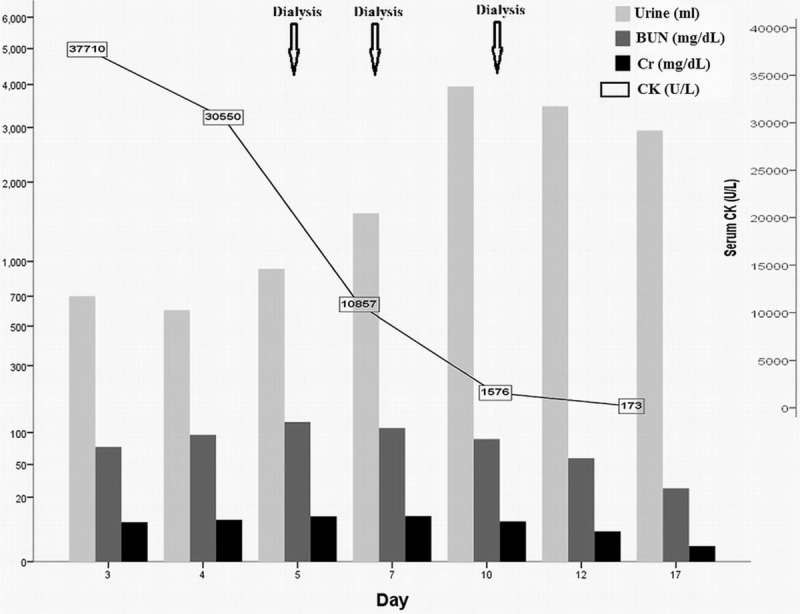
Clinical course of renal recovery of hyperosmolar hyperglycemic state-related rhabdomyolysis. Hemodialysis was started because of oliguria (urine amount < 1000 cc/day) and acute renal failure. Serum creatine kinase (CK) and creatinine (Cr) levels decreased rapidly after three hemodialysis sessions and urine volume gradually increased. BUN = Blood urea nitrogen, CK = creatine kinase, Cr = creatinine.
We have obtained informed consent from the patient and her parents for reporting this case. The authors thank the patient and her parents for agreeing to participate in the report and for providing the detailed medical history.
3. Discussion
Acute kidney injury is common and correctable in patients with a hyperglycemic crisis. In a study by Singhal et al, the incidence of acute renal failure in patients with diabetic crisis (DKA and HHS) was 25%.[6] As dehydration is more severe in patients with HHS than in DKA, the incidence of acute renal failure in HHS may be higher.[1] The cause of kidney injury is most often volume depletion, which can be reversed by adequate volume expansion, and renal replacement therapy is seldom necessary.[7]
In our case, the patient's urine output declined in spite of fluid resuscitation, and she subsequently suffered from pulmonary congestion and dependent edema. Careful re-evaluation of the etiology of acute renal failure is critical to allow for proper therapy of HHS with an unstable clinical course following standard treatment. In our case, continuing intravenous hydration alone would have resulted in inevitable respiratory failure because of pulmonary edema. Fortunately, rhabdomyolysis was diagnosed immediately, and her renal function had an excellent prognosis after temporary hemodialysis therapy. The common causes of rhabdomyolysis include trauma, extreme exertion, epilepsy, and toxin-related myopathy (such as that caused by alcohol and statins).[8] Occasionally, hyperglycemic crisis can contribute to the development of rhabdomyolysis.[9–12] Inadequate muscle energy storage, hyperosmolarity, or severe hypophosphatemia during hyperglycemic crisis are thought to be associated with rhabdomyolysis, however, the mechanism is not well understood.[9–12] If physicians ignore this rare complication of HHS, then the risk of mortality for patients with HHS is increased.[5]
In our case, her critical condition, including pulmonary edema and electrolyte imbalance, was reversed after prompt hemodialysis following HHS and rhabdomyolysis-related oligouria and acute renal failure. Her renal function improved after heme pigment casts were removed by dialysis and tubular obstruction was relieved. Urine volume, as well as serum Cr and CK levels, were restored to normal range, and temporary hemodialysis was successfully stopped. In such cases, another etiology of rhabdomyolysis that should be considered is neuroleptic malignant syndrome caused by antipsychotic medications.[13] However, the dosages of antipsychotic medications in our case had not changed before the onset of HHS and rhabdomyolysis, and no limb rigidity or seizures were observed. Therefore, neuroleptic malignant syndrome was not suspected, and her antipsychotic medications were continued throughout the clinical course after consultation with a psychiatrist.
4. Conclusion
In conclusion, rhabdomyolysis is not an uncommon complication of HHS, but delayed detection can be fatal. The early recognition of rhabdomyolysis, followed by prompt fluid resuscitation and timely renal replacement therapy can result in an excellent prognosis of survival and renal recovery.
Author contributions
Data curation: Cheng-Wei Lin.
Supervision: Cheng-Wei Lin.
Writing – original draft: I-Wen Chen.
Writing – review & editing: Cheng-Wei Lin.
I-Wen Chen orcid: 0000-0002-8928-6417.
Footnotes
Abbreviations: CK = creatine kinase, Cr = creatinine, DKA = diabetic ketoacidosis, HHS = hyperosmolar hyperglycemic state.
The authors have no conflicts of interest to disclose.
References
- [1].Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014;37:3124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med 2010;48:749–56. [DOI] [PubMed] [Google Scholar]
- [4].Mercer S, Hanks L, Ashraf A. Rhabdomyolysis in pediatric patients with diabetic ketoacidosis or hyperglycemic hyperosmolar state: a case series. Glob Pediatr Health 2016;3: 2333794X16671391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang LM, Tsai ST, Ho LT, et al. Rhabdomyolysis in diabetic emergencies. Diabetes Res Clin Pract 1994;26:209–14. [DOI] [PubMed] [Google Scholar]
- [6].Singhal PC, Abramovici M, Ayer S, et al. Determinants of rhabdomyolysis in the diabetic state. Am J Nephrol 1991;11:447–50. [DOI] [PubMed] [Google Scholar]
- [7].Orban JC, Maiziere EM, Ghaddab A, et al. Incidence and characteristics of acute kidney injury in severe diabetic ketoacidosis. PLoS One 2014;9:e110925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cervellin G, Comelli I, Benatti M, et al. Non-traumatic rhabdomyolysis: background, laboratory features, and acute clinical management. Clin Biochem 2017;50:656–62. [DOI] [PubMed] [Google Scholar]
- [9].Rosa EC, Lopes AC, Filho AWL, et al. Rhabdomyolysis due to hyperosmolarity leading to acute renal failure. Ren Fail 2009;19:295–301. [DOI] [PubMed] [Google Scholar]
- [10].Al-Matrafi J, Vethamuthu J, Feber J. Severe acute renal failure in a patient with diabetic ketoacidosis. Saudi J Kidney Dis Transpl 2009;20:831–4. [PubMed] [Google Scholar]
- [11].Huang Z, Xu L, Li F, et al. Fulminant type 1 diabetes mellitus with rhabdomyolysis: have we overlooked the situation? Diabetes Res Clin Pract 2010;90:e47–9. [DOI] [PubMed] [Google Scholar]
- [12].Ka T, Takahashi S, Tsutsumi Z, et al. Hyperosmolar non-ketotic diabetic syndrome associated with rhabdomyolysis and acute renal failure: a case report and review of literature. Diabetes Nutr Metab 2003;16:317–22. [PubMed] [Google Scholar]
- [13].Sa YK, Yang H, Jung HK, et al. Olanzapine-induced diabetic ketoacidosis and neuroleptic malignant syndrome with rhabdomyolysis: a case report. Endocrinol Metab (Seoul) 2013;28:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


