Abstract
Background:
To perform a meta-analysis of retrospective studies exploring the association of C-reactive protein to albumin (CAR) with overall survival (OS) in patients with lung cancer.
Methods:
Relevant studies were enrolled by searching databases of PubMed, Cochrane Library, Web of Science, and Embase were searched until July 16, 2017. We combined the hazard ratios (HRs) and 95% confidence intervals (CIs) to assess the correlation between CAR and OS in patients with lung cancer
Results:
Four studies involving 1257 participants from several countries were involved in the meta-analysis. In a pooled analysis of all studies, elevated CAR predicted poor OS (HR: 2.13; 95% CI: 1.52–2.97; P < .05). Subgroup analysis showed that high level of CAR predicted poor OS in patients with lung cancer though multivariate analyses on 1092 participants (HR: 1.63; 95% CI: 1.24–2.51; P < .001) and the heterogeneity decreased to 45.4%. Moreover, a similar trend was observed in patients receiving surgery (HR: 2.64; 95% CI: 2.08–3.35; P < .001) and chemotherapy (HR: 1.75; 95% CI: 1.93–2.57; P = .004). And the HRs for patients receiving surgery was moderately higher than that for patients receiving chemotherapy.
Conclusion:
Our findings indicate that CAR may have a prognostic value in lung cancer as we detected a significant association between elevated CAR and poorer OS. However, further studies are warranted to draw firm conclusions.
Keywords: CAR, lung cancer, prognosis, systemic inflammation
1. Introduction
Lung cancer is one of most common cancers and the leading cause of death in all cancers.[1] Among people with smoking history, the death rate of lung cancer reduced 16% to 20% though computed tomography.[2] Although the treatments for lung cancer have improved and the 5-year survival rate has decreased recent years, the ideal method to predict the prognosis of lung cancer remains unavailable.
Mounting evidence supported that high level of systemic inflammation is associated with poor survival in patients by promoting cancer cell proliferation and survival, angiogenesis, tumor metastasis in many kinds of cancers.[3,4] Inflammation-based prognostic scores, including neutrophil lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), Glasgow Prognostic Score (GPS) have been reported to have prognosis effect on patients with cancers.[5–9] Recently, CAR showed its impact on a large variety of tumor types.[10–18] CAR based on C-reactive protein and albumin, which not only presenting the inflammatory status but also the nutritional status may be a potential prognostic predictor for lung cancer. However, there were few studies regarding the effect of the CAR on prognosis in patients with lung cancer.[19–23] Therefore, we collected the available publications and conducted a meta-analysis to investigate whether CAR has a prognostic value in patients with lung cancer in this present study.
2. Method
2.1. Search strategy
The databases of PubMed, Cochrane Library, Web of Science, and Embase were searched until July 16, 2017. The key words such as “lung carcinoma,” “Pulmonary Neoplasms,” “Pulmonary Cancer,” “Albumin, serum,” and “C-Reactive Protein” were used in combination. Reviews and reference lists were also manually retrieved for additional publications. The publication language was limited to English. The titles and abstracts were screened to identify related studies, and full texts were evaluated carefully. The published studies were sought with no restrictions of the minimum number of patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendment.
2.2. Eligibility criteria
The following inclusion criteria were used: retrospective or prospective study design; published before July 2017; patients were pathologically diagnosed as lung cancer; CAR was measured based on C-reactive protein and albumin of serum; provision of HRs and 95% CIs for CAR in OS or data necessary to calculate them; full text papers published in English.
The exclusion criteria were as follows: nonhuman studies; review, meeting abstract, and letter, not full text in English; did not present the value for CAR.
If data sets were overlapped or duplicated, only the most recent information was included in this meta-analysis. All identified studies were reviewed by 2 authors independently for eligibility.
2.3. Data extraction
The following information such as the surname of the first author, study country, year of publication, sample size, treatment methods, and survival data were extracted from the eligible studies by 2 independent reviewers and any disagreement between them was resolved by discussion until consensus was reached.
2.4. Quality assessment
The quality assessment of primary studies was performed according to Newcastle-Ottawa quality assessment Scale (NOS). The full mark is 9 points and studies labeled with more than 6 points were regarded as high-quality researches (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp).
2.5. Statistical analysis
Statistical analysis was conducted using STATA 14.0 (STATA Corp, College Station, TX).[18] We used the hazards ratios in univariate models to calculate the pooled HRs. HRs with 95% CIs were directly obtained from the articles or estimated from the K-M curves according to the methods reported by Tierney et al.[19] Statistical heterogeneity among the studies was evaluated with Q and I2 statistics, with the significance level set at P < .05.[20] If there was significant heterogeneity among the studies, a random effects model was used to calculate the pooled HRs and 95% CIs.[21] Sensitivity analyses to rule out overrepresentation of results from a single study in the meta-analysis were performed by excluding each study individually from the meta-analysis. The potential publication bias was evaluated using Begg's test.[22]P < .10 was considered as statistically significant.
3. Result
3.1. The characteristics of included studies
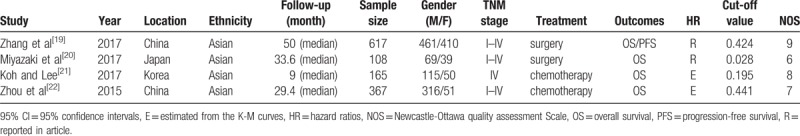
The flow diagram of this study was presented in Fig. 1. A total of 4 studies with 1257 patients were enrolled in the meta-analysis.[19–22] The sample sizes ranged from 108 to 617. These publications were retrospective studies and were conducted in Asia. Two of them were carried out in China.[19,22] One of the remaining researches was conducted in Japan[20] and another one was conducted in Korea.[21] Seven hundred twenty-five patients from 2 studies,[19,20] received the treatment of surgery for lung cancer and 532 patients from another 2 researches received the treatment of chemotherapy or other palliative treatment. Only 1 study involved patients with small cell lung cancer (SCLC)[22] and the remaining studies included patients with nonsmall cell lung cancer (NSCLC). Different studies use the different cut-off value of CAR, which is shown in Table 1. Almost all studies provided the HRs. we calculated them from K-M curve though the method mentioned before if there was no HRs was reported in articles. Moreover, all studies defined OS as the time from diagnosis to day of death or last follow-up. The researches were published from 2015 to 2017 and the NOS scores of the included studies ranged from 6 to 9. The detail information is provided in Table 1.
Figure 1.
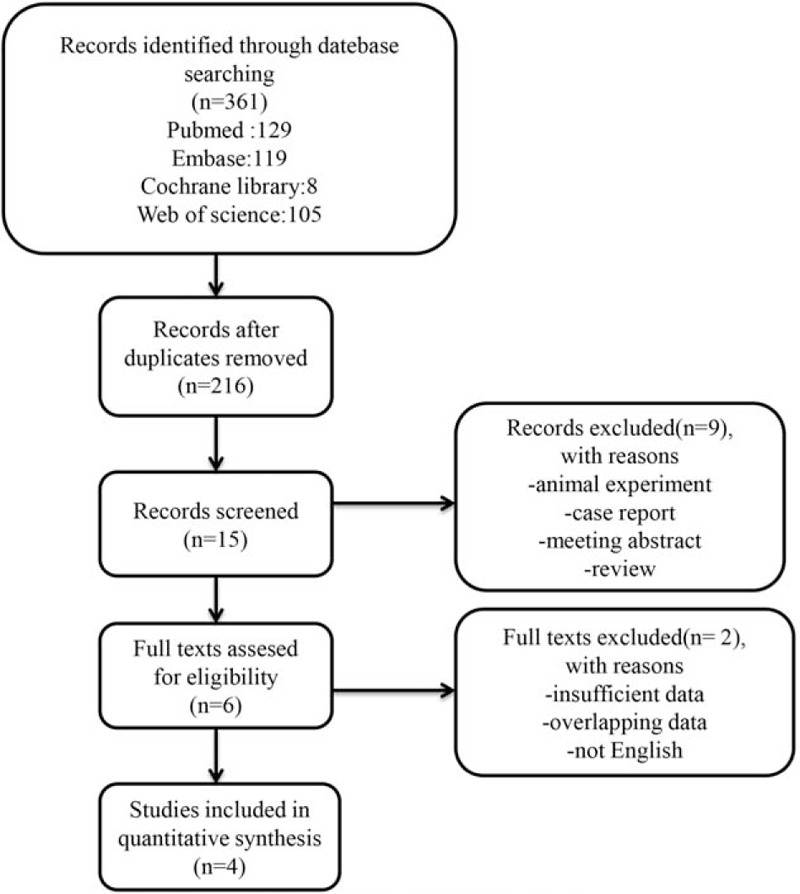
Flow chart of the study selection.
Table 1.
Characteristics of all included studies.
3.2. Relationship between CAR and OS in lung cancer
Four researches with 1257 enrolled population provide the data of CAR before treatment and OS. The random effects model showed a significant relationship between elevated CAR and OS in patients with lung cancer (HR: 2.13; 95% CI: 1.52–2.97; P < .05) with high heterogeneity (I2 = 76.4%, P < .001, Fig. 2).
Figure 2.
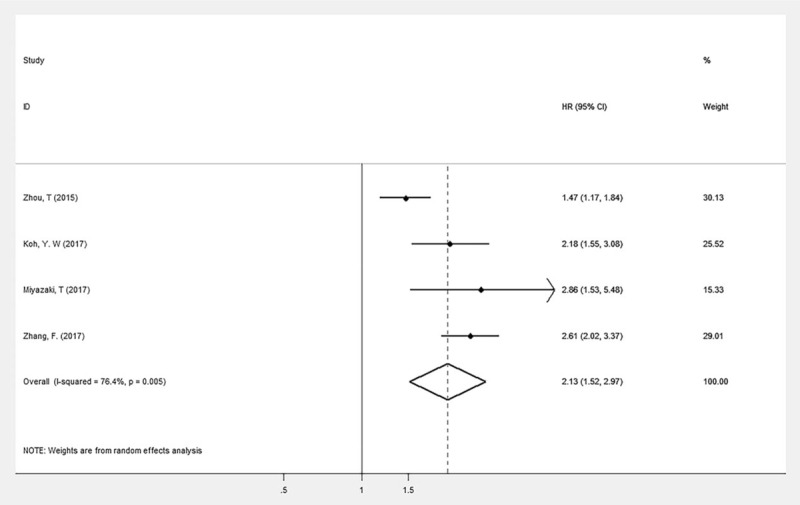
Forest plot of the association between CAR and OS in patients with lung cancer.
3.3. Subgroup analyses
To detect the potential source of heterogeneity, subgroup analyses stratified by different adjustment, treatment and sample size were conducted (Table 2, Fig. 3). The HRs of 3 studies were adjusted by different confounding factors, such as sex, age, and so on. As shown in Table 2, multivariate analyses on 1092 participants showed that high level of CAR predicted poor OS in patients with lung cancer (HR: 1.63; 95% CI: 1.24–2.51; P < .001) and the heterogeneity was decreased to 45.4%. We also conducted subgroup analysis based on treatment to further explain the results of this meta-analysis. A similar trend was observed in patients receiving chemotherapy and surgery (Table 2). And the HRs for patients receiving surgery was moderately higher than that for patients receiving chemotherapy (HR for surgery vs chemotherapy; 2.64 vs 1.75).
Table 2.
A summary of HR for the overall and subgroup analyses of GPS and lung cancer.
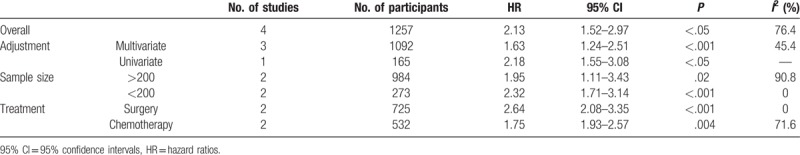
Figure 3.
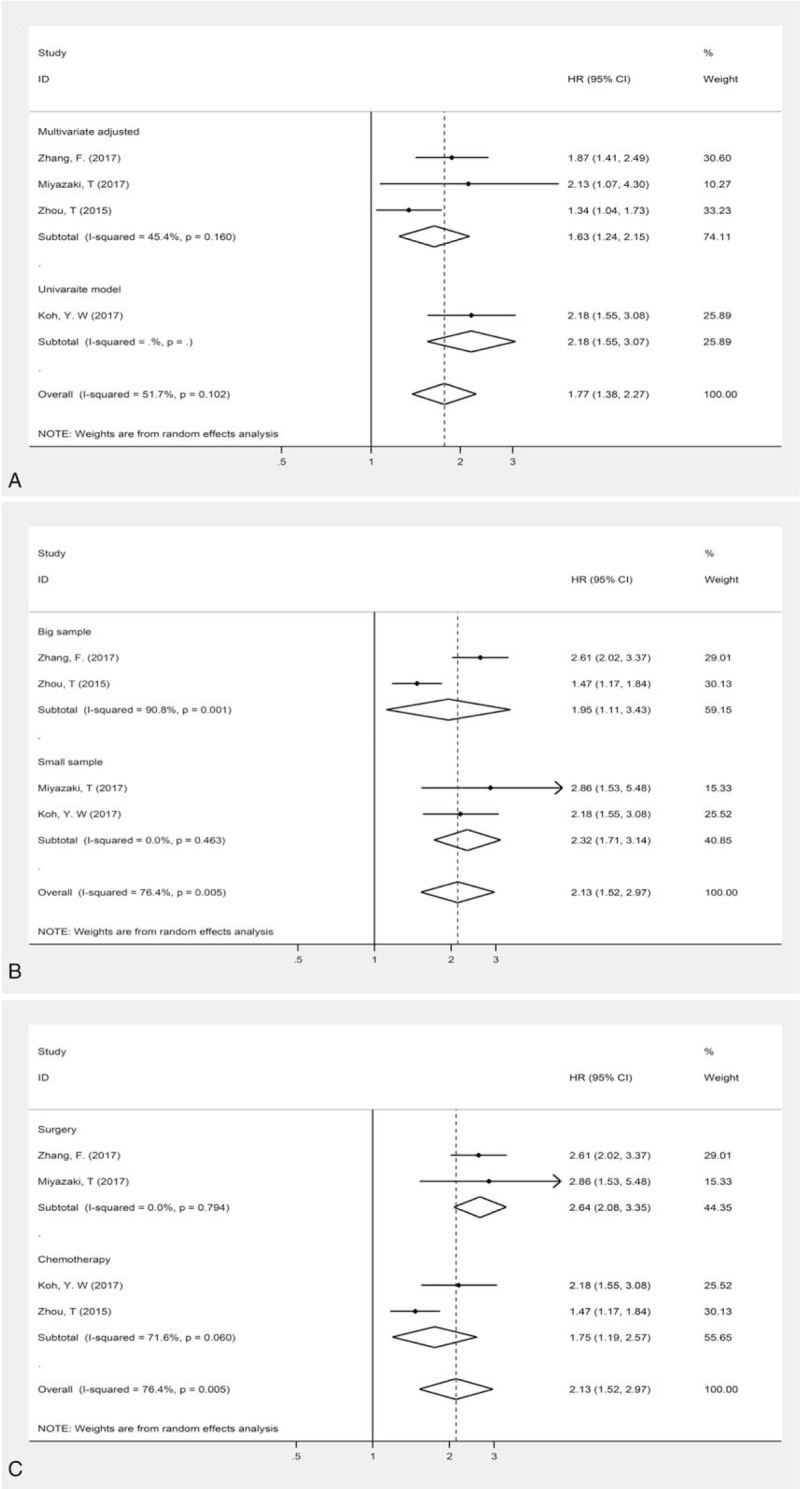
Forest plot of the association between CAR and OS in patients with lung cancer stratified by adjustment, sample size, and treatment.
If enrolled patients were more than 200 in study, we defined the study as the one with big sample size. Then we perform subgroup analysis based on different sample size. Almost the same result was observed in studies with big or small sample size (Table 2).
3.4. Sensitivity analysis and publication bias
Significant heterogeneity was discovered among all studies (I2 = 76.4%, P < .01). However, heterogeneity decreased after subgroup analysis. The influence of each single study was evaluated by excluding each study individually from the meta-analysis. The results showed that the pooled HRs for OS were robust in our study (Fig. 4). Moreover, Begg's test and the funnel plot showed no evidence of obvious publication bias (P = .500) (Fig. 5).
Figure 4.
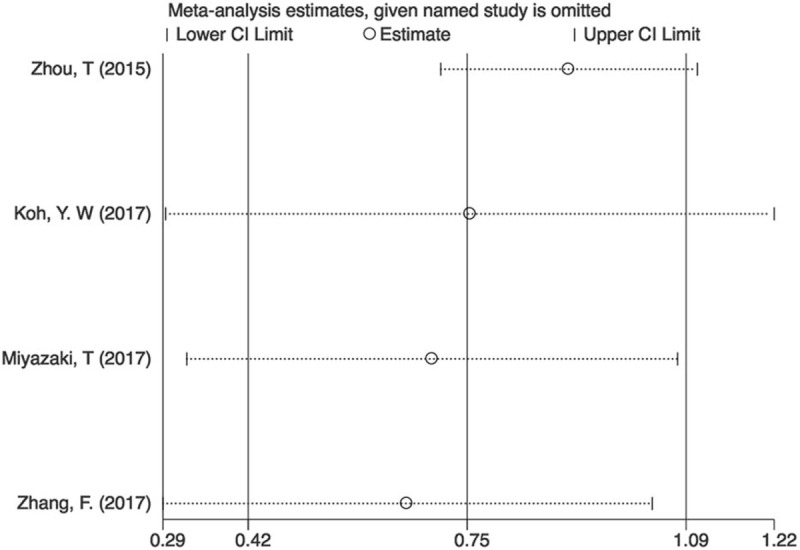
Sensitivity analysis on the relationship between CAR and OS in lung cancer.
Figure 5.
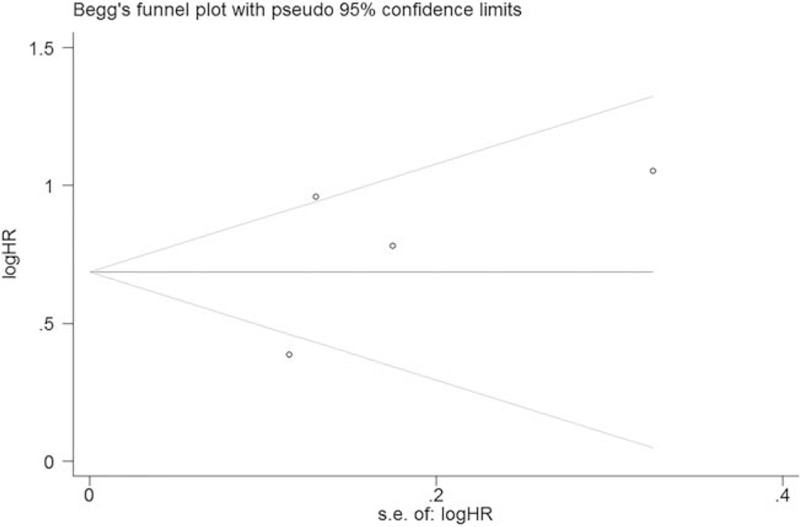
Begg's funnel plot of publication bias test for CAR and OS in lung cancer.
4. Discussion
In this study, we investigated utility of CAR as prognostic factors in lung cancer by meta-analysis of 1257 patients. We found that elevated level of CAR was significantly correlated with poor OS before treatment. Moreover, when we conducted subgroup based on treatment and different adjustment, the heterogeneity decreased and for patients receiving surgery, the poor OS was more closely associated with elevated CAR than those receiving chemotherapy. The results of different subgroup still showed that elevated CAR was a prognostic factor for OS in lung cancer.
More and more researches revealed that the host systemic inflammatory response plays a critical role in the development and progression of many cancers.[24,25] However, the mechanism by which inflammatory factors may impact prognosis remains unclear. As a biomarker of system inflammation, C-reactive protein (CRP) is a kind of acute reactive protein synthesized by liver cells, which is caused by microbial invasion and tissue injury.[26] And its prognostic value in patients with various types of cancer was investigated in many researches.[27–33] The CAR was primarily used to predict 90-day mortality in sepsis by Ranzani et al.[34] Then its prognostic value was explored in varies of cancers.[10–16,18,22] More recently, Kinoshita et al[35] and Chen et al[10] found that CAR has better performance in predicting prognosis than other inflammation-based factors in renal and hepatocellular carcinoma. Then, CAR that not only indicate the inflammatory status but also the nutritional status showed its potential prognostic value for lung cancer in some researches[19–22] and we assessed this in our study. Our result showed that elevated CAR indicated a worse outcome in patients receiving surgery and chemotherapy though multivariate and univariate analyses, which was in accordance with the previous studies.[19,20,22]
There were some limitations in our study. First, only 4 retrospective studies were included in this current meta-analysis and high heterogeneity existed in this meta-analysis, even after subgroup analyses. Though there was no significant bias after careful evaluation and our result remained stable. Second, participants from enrolled studies were at different clinical stages. Four hundred ninety patients with advanced stages (III and IV) and 767 patients with early stages (I and II) were involved in this meta-analysis, but we were not able to perform a subgroup analysis according to different clinical stages due to the insufficient data we can get from the article. Besides, we were not able to conduct a subgroup analysis according the pathological type of lung cancer, also due to the insufficient data. Finally, different studies use different cut-off value for CAR, which may be an important factor affecting the outcome.
To sum up, our study was the first to demonstrate the prognostic role of increased CAR for poor OS, and for patients receiving surgery, the poor OS was more closely associated with elevated CAR than those receiving chemotherapy. However, given the limitations mentioned above, these findings should be treated with caution when applied to clinical practice. More prospective cohort studies are warranted to test our results.
Author contributions
Conceptualization: Jing Zhang, Weimin Li.
Data curation: Jing Zhang, Yong-Zhao Zhou, Weimin Li.
Formal analysis: Tai-Bing Deng, Jing Zhang.
Investigation: Jing Zhang.
Methodology: Tai-Bing Deng, Jing Zhang, Yong-Zhao Zhou, Weimin Li.
Project administration: Jing Zhang.
Resources: Jing Zhang.
Software: Jing Zhang.
Writing – original draft: Tai-Bing Deng, Jing Zhang, Yong-Zhao Zhou, Weimin Li.
Writing – review & editing: Tai-Bing Deng, Jing Zhang, Yong-Zhao Zhou, Weimin Li.
Footnotes
Abbreviations: CAR = C-reactive protein to albumin ratio, CIs = confidence intervals, GPS = Glasgow Prognostic Score, HRs = hazard ratios, NLR = neutrophil lymphocyte ratio, NOS = Newcastle-Ottawa quality assessment Scale, NSCLC = nonsmall cell lung cancer, OS = overall survival, PLR = platelet to lymphocyte ratio, SCLC = small cell lung cancer.
T-BD and JZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roxburgh CS, Mcmillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. [DOI] [PubMed] [Google Scholar]
- [4].Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol 2013;114:149–54. [DOI] [PubMed] [Google Scholar]
- [5].Gu X, Sun S, Gao XS, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep 2016;6:23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nikolić I, Kukulj S, Samaržija M, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio help identify patients with lung cancer, but do not differentiate between lung cancer subtypes. Croat Med J 2016;57:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sanchez-Salcedo P, de-Torres JP, Martinez-Urbistondo D, et al. The neutrophil to lymphocyte and platelet to lymphocyte ratios as biomarkers for lung cancer development. Lung Cancer 2016;97:28–34. [DOI] [PubMed] [Google Scholar]
- [8].Li ZS, Chen P, Yao K, et al. Development of a new outcome prediction model for Chinese patients with penile squamous cell carcinoma based on preoperative serum C-reactive protein, body mass index, and standard pathological risk factors: the TNCB score group system. Oncotarget 2016;7:21023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol 2016;142:1285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol 2014;8:14893–900. [PMC free article] [PubMed] [Google Scholar]
- [11].Haruki K, Shiba H, Shirai Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg 2016;40:2254–60. [DOI] [PubMed] [Google Scholar]
- [12].Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol 2015;8:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2016;24:561–8. [DOI] [PubMed] [Google Scholar]
- [14].Park HC, Kim MY, Kim CH. C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg 2016;42:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong TC, Su HY, Chen YT, et al. Ratio of C-reactive protein to albumin predicts muscle mass in adult patients undergoing hemodialysis. PLoS ONE 2016;11:e0165403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Y, Zhou GQ, Liu X, et al. Exploration and validation of C-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer 2016;7:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miyazaki T, Yamasaki N, Tsuchiya T, et al. Ratio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patients. Surg Today 2017;47:836–43. [DOI] [PubMed] [Google Scholar]
- [21].Koh YW, Lee HW. Prognostic impact of C-reactive protein/albumin ratio on the overall survival of patients with advanced nonsmall cell lung cancers receiving palliative chemotherapy. Medicine 2017;96:e6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tolia M, Tsoukalas N, Kyrgias G, et al. Prognostic significance of serum inflammatory response markers in newly diagnosed non-small cell lung cancer before chemoirradiation. BioMed Res Int 2015;2015:485732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gioulbasanis I, Pallis A, Vlachostergios PJ, et al. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer 2012;77:383–8. [DOI] [PubMed] [Google Scholar]
- [25].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [26].Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. Eur J Surg Oncol 2008;34:727–9. [DOI] [PubMed] [Google Scholar]
- [27].Alifano M, Falcoz PE, Seegers V, et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:1161–7. [DOI] [PubMed] [Google Scholar]
- [28].Eggers H, Seidel C, Schrader AJ, et al. Serum C-reactive protein: a prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol 2013;30:705–10. [DOI] [PubMed] [Google Scholar]
- [29].Kinoshita A, Onoda H, Takano K, et al. Pretreatment serum C-reactive protein level predicts poor prognosis in patients with hepatocellular carcinoma. Med Oncol 2012;29:2800–8. [DOI] [PubMed] [Google Scholar]
- [30].Li YJ, Li ZM, Xia Y, et al. Serum C-reactive protein (CRP) as a simple and independent prognostic factor in extranodal natural killer/T-cell lymphoma, nasal type. PLoS ONE 2013;8:e64158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Oh BS, Jang JW, Kwon JH, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer 2013;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shao N, Cai Q. High pretreatment serum C-reactive protein level predicts a poor prognosis for combined small-cell lung cancer. Tumor Biol 2015;36:1–6. [DOI] [PubMed] [Google Scholar]
- [33].Tomita M, Shimizu T, Ayabe T, et al. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012;32:3535–8. [PubMed] [Google Scholar]
- [34].Ranzani OT, Zampieri FG, Forte DN, et al. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS ONE 2013;8:e59321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]


