Abstract
Rationale:
Primary iliopsoas abscess (IPA), an uncommon clinical entity, often has no specific clinical features, and advanced imaging techniques are often required for diagnosis.
Patient Concerns:
We successfully treated 3 patients with primary IPA complicated by rapid development of septic shock within 2 months.
Diagnosis:
All patients were in shock at the time of admission and were diagnosed with primary IPA by history, clinical examination and imaging findings.
Interventions:
All patients were treated by surgical drainage and sensitive antibiotics based on culture results.
Outcomes:
The patients eventually recovered and were discharged within 2 months.
Lessons:
An IPA may not be diagnosed in a timely manner because it has no specific symptoms or signs. Therefore, special attention must be given to patients with sudden onset of abdominal pain, hip pain, or high fever without an obvious cause, a primary IPA should be highly suspected in such patients.
Keywords: iliopsoas abscess, infection, primary, secondary, septic shock
1. Introduction
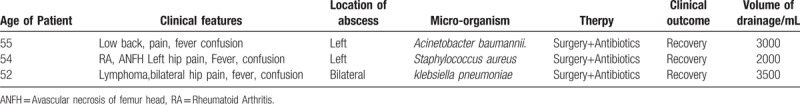
An iliopsoas abscess (IPA) can be either primary or secondary. Secondary IPAs are often caused by localized infections such as spinal lesions (eg, spondylitis and spinal tuberculosis), pancreatitis, urinary tract infections and stones (eg, within the kidneys or ureters), appendicitis, and intestinal infections (mainly Crohn disease). In contrast, primary IPAs are mainly caused by blood-borne infections, however, the specific etiologies remain unclear.[1] Despite its rarity, a primary IPA is a highly fatal condition, and affected patients often die of complicating sepsis. As a result of the extensive use of computed tomography (CT) and other diagnostic techniques in recent years, an increasing number of primary IPAs have been diagnosed.[2] Early diagnosis of a primary IPA is difficult due to the lack of specific clinical manifestations. However, both misdiagnosis and delayed diagnosis drastically increase the case-fatality rate. According to the existing literature, primary IPAs are mostly confined to the iliac abdomen and rarely spread to other tissues. We recently treated 3 cases of primary IPA, both of which had certain clinical characteristics (Table 1). The treatment process is described below.
Table 1.
Clinical features, microbiology, therapy, outcomes, and abscess volumes of patients with primary iliopsoas abscesses.
2. Ethical review
Because this case report does not breach the patients’ privacy, approval by an ethics committee was not required. All patients provided written informed consent.
3. Case 1
A 55-year-old man had a 7-day history of low back pain. The pain became aggravated with fever for 4 days, and he was hospitalized. Seven days before admission, the patient had developed sudden-onset left waist pain of no apparent cause, and no special treatment was given. Four days before admission, the low back pain worsened and was accompanied by fever, and the patient's body temperature fluctuated from 38.0 °C to 41.0 °C. He visited a local hospital for symptomatic treatment. One day before admission, the patient developed chills, fever, and unconsciousness and was transferred to our hospital for treatment. Physical examination revealed a temperature of 38.1 °C, heart rate of 108 beats/min, respiratory rate of 27 breaths/min, blood pressure of 84/60 mmHg, and unconsciousness. Laboratory data showed anemia, leukocytes, platelets, blood lactic acid, and C-reactive protein (Table 2). Major examinations including ultrasound, CT, and magnetic resonance imaging (MRI) confirmed the presence of a large abscess (Fig. 1A, B, and C1/C2). The left abdomen was punctured and pus was extracted and submitted for bacterial culture and drug sensitivity testing. The diagnoses were septic shock and left IPA.
Table 2.
Blood test results of patients with primary iliopsoas abscesses.
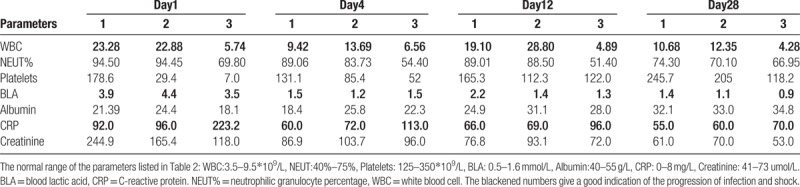
Figure 1.
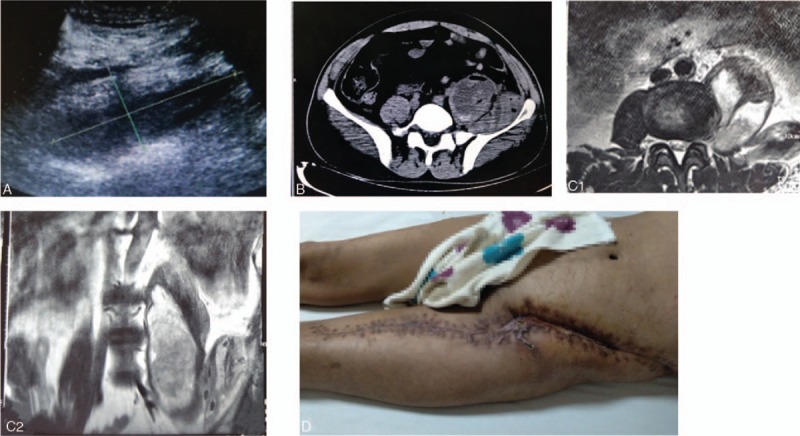
(A) Ultrasound showed an approximately 15.1- × 6.4- × 5.7-cm liquid dark area from the left retroperitoneal area to the left inner thigh. (B) Computed tomography showed a massive abscess in the left abdomen extending downward to the left iliac and inguinal areas, where air bubbles were visible. (C1/C2) Magnetic resonance imaging showed a double-chambered abscess in the left abdomen. (D) The picture depicts the healed wound after discharge.
On the second day after admission, the patient underwent surgical incision drainage. The anteromedial lumbar retroperitoneal approach was used, and 3000 mL of dark red, purulent, bloody liquid was drained from the extraperitoneal space. The wound was partially closed and drained with a vacuum sealing drainage (VSD) device. After surgery, broad-spectrum antibiotics were given, and sensitive antibiotics were used when the culture results were confirmed. The patient was hospitalized for 58 days and underwent 5 operations. The wound healed well and the patient was discharged on the 50th day (Fig. 1D). Two months after discharge, the patient reported self-sufficiency in daily activities without recurrence of inflammation.
4. Case 2
A 54-year-old women had a 2-month history of left hip pain. The pain became aggravated for 2 days, and she was hospitalized. She had a 14-year history of rheumatoid arthritis and a 6-month history of left femoral head necrosis. He had repeatedly received apitherapy for the last 6 months. Two months before admission, the patient developed left hip pain with no apparent cause, and no special treatment was given. Two days before admission, the left hip pain worsened and the patient was admitted to our hospital. Physical examination showed a body temperature of 36.5 °C, heart rate of 100 beats/min, respiratory rate of 25 breaths/min, blood pressure of 92/71 mmHg, and unconsciousness. Both sides of the hip and lower extremity had a wide range of old and new staggered needle-like scars. The patient's laboratory test results are shown in Table 2. Radiography, CT, and MRI of the hip showed that an abscess had formed in the left iliopsoas (Fig. 2A1/A2, B, and C). The patient was diagnosed with infection with toxic shock, multiple organ dysfunction syndrome, primary IPA, pneumonia, and left femoral head necrosis.
Figure 2.
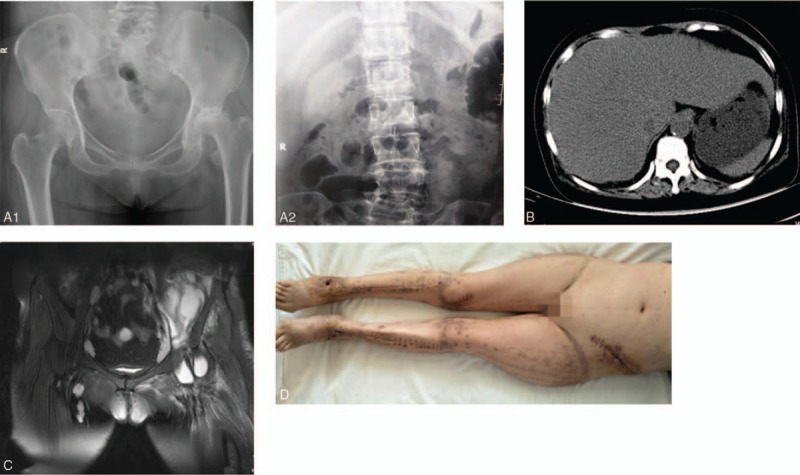
(A1/A2) Radiographic examination showed that the left femoral head was flat, the left hip joint space was significantly narrow, the left acetabulum was slightly hardened, and a huge oval soft tissue density was present near the vertebral body. (B) Computed tomography revealed a giant oval abscess of about 11.0 × 3.0 cm in the left abdomen, with the upper end reaching the level of T12 and extending down to the level of the iliac fossa. The abscess contained bubbles. (C) Magnetic resonance imaging of the hip showed that the abscess, which was in the multi-chamber, was interlinked with the joint capsule, (D) The wound healed completely.
The patient was treated with surgical incision drainage and sensitive antibiotics. The bacterial culture grew Staphylococcus aureus sensitive to levofloxacin, gentamicin, and vancomycin. The left lumbar anterolateral retroperitoneal approach was used, and 2000 mL of yellow pus mixed with viscous liquid was drained from the extraperitoneal space. The cavity was drained by a VSD device. Sensitive antibiotics were administered for 6 weeks after surgery. The patient was hospitalized for 55 days and underwent 5 operations. The wound healed well and the patient was discharged on the 55th day (Fig. 2D). One month after discharge, the wound was completely healed without recurrence of infection.
5. Case 3
A 52-year-old woman presented with a 15-day history of pain in the right hip with a high-grade fever that had become aggravated during the past day. The patient had a history of lymphadenoma and had received chemotherapy several times a month. Fifteen days before admission, the right hip pain of no obvious cause had developed with a high fever and her body temperature fluctuated from 38.1 °C to 41.0 °C. Abdominal MRI at a local hospital revealed the formation of an oval abscess of 8.5 × 2.7 cm in the right iliopsoas muscle (Fig. 3A). The patient's condition slightly improved after local treatment with ultrasound-guided percutaneous catheter drainage and broad-spectrum antibiotics. One day before admission, the patient's right hip pain worsened, her body temperature increased to 40.1 °C, and she lost consciousness, she was then transferred to our hospital for treatment. Physical examination revealed a temperature of 40.1 °C, heart rate of 110 beats/min, respiratory rate of 27 breaths/min, blood pressure of 90/60 mmHg, and bilateral lower abdominal swelling. The patient's laboratory test results are shown in Table 2. Abdominal MRI revealed an abscess in the iliopsoas muscle on both sides (Fig. 3B). The left abdomen was punctured and pus was extracted and submitted for bacterial culture and drug sensitivity testing. The patient was diagnosed with infection with toxic shock, bilateral primary IPA, and pneumonia. She underwent surgical incision drainage using the anterior and lateral retroperitoneal approach to the lumbar spine, and 3500 mL of yellow pus mixed with viscous liquid was drained from the extraperitoneal space and iliac fossa. The cavity was drained by a VSD device. Sensitive antibiotics were administered for 6 weeks after surgery. She was hospitalized for 50 days and underwent 4 operations. The wound healed well and the patient was discharged on the 50th day (Fig. 3C). One month after discharge, the wound was completely healed without recurrence of infection.
Figure 3.
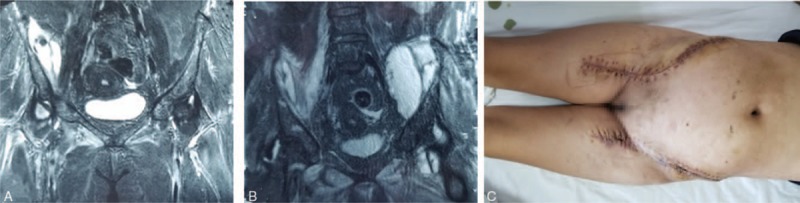
(A) Magnetic resonance imaging showed that the abscess was only present on the right side. (B) Magnetic resonance imaging showed that the bilateral iliopsoas contained large abscesses and was multiventricula. (C) The wound healed completely.
6. Discussion
A primary IPA is an extremely rare infectious disease, and most such abscesses have been described in case reports.[3,4] In their retrospective analysis of 24 cases of IPA, Huang et al[5] concluded that only 6 cases were classified as primary IPAs, in addition, 24% of the IPAs occurred on the left side, 60% on the right side, and 16% on both sides. Navarro et al[6] indicated that among 124 IPAs, only 27 were primary IPAs, the IPA was on the right side in 43.6% of patients, on the left side in 52.4%, and bilateral in 4.0%. In past reports, primary IPAs typically occurred in adolescent patients, among whom 70% were <20 years of age.[7,8] According to more recent literature, however, the average age of patients with a primary IPA is >40 years.[7] The age at onset in our 3 patients with primary IPA was consistent with the literature.
A primary IPA is caused by a blood-borne infection. Because abundant blood vessels are present around the iliopsoas muscle and rich lymphatic vessels are present behind the peritoneum, bacteria can easily travel to these locations through the circulation and form an abscess.[9,10] Recent studies have shown that primary IPAs often occur in patients with compromised immunity, human immunodeficiency virus infection, malnutrition, drug addiction, chronic diseases (such as diabetes), and malignant tumors.[4,11] Once the body's natural defense against illness has declined, the body is susceptible to attacks of bacteria from the blood and resultant infectious disease. In Case 2 of the present report, the patient had a history of long-term use of immunosuppressive agents and repeated “bee therapy” for his rheumatoid condition, which might explain the development of his primary IPA and multiple abscesses. In Case 3, the patient had received multiple chemotherapy treatments for lymphoma, resulting in extremely low immunity, eventually, bacteria became able to easily and quickly pass through the blood, causing disease.
Fever, abdominal pain, and restricted hip joint movement are the most common symptoms of a primary IPA.[12] However, this disease is often misdiagnosed due to the lack of specific symptoms in its early stage. In our patients, we failed to make an early diagnosis of IPA because all 3 patients had only abdominal pain, fever, and disturbance of consciousness with no other specific symptoms or signs. As a result, the 3 patients soon developed toxic shock.
In addition to physical examination findings and clinical manifestations, medical imaging plays a key role in the diagnosis of IPA. Ultrasound has an accuracy of only 60% in detecting abscesses, for some small abscesses or diffuse cellulitis, its accuracy is greatly reduced, and some abscesses may not even be visible. At present, CT is the gold standard technique for diagnosing IPA, and its accuracy can reach 100%. CT scans can reveal the size, depth, and range of the abscess.[5,8,13,14] MRI often clearly shows the location of the lesion and its surrounding soft tissue structures and is therefore also helpful in the diagnosis of IPA.[15] Radiographs can be used to rule out a secondary IPA caused by vertebral lesions and can sometimes also show the approximate location of the abscess, however, they cannot be used as the main diagnostic technique.
Primary IPAs are reportedly mainly caused by staphylococcus infections, especially S. aureus, which accounts for 88% of IPAs.[4]Acinetobacter baumannii, Proteus spp. (especially P. mirabilis), and Serratia marcescens infections may also be the etiologies of primary IPA.[10,16,17] The main pathogen of secondary IPAs is Escherichia coli.[10] In our patients, the pus cultures showed that the IPA was caused by A. baumannii in Case 1, S. aureus in Case 2, and Klebsiella pneumoniae in Case 3.
No standard treatment protocol has been established for primary IPAs.[3] Treatments often differ due to differences in the abscess size, pathogen type, invasion extent, and infection severity. According to Yacoub et al,[18] when the abscess is <3 cm in diameter, treatment with broad-spectrum antibiotics alone for 6 weeks is adequate; however, when the abscess is >3 cm, ultrasound- or CT-guided percutaneous catheter drainage combined with broad-spectrum antibiotics is required. Van et al[4] suggested that when the abscess is 2.5 to 7.5 cm in diameter, oral broad-spectrum antibiotics alone for 6 weeks can be curative. Other authors have stated that open-access drainage combined with antibiotics is needed even for small abscesses.[19] Santaella et al[10] proposed that open-access drainage of pus is more effective than ultrasound- or CT-guided percutaneous catheter drainage, especially in patients with multi-loculated abscesses or gas formation within the pus. A recent study suggested that the retroperitoneal laparoscopic posterior approach is more thorough in removing abscesses; in particular, it can completely remove the pus-moss and necrotic tissue from the abscess wall.[20] Our patients had the following 2 unique features:
-
(1)
the disease progressed rapidly and septic shock occurred within a short period of time (3 days), and
-
(2)
the abscesses were huge, involving large areas.
In Case 1, the abscess had spread to the medial thigh muscle tissue and extended to the distal thigh. In Case 2, the abscess affected both ankles, both calves, and the right knee (the left knee was normal), manifesting as multiple abscesses. In Case 3, the abscess invaded both the iliac fossa and the ischial tubercle. Complete removal of the abscess is often difficult with minimally invasive drainage, which has become a popular technique in recent years, however, the residual necrotic tissue is an important cause of recurrence of a primary IPA.[20] In our three patients, therefore, we adopted the conventional large-incision repeated debridement and drainage technique. The patients’ general condition was remarkably improved within 48 h after the procedure, and all 3 patients were successfully discharged within 2 months, which justifies our strategy.
In conclusion, a primary IPA is a rare infectious disease that can be easily misdiagnosed in its early stage. The lesson provided by these cases is that if the diagnosis and cause are not ascertained as soon as possible, the patient will soon develop septic shock. Thus, less experienced doctors should be vigilant. Ultrasound, CT, and MRI should be performed in a timely manner for patients with sudden waist, abdomen, and hip pain accompanied by fever to rule out various disease conditions or achieve a diagnosis in the early stage and administer optimal treatments.
Acknowledgment
None.
Author contributions
Investigation: Yingying Deng, Lichuang Zhang.
Writing – original draft: Yingying Deng, Aqin Peng.
Writing – review & editing: Yingying Deng, Yanlong Zhang, Lianxin Song, Xuebin Zhang, Zheyuan Shen, Zhengqiang Li, Aqin Peng.
Footnotes
Abbreviations: CT = computed tomography, IPA = iliopsoas abscess, MRI = magnetic resonance imaging, VSD = vacuum sealing drainage.
All authors have no conflicts of interest to disclose.
References
- [1].Mallick IH, Thoufeeq MH, Rajendran TP. Iliopsoas abscesses. Postgrad Med J 2004;80:459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tate H. Clinical study of iliopsoas abscess in 11 cases from 2005 to 2008. Kansenshogaku Zasshi 2009;83:652–7. [DOI] [PubMed] [Google Scholar]
- [3].Suzuki K, Yamaguchi T, Iwashita Y, et al. Case series of iliopsoas abscesses treated at a university hospital in japan: epidemiology, clinical manifestations, diagnosis and treatment. Intern Med 2015;54:2147–53. [DOI] [PubMed] [Google Scholar]
- [4].van den Berge M, de Marie S, Kuipers T, et al. Psoas abscess: report of a series and review of the literature. Neth J Med 2005;63:413–6. [PubMed] [Google Scholar]
- [5].Huang JJ, Ruaan MK, Lan RR, et al. Acute pyogenic iliopsoas abscess in Taiwan: clinical features, diagnosis, treatments and outcome. J Infect 2000;40:248–55. [DOI] [PubMed] [Google Scholar]
- [6].Navarro Lopez V, Ramos JM, Meseguer V, et al. Microbiology and outcome of iliopsoas abscess in 124 patients. Medicine (Baltimore) 2009;88:120–30. [DOI] [PubMed] [Google Scholar]
- [7].Tabrizian P, Nguyen SQ, Greenstein A, et al. Management and treatment of iliopsoas abscess. Arch Surg 2009;144:946–9. [DOI] [PubMed] [Google Scholar]
- [8].Absesi P, Tarhan Hs, Cakmak zrz, et al. editors. Psoas Abscess: Evaluation of 15 Cases and Review of the Literature. 2014. [Google Scholar]
- [9].Gruenwald I, Abrahamson J, Cohen O. Psoas abscess: case report and review of the literature. J Urol 1992;147:1624–6. [DOI] [PubMed] [Google Scholar]
- [10].Santaella RO, Fishman EK, Lipsett PA. Primary vs secondary iliopsoas abscess. Presentation, microbiology, and treatment. Arch Surg 1995;130:1309–13. [DOI] [PubMed] [Google Scholar]
- [11].Walsh TR, Reilly JR, Hanley E, et al. Changing etiology of iliopsoas abscess. Am J Surg 1992;163:413–6. [DOI] [PubMed] [Google Scholar]
- [12].Chern CH, Hu SC, Kao WF, et al. Psoas abscess: making an early diagnosis in the ED. Am J Emerg Med 1997;15:83–8. [DOI] [PubMed] [Google Scholar]
- [13].Zissin R, Gayer G, Kots E, et al. Iliopsoas abscess: a report of 24 patients diagnosed by CT. Abdom Imaging 2001;26:533–9. [DOI] [PubMed] [Google Scholar]
- [14].Lowe BA, Smith AY. Primary psoas abscess. J Urol 1987;137:485–6. [DOI] [PubMed] [Google Scholar]
- [15].Hsieh MS, Huang SC, Loh E, et al. Features and treatment modality of iliopsoas abscess and its outcome: a 6-year hospital-based study. BMC Infect Dis 2013;13:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nakazato T, Kitahara M, Watanabe K, et al. Pneumococcal psoas abscess. Intern Med 1999;38:63–6. [DOI] [PubMed] [Google Scholar]
- [17].Alan C, Ataus S, Tunc B. Xanthogranulamatous pyelonephritis with psoas abscess: 2 cases and review of the literature. Int Urol Nephrol 2004;36:489–93. [DOI] [PubMed] [Google Scholar]
- [18].Yacoub WN, Sohn HJ, Chan S, et al. Psoas abscess rarely requires surgical intervention. Am J Surg 2008;196:223–7. [DOI] [PubMed] [Google Scholar]
- [19].Baier PK, Arampatzis G, Imdahl A, et al. The iliopsoas abscess: aetiology, therapy, and outcome. Langenbecks Arch Surg 2006;391:411–7. [DOI] [PubMed] [Google Scholar]
- [20].Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012;379:1902–13. [DOI] [PubMed] [Google Scholar]


