Abstract
In this longitudinal study, we examined intelligence in a group of Vietnam veterans in their 60 s who suffered combat-related penetrating traumatic brain injuries (pTBI) in their 20 s (n = 120), as well as matched veterans with no brain damage (n = 33). Intelligence was evaluated using the Armed Forces Qualification Test (AFQT) administered before the injury occurred and then again at three points in time over the following 45 years. We tested for potential predictors and correlates of late midlife intelligence score, as well as the recent change in score over the seventh decade. The pTBI group had lower intelligence scores than the control group when currently evaluated. Pre-injury intelligence and the presence of a pTBI were the most consistent predictors of current intelligence scores. While exacerbated intellectual decline occurs following a young-adulthood pTBI and affects everyday life, no evidence for late midlife accelerated cognitive decline or dementia was found.
Keywords: Intelligence, Penetrating traumatic brain injury, Exacerbated cognitive decline
1. Introduction
The concept of intelligence has been the focus of much research and scientific debate since it was introduce more than a century ago. While most studies of intelligence have emphasized its substantial stability through midlife [1–3], a number of variables that can influence adult cognitive skills have been identified, including age, genetic polymorphism, health and environmental factors.
Not surprisingly, traumatic brain injury (TBI) can also affect intelligence scores. A recent meta-analysis [4] revealed that impairments in intelligence persist after TBI in both children and adults, and that injury severity can predict post-injury intelligence scores. A different study followed individuals who had brain injury for 16 years and reported impairment in intellectual performance throughout that time span [5]. Nonetheless, the long-term effect of young-adulthood brain injury on later midlife intelligence remains generally unknown. Two different studies, which tested war veterans some 30 years post penetrating traumatic brain injury (pTBI) [6,7], reported exacerbated decline in intelligence compared to matched controls, mostly among veterans with left hemisphere damage. According to both studies, the strongest predictors of a slower rate of cognitive decline following TBI were higher pre-injury intelligence and more years of education.
Genetic factors were also shown to play a significant role in modulating the exacerbated cognitive decline or dementia following TBI. The most researched gene in this context is the Apolipoprotein E (ApoE). The ε4 allele of the ApoE gene is associated with increased risk of Alzheimer’s Disease (AD) and an earlier age of AD onset [8–10]. It has also been associated with unfavorable outcomes following TBI, including poorer recovery of cognitive function over a 6 month period [11], impaired long-term verbal memory [12], and impaired frontal lobe function [13].
Another gene that plays a significant role in cognitive recovery following TBI is the Brain-Derived Neurotrophic Factor (BDNF). This gene is involved in neuronal plasticity and was previously shown to regulate processes related to cognitive recovery following TBI; for example, a recent study showed that the BDNF single-nucleotide polymorphism (SNP) rsll57659 interacted with mild TBI to predict reduced hippocampal volume [14]. In addition, studying the same sample of combat veterans that were included in the current study, our group have previously shown that BDNF polymorphism predicated poorer performance in general intelligence [15] as well as poorer recovery of executive functioning [16] 35 years post injury.
Other studies have shown an association between experiencing an earlier single TBI and the later development of neurodegenerative disease [17]. For example, Barnes and colleagues [18] followed veterans with and without TBI for 9 years during their eighth decade and found a 60% increase in the risk of developing dementia in the TBI group, as well as an earlier dementia onset. On the other hand, earlier findings reported by our group [7] concluded that there is no increased risk for developing dementia in the sixth decade after pTBI, and the association between TBI and an increased risk for dementia remains tentative.
In order to clarify the long-term impact of young adulthood brain injury on later-midlife intelligence we examine a group of veterans participating in a prospective, long-term follow-up study of Vietnam combat veterans with pTBI (the Vietnam Head Injury Study; VHIS [19]). Military personnel represent an ideal population when studying changes in intelligence scores after pTBI, since pre-injury data are available in the form of the Armed Force Qualification Test (AFQT) which is highly correlated with the Wechsler Adult Intelligence Scale (WAIS) [20]. After completing the AFQT upon enlistment, veterans participating in our study were assessed with the same test three more times during three study phases: Phase 2 (P2) of the VHIS was conducted between 1981 and 1984, some 12–15 years following the brain injury; Phase 3 (P3) was conducted between 2003 and 2006, some 36–39 years post-injury and Phase 4 (P4) was conducted between 2008 and 2012, some 40–45 years post-injury. In each phase participants were also assessed with other neuropsychological testing, a neurological exam, a psychiatric evaluation, genetic testing, and neuroimaging (CT scans).
Previous findings from P2 of the VHIS study shown that the most important determinant of post-injury intelligence was pre-injury intellectual ability as assessed by the AFQT [20]. Later analyses revealed that pTBI patients have an exacerbated decline in intelligence scores between P2 and P3 when compared to matched controls [7].
The present study aims to investigate the changes in intelligence scores between P3 and P4, as well as intelligence scores at P4. This latest phase of the study is of special interest since participants in P4 are in their 60 s, an age range which is often characterized by age related cognitive decline or the first signs of a neurodegenerative disorder.
We evaluated whether pre-injury intelligence, educational level, total brain volume loss, lesion location, and genetic polymorphisms affected current intelligence score or recent change in intelligence, as well as behavior and caregivers burden level. We hypothesized that pTBI will cause exacerbated cognitive decline later in life as a result of the combined effects of TBI-related brain damage and neuronal loss due to normal aging [21].
2. Method
2.1. Participants
One hundred and fifty-three veterans were drawn from P4 of the VHIS registry. Of the 199 pTBI patients who were assessed in P3, 120 attended P4 of the study; of the original 54 control participants without brain injuries recruited in P3, 33 attended P4. At P4, participants were assessed for 5 days at the National Institute of Neurological Disorders and Stroke. There were no significant differences between the pTBI and control participants attending P4 in terms of age or total years of education (Table 1).
Table 1.
Comparison of head injured and control participants at P4 [Mean(SD)].
| Control | Head injured | Statistics | |
|---|---|---|---|
| Age (years) | 63.33(2.79) | 63.42(2.94) | t(148)= 0.13, p = 0.89 |
| Education (years) | 15.22(2.33) | 14.88(2.36) | t(150) = −0.74, p = 0.45 |
| Pre-injury intelligence | 72.90(17.05) | 64.81(23.34) | t(134) = −1.54, p = 0.12 |
2.2. Tests
Armed Forces Qualification Test (AFQT-7A, DoD 1960).
The AFQT is a 50-min paper-and-pencil test consisting of 100 multiple-choice items that was originally administered by the Department of Defense just before military induction to determine qualification to enter the military. The AFQT is composed of four subtests measuring vocabulary knowledge, arithmetic word problem solving, knowledge and reasoning about tools and mechanical relations, and visual-spatial processing. We administered the same AFQT version given to participants at the time of military induction. AFQT scores were recorded as percentiles based on military norms. Knowing that AFQT scores are traditionally highly correlated (~.85) with traditional measures of IQ [20,22], we tested whether current AFQT score are correlated with standard intelligence scores in our sample (as measured by the WAIS-III Full-Scale IQ in P3, and the WASI full IQ percentile score in P4). In both pTBI and control participants, the two were significantly correlated (P3: r = 0.845, p < 0.001 pTBI; r = 0.816, p < 0.001 controls; P4: r = 0.728, p < 0.001 pTBI; r = 0.830, p < 0.001 controls).
Neurobehavioral Rating Scale (NBRS, [23]).
This 27-item scale is used to measure the severity of behavioral sequelae following TBI. Each item required a rating on a scale from 1 (not present) to 7 (extremely severe). Total pathology score is the sum of all items, ranging from 27 to 189. After approximately 25 hours of formal testing and general interactions, an experienced research assistant rated each participant using the NBRS based on observations of the participant’s spontaneous behavior.
Zarit Burden Interview (ZBI [24],).
To evaluate caregiver burden, we used the Zarit Burden Interview (ZBI) which comprises 22 questions completed by the caregiver. Caregivers have to rate their feelings on a 5-point scale (0 = never and 4 = nearly always). The total score sums the first 21 questions excluding the last one that pertains to the overall burden felt by the caregiver. Higher scores mean a greater burden.
Genetic testing.
We preformed genetic testing to identify polymorphisms in the Apo E and BDNF genes (for a full description of the methods see previous publications on this sample [7,15]). None of the alleles/SNPs tested was associated with intelligence in P4 or with the change in intelligence between P3 and P4. Since the genetic analyses were negative they will not be presented in the results section. We address the negative findings in the discussion.
2.3. Computed tomography (CT) acquisition and analysis
The neuroimaging analysis reported here was performed on CT images acquired at P3. Additional CT scans were completed at P4 for clinical purposes and no new lesions or significant pathological changes were reported by an NIH staff radiologist who viewed them. The axial CT scans were acquired without contrast in helical mode on a GE Electric Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital. Structural neuroimaging data was reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, and a 1 mm slice interval. The lesion location and volume were determined from CT images using the interactive Analysis of Brain Lesions (ABLe) software implemented in MEDx v3.44 (Medical Numerics) [25,26]. Lesion volume was calculated by manually tracing the lesion in all relevant slices of the CT image in native space, and then summing the trace areas and multiplying by slice thickness. Manual tracing was performed by a trained neuropsychiatrist with clinical experience in reading CT scans. The lesion tracing was then reviewed by an observer who was blind to the results of the clinical evaluation and neuropsychological testing (J.G.) enabling a consensus decision to be reached regarding the limits of each lesion. The CT image of each individual’s brain was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. Afterwards, the percentage of Automated Anatomical Labeling (AAL) structures that were intersected by the lesion was determined by analyzing the overlap of the spatially normalized lesion image with the AAL atlas [27].
2.4. Statistical analysis
Behavioral data analysis was carried out using IBM SPSS Statistics 21.0 software with an alpha level set to p < 0.05 (two-tailed). Multiple comparisons with Bonferroni correction were included in all analyzes. A variety of parametric procedures were used in this study including analysis of variance (ANOVAs) and linear logistic and stepwise multiple-regression procedures to assess the impact of education, pre-injury intelligence, brain volume loss, lesion location and genetic markers on cognitive ability 40 to 45-years post-injury, and to examine evidence of intellectual decline between P3 and P4. A significance level of p ≤ 0.05 or less was required to enter and remain in the stepwise regression procedures. This analysis allowed an estimation of the relative contribution of each predictor to our main dependent measure of intellectual decline. T-tests (two tailed) were used to compare between participants in P3 and in P4, as well as between pTBI and control groups. Finally, bivariate Spearman correlations between AFQT scores and behavioral measures (NBRS and caregiver burden scores) within subgroup were preformed (p < 0.05, two-tailed).
3. Results
We report the results in the context of a series of questions that address our main a priori hypotheses regarding cognitive decline in this sample.
3.1. Are participants who attended P4 comparable to those who attended P3?
A t-test (two-tailed) was run to compare those participants who underwent assessment only in P3 (194 pTBI and 54 controls) compared with those who attended both P3 and P4 (120 pTBI and 33 controls). There were no significant differences in age, total years of education and total lesion volume loss between P4 attenders and non-attenders. However, those that attended P4 had a significantly higher pre-injury AFQT score (t(214)= 3.39, p < 0.001) than P4 non-attendees.
In terms of differences in demographic variables at P4, t-tests showed no significant differences between the pTBI and control groups regarding age, education, and pre-injury AFQT score (Table 1).
3.2. How do pTBI and control participants’ AFQT scores compare?
The mean AFQT percentile score at P4 for the entire sample was 58.50. In controls (n = 33), the AFQT percentile score (M = 71.39, SD = 19.54) was significantly higher than that of pTBI patients (n = 120, M = 54.95, SD = 26.11; t(151)=3.36, p = 0.001, d = 0.71). The decrease in AFQT percentile score from pre-injury to P4 was significantly greater among pTBI patients (M = −10.43. SD = 19.74) compared with controls (M = −0.18, SD = 12.36; t(134) = 2.34, p = 0.02, d = 0.62; see Fig 1). However, the change in AFQT percentile score from P3 to P4 was no different for the pTBI (M = −1.65, SD = 13.11) and control (M = 1.03, SD = 9.7) groups (t(151)= 1.09, p = 0.27, d = 0.23).
Fig 1.
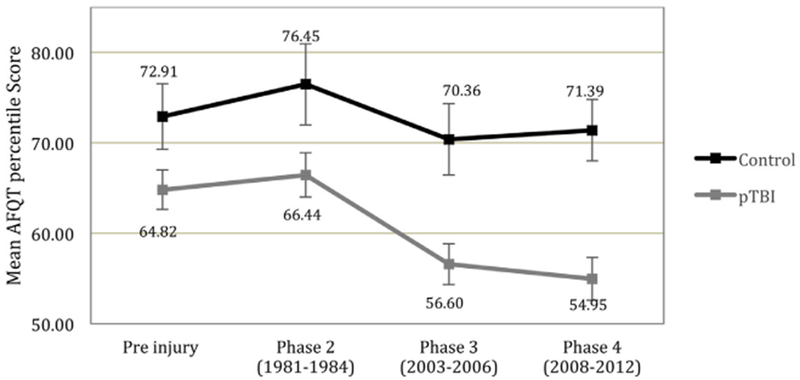
Mean AFQT percentile scores pre-injury, at P2, at P3 and at P4. Error bars represents + −1 standard error.
3.3. What predicts current intelligence level?
3.3.1. Demographic measures
A univariate linear regression procedure was performed to assess the predictability of P4 AFQT score, with race, age, military rank, education and experimental group (i.e. whether a participant was a pTBI participants or a control) as covariates. Years of education (F(1,151)= 11.20, p = 0.001) and rank (F(1,143) = 5.96, p = 0.016) significantly predicted P4 AFQT scores. VHIS group (F(1,143) = 3.54, p = 0.062) and age (F(1,143) = 3.29, p = 0.072) were marginally significant predictors. However, when pre-injury AFQT score was added as a covariate, only pre-injury AFQT score (with a higher pre-injury AFQT score predicting a higher score at P4; F(1,126) = 87.31, p < 0.001) and presence of pTBI (F(1,126) = 4.78, p = 0.031) were found to have a significant impact on the P4 AFQT percentile score.
3.3.2. Brain volume loss and lesion location
A stepwise regression analysis was performed to look for lesion variables predicting the participants’ current AFQT percentile score. The dependent variables included right hemisphere volume loss, left hemisphere volume loss, total brain volume loss and the overall involvement of the frontal lobe, as well as the lateral involvement of the dorsolateral prefrontal cortex (dlPFC) and the ventromedial (vm) PFC. The volume of brain tissue loss at P4 as judged by a board-certified neurologist familiar with our participants was not affected by age-related atrophy, hence, these measurements focused on the primary lesion, rather than any atrophic change. As total brain volume loss was the only predictor of P4 AFQT score (β = −0.213, t= −4.36, p < 0.001), all other areas of brain involvement were excluded from further analyses.
AFQT scores at P4 were negatively correlated with total brain volume loss (r = −0.375, p < 0.001), such that greater loss of brain tissue was associated with lower AFQT percentile scores.
3.4. What can we learn from the individual subtests of the AFQT?
The AFQT test has four main measures that assess verbal comprehension (vocabulary subtest), visual-spatial imagery (boxes subtest), arithmetic word problems (math subtest) and object-function matching (tools subtest). Pearson correlation between the four subtest scores ranged between 0.51- 0.69, and the overall scale reliability was high (Cronbach’s alpha = 0.84).
A univariate linear regression analyses was completed to assess whether performance in the individual subtests of the AFQT at P4 could predict change in AFQT over time. Scores in the Boxes subtest significantly predicted changes in intelligence from P3 to P4 (F(1,148) = 4.15, p = 0.043), such that grater decline between P3 and P4 associated with lower score on the boxes subtest in P4. From pre-injury to P4, performance on both boxes (F(1,131) = 7.62, p = 0.007) and tools (F(1,131)= 6.98, p = 0.009) subtests predicted decline in intelligence.
A stepwise regression analysis was performed to look for lesion location predicting the score on individual subtests of the AFQT in P4. The independent variables included total volume loss, right hemisphere volume loss, left hemisphere volume loss, frontal, parietal, temporal and occipital lobes volume loss, and the lateral involvement of the dlPFC and the vmPFC. Total brain volume lost was the sole predictor of scores in boxes (t = −4.03, p < 0.001), math (t = −4.661, p < 0.001), and tools subtest (t = −4.121, p < 0.001). Scores in the vocabulary subtest were best predicted by volume loss in the left hemisphere (t = −5.541, p < 0.001).
3.5. What predicts recent change in intelligence level?
pTBI and control groups did not differ in how their AFQT percentile scores changed from P3 to P4. A stepwise regression analysis was performed to look for predictors of the change in AFQT scores from P3 to P4 in our patients. The dependent variables included right hemisphere volume loss, left hemisphere volume loss, total brain volume loss and the overall involvement of the frontal lobe, as well as the lateral involvement of the dlPFC and the vmPFC. None of the variables significantly predicted the change in AFQT scores from P3 to P4. However, we found that the group of veterans who suffered significant damage to the left vmPFC (more than 15% damage to this region, n = 18; see Fig. 2), showed exacerbated decline from P3 to P4 compared to the control group (ML vmPFC = −7.00, SD = 9.9, Mcontrol==1.03, SD = 9.70, t(49)= −2.803, p = 0.007, d = 0.81), and a marginal difference compared to the group of pTBI patients with no damage to the left vmPFC (M = −0.9, SD = 13.38, t(105)= −1.82, p = 0.071, d = 0.51) (see Fig. 3).
Fig. 2.
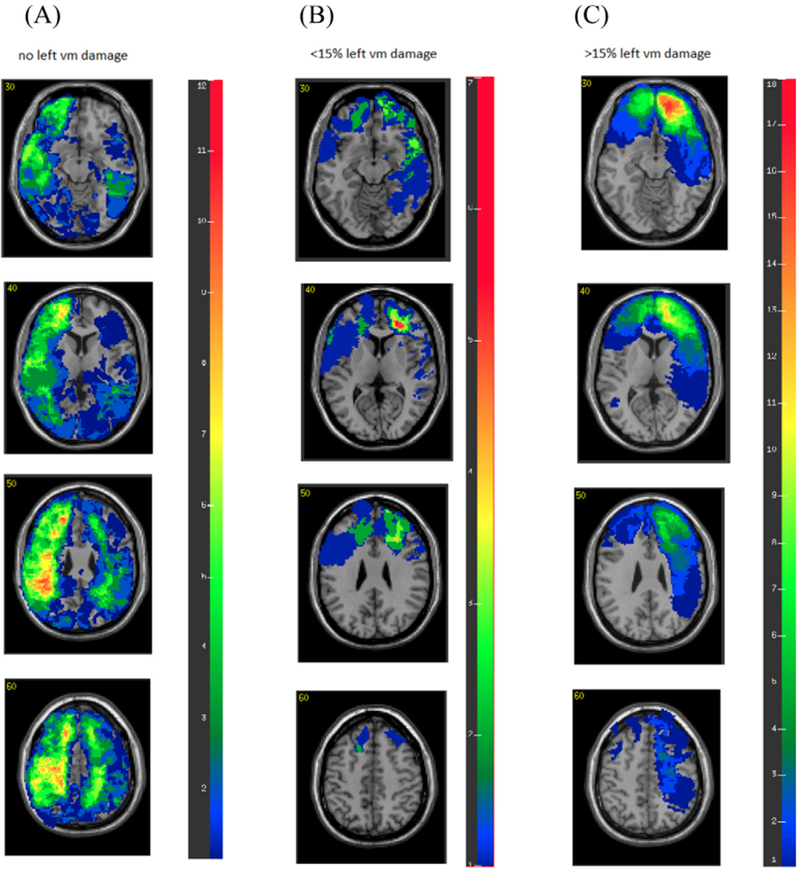
Overlay brain lesion maps of pTBI group. (A) Participants with no left vmPFC damage (n = 88). (B) Participants with less than 15% left vmPFC damage (n = 13). (C) Participants with more than 15% left vmPFC damage (n = 18). In each slice, the right hemisphere is on the reader’s left.
Fig. 3.
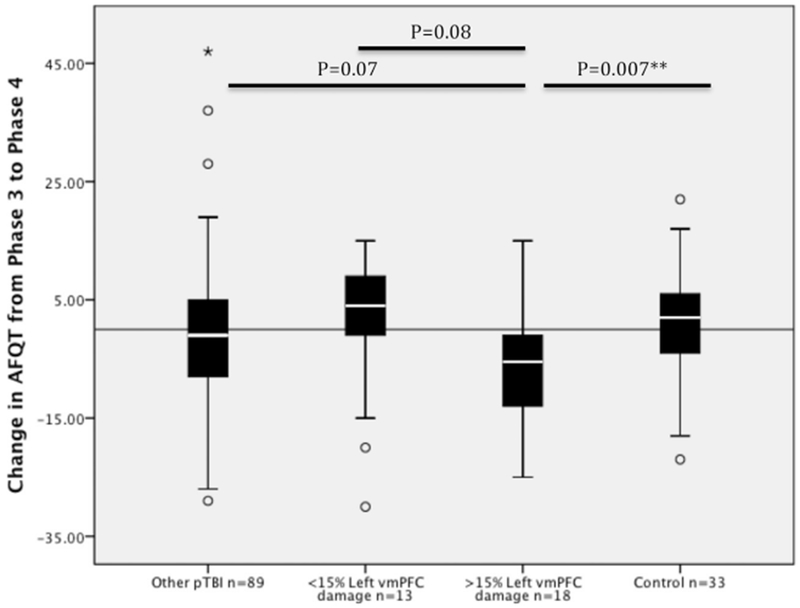
Box plot of mean change in AFQT score from P3 to P4 according to subject group. The box shows the Inter Quartile Range (IQR) of the difference score between P3 and P4, and the white bar in the boxes shows the median. Black solid line across the graph represents no change in AFQT scores from P3 to P4.
◯ Outliers with values between 1.5 and 3 box lengths from the upper or lower edge of the box.
*An extreme outlier (a value more than 3 times the inter-quartile range from a quartile).
**p < 0.01.
While exacerbated decline in intelligence (as measured by change in AFQT score from P3 to P4) was negatively correlated with left vmPFC brain damage (r = −0.18, p = 0.04), it was not significantly correlated with total brain volume loss or a brain lesion in any other frontal lobe region.
3.6. Is there evidence of dementia?
The Mini-Mental State Examination (MMSE [28];) is a commonly used screening tool designed to detect significant cognitive decline and dementia. A score below 24 out of a possible 30 is considered indicative of likely dementia, yet only 3.5% (n = 4) of our participants had a recorded score of below 24 out of 30 on the MMSE, all of which in the pTBI group. None of these 4 participants was a ApoE ε4 carrier. An MMSE score below 27 out of 30 is sometimes used to indicate mild cognitive decline [29]. Of our participants, 9.7% (n = 11) had a recorded score of below 27 on the MMSE, again all in the pTBI group. Two of these participants were ApoE ε4 carriers. Not surprisingly, these participants with a score of below 27 on the MMSE had a significantly lower AFQT score at P4 (t(111)= −2.68, p = 0.008, d = 0.84), but they were not different in the level of decline in intelligence from P3 to P4 compared to those with higher scores (t(111) = 0.89, p = 0.92, d = 0.02), suggesting that their cognitive decline was not reflecting a recent exacerbation predictive of dementia.
Those with MMSE scores below 27 had significantly larger lesions (mean total volume loss = 66.10cc for those with lower scores versus 32.27cc to those with higher scores; t(87)= 2.73, p = 0.008, d = 0.89). Further analysis revealed that this effect was primarily driven by a difference in brain volume loss in the left hemisphere (left volume loss = 58.62cc for those with lower scores versus 12.02cc for those with higher scores; t(87)= 5.96, p < 0.001, d = 1.46). Of note, the MMSE score did not correlate with age or with total years of education.
We also recorded if participants had any family history of dementia. This included 15.8% of pTBI participants and 6.1% of controls. No significant differences in terms of current MMSE score or decline in intelligence from P3 to P4 between those with and without a family history of dementia were found.
The univariate linear regression procedure containing pre-injury intelligence, race, age, rank, education and experimental group as covariates was repeated, adding MMSE score and family history of dementia into the model. Neither MMSE score or family history of dementia significantly affected the change in AFQT percentile scores between P3 to P4.
3.7. How do AFQT scores relate to behavioral measures?
3.7.1. Neurobehavioral rating scale (NBRS)
Significant rank order correlations were found between AFQT percentile scores at P4 and the NBRS total pathology score, both for the pTBI (rho = −0.587, p < 0.001) and control groups (rho = −0.474, p = 0.005), reflecting a strong association between lower intelligence score and more severe behavioral pathology. We did not find a significant correlation between the change in intelligence over the last decade (P4–P3) and behavioral pathology in the pTBI group (rho = −0.10, p = 0.27). Surprisingly, in the control group we found a positive correlation, (rho = 0.380, p = 0.029) reflecting an association between decline in intelligence over the past decade and less behavioral pathology.
3.7.2. Caregiver burden
A lower AFQT percentile score at P4 correlated with increased caregiver burden for the pTBI group (r = −0.29, p = 0.003). However, we did not find a similar association among the healthy control group participants (r = −0.081, p = 0.68). Ratings of caregiver burden were not correlated with the change in AFQT scores between P3 to P4 in either group.
4. Discussion
In this study we investigated the effects of brain injury acquired in young adulthood on late-midlife intelligence. Several studies have argued that a history of TBI, combined with brain changes associated with normal aging, might lead to exacerbated cognitive decline in older adults [6,7,21,30]. This study supports this hypothesis by showing that participants who had pTBI in their 20 s have lower intelligence scores in their seventh decade of life compared to matched controls. This effect remains significant after controlling for age, years of education and pre injury intelligence score.
Importantly, AFQT scores of both the pTBI (54%ile) and control (71%ile) groups at P4 fall within the normal range, and neither group had evidence of dementia 45 years post injury. Hence, findings from this study do not support the idea that a history of pTBI increases a person’s risk of developing AD. In our sample, 102 out of 113 combat veterans with a history of pTBI had normal MMSE scores in their 60 s, even when the strict cut of > 27 points was employed. This lack of evidence for dementia in this population might question the sensitivity of the MMSE as a measure of dementia in this age range. A more comprehensive screening test such as the Montreal Cognitive Assessment (MoCA) [31] is advised for use in future research with participants in their 60 s. Nonetheless, it is important to note that all 11 participants who did have abnormal MMSE scores were pTBI patients. This heightened risk for cognitive decline might be an expected outcome when brain changes associated with normal aging start to occur in a neural system that has already experienced pTBI-related damage.
Our data is consistent with the claim that individuals with dementia do not usually have a history of TBI, and survivors of TBI do not invariably acquire dementia later in life [32]. In fact, many of the studies reporting a higher risk of dementia after TBI do not include genetic risk (ApoE ε4 gene) as a factor in their analysis, and do not consider exacerbated decline as an alternative diagnosis.
Yet, the sample in this study is fairly young as the mean age of participants is 63.4 years. While many of the studies, reporting a higher risk of dementia after TBI, found that at a younger age (e.g. [33],) the risk for AD diagnosis increase dramatically after 65 years of age and there are some evidence to suggest that a history of TBI increases the risk of AD for people in their 80s [34]. Further investigation of the risk among older participants is needed to draw clear conclusions.
Lastly, the reported lack of evidence of early-stage dementia in our study might be an under-estimation of the risk in the general population since pTBI participants with the lowest pre-injury AFQT scores at P3 tended not to attend P4 of the study. This implies that we were only able to review the long-term predictors among veterans who were probably least at risk for exacerbated cognitive decline [20]. Based on the assumption that individuals with depleted cognitive resources are more prone to future decline, it is possible that if we had tested the entire larger P2 cohort in P4 of the study, we would have found even greater levels of cognitive decline.
Another goal of this study was to better understand the timeline of general cognitive decline following pTBI. We found that the gap in intelligence scores between the pTBI and control groups at P4 was similar to the one found seven years earlier at P3. In other words, the exacerbated decline we report at P4 is not a result of a recent, late-life deterioration, but is more likely to be an outcome of a slow gradual decline that follows brain injury and reflects the additional burden of neuronal loss with aging. An exception to this general trend is a subgroup of 18 participants who suffered significant injuries to the left vmPFC, and declined significantly in their AFQT scores between P3 and P4.
This intriguing finding is consistent with results form a PET study [35] in which less activation of a medial-frontal region correlated with diminished cognitive performance in a sample of healthy adults in their late 50 s, suggesting a special role for the midfrontal cortex in age-related and exacerbated cognitive decline. In addition, the reported laterality effect is consistent with a previous study suggesting that left hemisphere injuries have a greater impact than right hemisphere injuries on exacerbated cognitive decline [6]. Moreover, a recent study [36] found exacerbated executive decline among patients with frontal- but not non-frontal- brain damage, suggesting that when impaired, the cognitive processes mediated by the frontal lobes are more likely to results in a later life exacerbated cognitive decline in intelligence. Nonetheless, note that this is a relatively small group of participants with left vmPC lesions, and further support for this finding is needed.
As found in prior studies, we confirmed that the AFQT percentile score obtained at induction into the military is the most consistent predictor of late-life AFQT score, together with the presence of a TBI [7,15,37,38]. Higher pre-injury scores are associated with a lesser degree of decline 40 years post injury, as are years of education and military rank, supporting the idea of cognitive reserve as a protective factor. Cognitive reserve is a theoretical concept that proposes that greater lifetime engagement in cognitively stimulating activities modifies the brain in such a way that the negative effects of brain pathology associated with normal aging or dementia are reduced [39,40].
A previous study from our group indicated that a BDNF polymorphism has an effect on the early-recovery of general intelligence after pTBI (P2 AFQT scores), although it has no influence on pre-injury intelligence [15]. Since BDNF polymorphism was only weakly associated with intelligence scores at P3, the authors proposed a mechanism of lesion-induced plasticity that is decreased over time. The negative genetic findings in the current study are consistent with this hypothesis since no association was found between BDNF polymorphism and AFQT scores at P4. While previous findings suggest the ApoE ε4 allele can predict poor outcome following TBI [13,41–43], note that most of this literature refers to participants with closed - not penetrating- TBI.
In terms of daily functioning, we found some evidence suggesting that lower AFQT scores were associated with notable behavioral sequelae following TBI. To our knowledge there are no previous reports on association between intelligence score and neurobehavioral performance, and further research will help identify the nature of this association. Moreover, lower intelligence was associated with higher levels of burden reported by the patient’s caregiver. The primary caregiver plays a crucial role attending to the needs of the patient, and exacerbated cognitive decline may lead to increased emotional, social, and economic long-term burden as a result.
Lastly, it is important to note that the finding reported in this study are limited by studying a self-selected male sample who agreed to come to Bethesda, Maryland from all over the United States, and the results may have been different if we had been able to assess a more representative group at all phases of the study.
3.1. Conclusions
In conclusion, our findings suggest that patients with TBI are at risk of later life exacerbated decline in intelligence, however the rate of late-life decline in the seventh decade over 7 years is similar for individuals with and without TBI. Pre-injury intelligence turned out to be the best predictor of outcome 45 years after the injury although the volume of brain damage predicts lower intelligence scores later in life. The presence of pTBI did not increase the risk for dementia. Professionals working with individuals who suffered TBI in their past need to be aware of the long term effects of TBI and should also consider the added burden to their caregivers when estimating their future health care needs.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. The authors are grateful to all the Vietnam veterans who participated in this study. Without their long-term commitment to improving the health care of veterans this study could not have been completed. We thank the National Naval Medical Center for their support and provision of their facilities.
Financial disclosure
Jordan Grafman received funds from the Smart Family Foundation of New York and the Julius N. Frankel Foundation.
Barry Gordon and Jordan Grafman were supported by the Therapeutic Cognitive Neuroscience Fund.
Carola Salvi was supported by NIH grant no. T32 NS047987.
Footnotes
Note: Questions regarding the Vietnam Head Injury Study can be directed to Dr. Jordan Grafman.
Declarations of interest
None
Ethical statement
I testify on behalf of all co-authors that our article submitted to Trends in Neuroscience of Education Titled “Intelligence Across the Seventh Decade in Patients with Brain Injuries Acquired in Young Adulthood” has not been published in whole or in part elsewhere; the manuscript is not currently being considered for publication in another journal, and all authors have been personally and actively involved in substantive work leading to the manuscript, and will hold themselves jointly and individually responsible for its content.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tine.2018.08.001.
References
- [1].Lyons MJ, Panizzon MS, Liu W, McKenzie R, Bluestone NJ, Grant MD, Franz CE, Vuoksimaa EP, Toomey R, Jacobson KC, Reynolds CA, Kremen WS, Xian H, A longitudinal twin study of general cognitive ability over four decades, Dev. Psychol 53 (2017) 1170–1177, 10.1037/dev0000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Larsen L, Hartmann P, Nyborg H, The stability of general intelligence from early adulthood to middle-age, Intelligence 36 (2008) 29–34, 10.1016/j.intell.2007.01.001. [DOI] [Google Scholar]
- [3].Schwartzman AE, Gold D, Andres D, Arbuckle TY, Chaikelson J, Stability of intelligence: a 40-year follow-up, Can. J. Psychol. Can. Psychol 41 (1987) 244–256, 10.1037/h0084155. [DOI] [PubMed] [Google Scholar]
- [4].Königs M, Engenhorst PJ, Oosterlaan J, Intelligence after traumatic brain injury: meta-analysis of outcomes and prognosis, Eur. J. Neurol 23 (2016) 21–29, 10.1111/ene.12719. [DOI] [PubMed] [Google Scholar]
- [5].Wood RLI, Rutterford NA, Long-term effect of head trauma on intellectual abilities: a 16-year outcome study, J. Neurol. Neurosurg. Psychiatry. 77 (2006) 1180 LP–1184 http://jnnp.bmj.com/content/77/10/1180.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Corkin S, Rosen TJ, V Sullivan E, Clegg RA, Penetrating head injury in young adulthood exacerbates cognitive decline in later years, J. Neurosci 9 (1989) 3876 LP–3883 http://www.jneurosci.org/content/9/11/3876.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J, Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury, Brain 131 (2008) 543–558, 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- [8].Moustafa AA, Hassan M, Hewedi D, Garami J, Alashwal H, Zaki N, Seo SY, Cutsuridis V, Angulo SL, Hewedi E, Natesh JY, Herzallah MM, Frydecka D, Misiak B, Hornberger M, Genetic underpinnings in Alzheimer’s disease – a review, Rev. Neurosci 29 (2018) 21–38. [DOI] [PubMed] [Google Scholar]
- [9].Yu J-T, Tan L, Hardy J, Apolipoprotein E in Alzheimer’s disease: an update, Annu. Rev. Neurosci 37 (2014) 79–100, 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- [10].Liu C-C, Kanekiyo T, Xu H, Bu G, Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy, Nat. Rev. Neurol 9 (2013) 106–118, 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Müller K, Ingebrigtsen T, Wilsgaard T, Wikran G, Fagerheim T, Romner B, Waterloo K, Prediction of time trends in recovery of cognitive function after mild head injury, Neurosurgery 64 (2009) 698–704 doi: 10.1227/01.NEU.0000340978.42892.78. [DOI] [PubMed] [Google Scholar]
- [12].Yue JK, Robinson CK, Burke JF, Winkler EA, Deng H, Cnossen MC, hester Lingsma F, adam Ferguson R, Mcallister TW, Rosand J, Burchard EG, marco Sorani D, Sharma S, Nielson JL, Satris GG, Talbott JF, Tarapore EP, Korley FK, Wang KWK, Yuh EL, Mukherjee P, Diaz-Arrastia R, alex Valadka B, Okonkwo DO, Manley GT, Apolipoprotein E epsilon 4 (APOE - ε4) genotype is associated with decreased 6 - month verbal memory performance after mild traumatic brain injury, Brain Behav (2017) 1–13, 10.1002/brb3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ariza M, Pueyo R, Matarín M.delM. , Junqué C, Mataró M, Clemente I, Moral P, Poca MA, Garnacho Á, Sahuquillo J, Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury, J. Neurol. Neurosurg. Psychiatry. 77 (2006) 1191 LP–1193 http://jnnp.bmj.com/content/77/10/1191.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hayes JP, Reagan A, Logue MW, Hayes SM, Sadeh N, Miller DR, Verfaellie M, Wolf EJ, McGlinchey RE, Milberg WP, Stone A, Schichman SA, Miller MW, BDNF genotype is associated with hippocampal volume in mild traumatic brain injury, Genes Brain Behav (2017), 10.1111/gbb.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rostami E, Krueger F, Zoubak S, Monte OD, Raymont V, Pardini M, Hodgkinson CA, Goldman D, Risling M, Grafman J, Bdnf polymorphism predicts general intelligence after penetrating traumatic brain injury, PLoS One 6 (2011) 1–13, 10.1371/journal.pone.0027389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krueger F, Pardini M, Huey ED, Raymont V, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J, The role of the Met66 brain-derived neurotrophic factor allele in the recovery of executive functioning after combatrelated traumatic brain injury, J. Neurosci 31 (2011) 598 LP–606 http://www.jneurosci.org/content/31/2/598.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A, Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication, J. Neurol. Neurosurg. Psychiatry. 74 (2003) 857 LP–862 http://jnnp.bmj.com/content/74/7/857.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K, Traumatic brain injury and risk of dementia in older veterans, Neurol. 83 (2014) 312–319 http://www.neurology.org/content/83/4/312.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raymont V, Salazar AM, Krueger F, Grafman J, “Studying injured minds” – the Vietnam head injury study and 40 years of brain injury research, Front. Neurol (2011) 1–13, 10.3389/fneur.2011.00015 March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Smutok MA, Vance SC, Intellectual function following penetrating head-injury in Vietnam veterans, Brain 111 (1988) 169–184. [DOI] [PubMed] [Google Scholar]
- [21].Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J, Cognitive decline in older adults with a history of traumatic brain injury, Lancet Neurol 11 (2012) 1103–1112, 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- [22].Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, Schaie KW, Panizzon MS, Boake C, Xian H, Toomey R, Eisen SA, Kremen WS, Genes Determine stability and the environment determines change in cognitive ability during 35 years of adulthood, Psychol. Sci 20 (2009) 1146–1152, 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levin HS, High WM, Goethe KE, Sisson RA, Overall JE, Rhoades HM, Eisenberg HM, Kalisky Z, Gary HE, The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician, J. Neurol. Neurosurg. Psychiatry. 50 (1987) 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zarit SH, Todd PA, Zarit JM, Subjective burden of husbands and wives as caregivers: a longitudinal study, Gerontologist 26 (1986) 260–266. [DOI] [PubMed] [Google Scholar]
- [25].Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J, Quantification of brain lesions using interactive automated software, Behav. Res. Methods Instrum. Comput 34 (2002) 6–18, 10.3758/BF03195419. [DOI] [PubMed] [Google Scholar]
- [26].Solomon J, Raymont V, Braun A, Butman JA, Grafman J, User-friendly software for the analysis of brain lesions (ABLe), Comput. Methods Progr. Biomed 86 (2007) 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain, Neuroimage 15 (2002) 273–289. [DOI] [PubMed] [Google Scholar]
- [28].Folstein MF, Folstein SE, McHugh PR, “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res. 12 (1975) 189–198. [DOI] [PubMed] [Google Scholar]
- [29].O’Bryant SE, Humphreys JD, Smith GE, Ivnik RJ, Graff-Radford NR, Petersen RC, Lucas JA, Detecting dementia with the mini-mental state examination (MMSE) in highly educated individuals, Arch. Neurol 65 (2008) 963–967, 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Klein M, Houx PJ, Jolles J, Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age, J. Nerv. Ment. Dis 184 (1996) 459–467. [DOI] [PubMed] [Google Scholar]
- [31].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment, J. Am. Geriatr. Soc 53 (2005) 695–699, 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [32].Plassman BL, Grafman J, Traumatic Brain Injury and Late-Life Dementia, First ed., Elsevier Ltd., 2015, 10.1016/B978-0-444-63521-1.00044-3. [DOI] [PubMed] [Google Scholar]
- [33].DE B, AL B, RC G, KH S, Boscardin W, Yaffe K, Association of mild traumatic brain injury with and without loss of consciousness with dementia in us military veterans, JAMA Neurol (2018), 10.1001/jamaneurol.2018.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barnes D, Krueger K, Byers A, Diaz-Arrastia R, Yaffe K, Traumatic brain injury and risk of dementia in older veterans, Alzheimer’s Dementia 7 (2011) S347, 10.1016/j.jalz.2011.05.1006. [DOI] [Google Scholar]
- [35].Pardo JV, Lee JT, Sheikh SA, Surerus-Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW, Where the brain grows old: decline in anterior cingulate and medial prefrontal function with normal aging, Neuroimage 35 (2007) 1231–1237, 10.1016/j.neuroimage.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cipolotti L, Healy C, Chan E, MacPherson SE, White M, Woollett K, Turner M, Robinson G, Spanò B, Bozzali M, Shallice T, The effect of age on cognitive performance of frontal patients, Neuropsychologia 75 (2015) 233–241, 10.1016/j.neuropsychologia.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grafman J, Salazar A, Weingartner H, Vance S, Amin D, The relationship of brain-tissue loss volume and lesion location to cognitive deficit, J. Neurosci 6 (1986) 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gao B, Jiang S, Wang X, Chen J, The role of pre-injury IQ in the determination of intellectual impairment from traumatic head injury, J. Neuropsychiatry Clin. Neurosci 12 (2000) 385–388, 10.1176/jnp.12.3.385. [DOI] [PubMed] [Google Scholar]
- [39].Soldan A, Pettigrew C, Cai Q, Wang J, Wang M-C, Moghekar A, Miller MI, Albert M, Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease, Neurobiol. Aging. 60 (2017) 164–172, 10.1016/j.neurobiolaging.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stern Y, Cognitive reserve, Neuropsychologia 47 (2009) 2015–2028, 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Teasdale GM, Murray GD, Nicoll JAR, The association between APOE ε4, age and outcome after head injury: a prospective cohort study, Brain 128 (2005) 2556–2561, 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- [42].Lichtman SW, Seliger G, Tycko B, Marder K, Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation, Neurol. 55 (2000) 1536–1539 http://www.neurology.org/content/55/10/1536.abstract. [DOI] [PubMed] [Google Scholar]
- [43].Davidson J, Cusimano MD, Bendena WG, Post-traumatic brain injury: genetic susceptibility to outcome, Neuroscience 21 (2014) 424–441, 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


