Abstract
Rationale:
Aim of this study is to evaluate the cognitive and motor outcomes after a combined rehabilitative training using a standard cognitive approach and virtual reality (VR), in a patient with spinal cord injury (SCI).
Patient's concerns:
A 60-year-old right-handed man, affected by incomplete cervical SCI, came to our observation for a moderate tetraparesis, mainly involving the left side, after about 6-months from the acute event. The neurological examination showed imbalance with upper limb incoordination, besides the paresis mainly involving the left side. At a neuropsychological evaluation, he presented important impairment in cognitive and behavioural status, with temporal and spatial disorientation, a reduction of attention and memory process, deficit of executive function and a severe depression of mood, which was not detected during the previous recovery.
Diagnosis:
Motor and cognitive deficits in SCI.
Interventions:
The patient was 1st submitted to standard cognitive training and traditional physiotherapy, and then to a combined therapeutic approach, in which virtual reality training was provided by means of the virtual reality rehabilitation system (VRRS, Khymeia, Italy).
Outcomes:
After the combined therapeutic approach with the VRRS training, we observed a significant improvement in different cognitive domains, a notable reduction of anxiety and depressive symptoms, as well as motor performance, and balance improvement.
Lessons:
Virtual reality can be considered a promising tool for the rehabilitation of different neurological disorders, including patients with both motor and cognitive deficits following SCI.
Keywords: neurorehabilitation, spinal cord injury, virtual reality, VRRS
1. Introduction
Spinal cord injury (SCI) consists of temporary or permanently loss of motor, sensory, or autonomic functions after either traumatic or non-traumatic spinal cord damage. Every year, spinal trauma, affects about an individual in a thousand. An Italian epidemiologic survey[1] has found 1014 new cases of spinal cord injury (SCI) in 2 years, most trauma (67.5%). Among these, almost the 57% showed a SCI close to the neck, which can cause total or partial quadriplegia and an alteration of the muscle tone, depending on the specific position, and the seriousness of the trauma.[2] The location and type of spinal cord damage determine the different clinical pictures (ranging from various levels of incomplete forms up to a complete SCI), and the functional and rehabilitation outcomes.[3]
The SCI can cause important disability, mainly involving the motor function. Sensory-motor as well as genito-urinary, neuro-vegetative or breathing alterations may also be present. Cognitive dysfunctions sometimes may be detected, but they are not related to the injury. The model spinal cord injury system (MSCIS)[4] observed that 28% of the patients affected by acute SCI tend to have minor brain injuries, and among these, only 12% presents cognitive or behavioral alterations. Kreutzer et al[5] have observed a chronic deficit in visual verbal learning, in the visual organization and attention in 30 patients affected by SCI, even if they did not reveal evident traumatic brain injuries. Moreover, patients affected by SCI shows high levels of depression and anxiety, which tend to decrease during their rehabilitative period even if their prevalence remains unexpectedly high.[6] In literature, some studies[7,8] highlighted that cognitive and emotional-behavioral symptoms in SCI (concentration, attention and memory deficits) can be a consequence of the loss of consciousness or of a post-traumatic amnesia. Nonetheless, these symptoms can also be a consequence of predisposing risk factors connected to some aspects of the patients’ everyday life,[9,10] to their level of education,[6] to their stress and other socio-demographic factors, or to prolonged anesthesia. Many traditional and innovative techniques can be useful for the rehabilitation of motor and cognitive deficits,[11,12] however; in the last decade, virtual reality (VR) has had a growing role in the treatment of different neurological disorders.[13,14]
The VR is the term used to indicate a simulated reality: it consists in the use of computer technologies to create artificial environments, similar to the real ones with which the patient can interact. The “virtual rehabilitation” puts together the advantages of a cognitive and motor stimulation with a recreational and highly ecologic role, so that the patients’ motivation and pleasure grows during the rehabilitative session, with a simulation of everyday life activities. These aspects have relevant consequences on both motor and cognitive abilities, with regard to attention, memory and executive processes,[15] supporting higher awareness and knowledge of results and performance. Indeed, thanks to these virtual scenarios, the central nervous system receives an increased sensory feedback, which is able to produce changes in the synaptic plasticity, to reinforce motor learning.[16]
Among the various tools using VR, the virtual reality rehabilitation system (VRRS) is a promising device for the rehabilitation of a wide range of neurological disorders, thanks to its modularity. The VRSS has 3 main rehabilitative modules: Neurologic (including motor, cognitive; and logopedic activities; Cardiorespiratory, and Orthopedic, whose use is giving promising results.[17–19]
The aim of this study is to evaluate the cognitive and motor outcomes after combined rehabilitative training using a standard cognitive approach and virtual reality, in a patient with SCI.
2. Case description
A 60-year-old right-handed man, affected by incomplete cervical vertebro-spinal trauma, presented with a moderate tetraparesis, mainly involving the left side. The patient, an oncologist lining in Messina (Sicily, Italy), was married and has 2 children (a 27-year-old male and 23-year-old female). His parents were both dead: the father for natural causes and mother from breast cancer, while he and her older sisters were healthy. The psychomotor development of the patient was normal and he practiced sport regularly; at a young age, he was a soccer player, and he liked cycling. The patient also liked reading, swimming and travelling, and he had a very intense social life. He was a moderate coffee drinker; he did not drink alcohol and was a non-smoker.
In November 2016, following a severe car accident resulting in SCI, he was admitted to a neurosurgical ward for a total decompressive laminectomy at C3–4 level. He attended the rehabilitation center for about 4 months, with moderate motor improvements. At discharge, an assessment of cognitive status was not performed. The patient was then admitted to the (blinded), to undergo a specific neurorehabilitation cycle (i.e. around 6 months after the SCI).
At our observation, the neurological examination showed a moderate left-hemiparesis and imbalance with upper limb incoordination (that was present only in the eye-closed condition) and reduced sensitivity (hypoesthesia). At a neuropsychological evaluation, he presented several alterations in the cognitive and behavioral status, with temporal, spatial and autobiographic disorientation, reduction of attention and memory processes, deficit of the executive function and severe anxious-depressive symptoms. No other medical comorbidities were detected, and hematochemical tests, as well as cardiac evaluation, and cerebral magnetic resonance, were normal.
Patient gave informed consent for the diagnostic procedures, treatment, and publication of the case.
2.1. Procedures
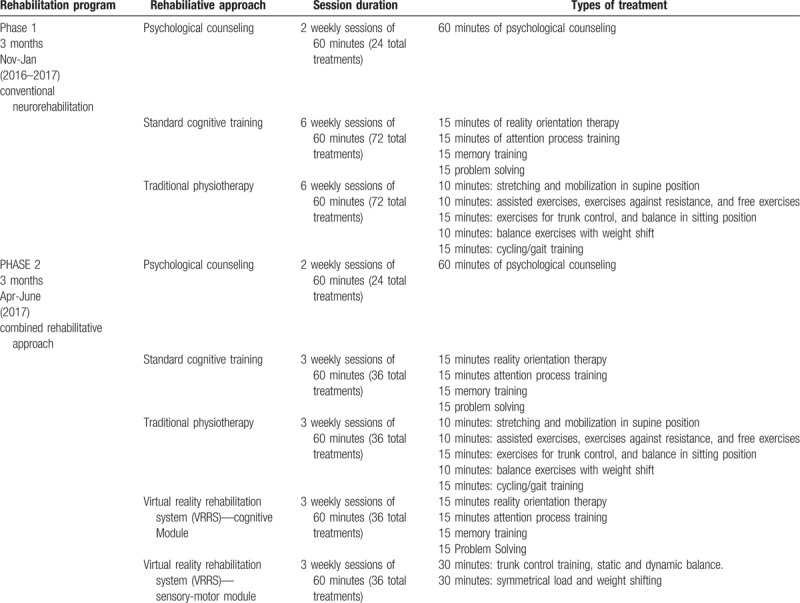
As the patient was in a post-traumatic chronic phase, to manage his cognitive, motor and emotional status, we decided to treat him by using a specific rehabilitation protocol, including 2 different types of training. The patient was adequately informed about the study and offered his collaboration and written consent. In the 1st phase, the patient was submitted to a conventional treatment, including psychological counselling, standard cognitive training (SCT) and physiotherapy (PT). After the 1st phase, 4 weeks of latency have elapsed before the next phase, in which the patient was only treated with physiotherapy (to avoid a cumulative effect). Then, the patient was submitted to the experimental protocol (second phase; i.e. a combined therapeutic approach, cognitive and sensory-motor training was provided by means of the virtual reality rehabilitation system (VRRS, by Khymeia, Italy), besides the standard Bobath physical therapy).
However, the patient was provided with the same amount of treatment both in phase 1 and 2; in particular, in the 1st phase the patient underwent 72 sessions of PT and 72 of SCT, whereas in the 2nd phase the patient was submitted to 36 PT sessions and 36 SCT sessions, besides 72 VRRS sessions (both cognitive and motor). The entire rehabilitation treatment lasted about 6 months: the patient was hospitalized for 3 months, then, he stayed at home for 1 month, and came back as inpatient for other 3 months (for more details see Table 1).
Table 1.
Standard and experimental training performed by the patient.
2.2. Functional assessment
The patient was evaluated by a skilled neuropsychologist through the administration of specific neuropsychological battery, including Montreal cognitive assessment (MoCA) to evaluate general cognitive status, attentive matrices test (CAM); trail making test (TMT), digit span and the Rey auditory verbal learning immediate and recall (RAVLI, RAVLR), Weigl's sorting test, Raven's coloured matrices; verbal fluency test (VFT) and semantic fluency test (SFT) to assess spontaneous speech, comprehension and communication skills, Hamilton rating scale for depression (HRS-D) and Hamilton rating scale for anxiety (HRS-A).
A physiotherapist administered the functional independence measure (FIM) (after discussion with the rehabilitation team), and the Berg balance scale (BBS) to assess functional recovery and balance (Table 2). Both the neuropsychologist and physiotherapist were blinded to the patient's treatment. We evaluated the patient's cognitive and motor profile in 2 separate phases, before and after the 2 different trainings (T0: baseline; T1: at the end of the traditional neurorehabilitation training; T2: at the beginning of the combined experimental approach; and T3: after the combined approach). Reliable change index (RCI) was used to evaluate whether a change in an individual's score (i.e., between T0 and T1, and T2 and T3) was significant or not (based on how reliable the measure is). The RCI allows to define if the change observed in a patient is clinically and practically significant, based on the amount of change that a patient has to show on a specific psychometric instrument between the measurement occasions in such a way that a difference is more significant than reasonably expected for any measurement errors. The RCI is a statistic used to determine if a change occurring in the score of an individual (or group) is statistically significant based on the test-retest reliability of the measurement.[20] This provides information about the likelihood that a change in test scores “results from” true or reliable change or results from the case. To calculate RCI as suggested by Bauer et al[21] we used a specific formula. In this study, we added other scores. We administered same criteria to a half-treatment evaluation, to monitor the changes, and in the end the post-treatment scores. We used the standard deviation (SD) from the functional normative sample, and coefficient alpha from the normative sample for the scale to obtain the minimum change in a scale score calculation. The standard error of the difference will be dependent on the measure's standard error, which involves the standard deviation of the normative sample of the instrument and the test reliability. The result represents a standard score. Values ≥ 1.96 or ≤ 1.96 are representative of a true change at a 95% confidence level (P-value = .05), unlikely to be explained by measurement error. Given that it has been demonstrated that RCI could be useful in assessing changes in the neuropsychological field,[22] we used this index also for motor functional outcomes.
Table 2.
shows the patient's neuropsychological and motor assessment.
2.3. Conventional rehabilitation training
The conventional treatment consisted of psychological counselling, standard cognitive training (SCT) and physiotherapy (PT). Counselling was focused to reduce anxiety and depression symptoms, using a narrative and introspective training to help both the patient and his family. The SCT was based on a face-to-face approach between the therapist and the patient using paper and pencil tools. It was mainly focused on strengthening orientation with a specific cognitive program based on the reality orientation therapy (ROT); autobiographic memory, temporal and spatial orientation, and simple relationships and logic associations were also trained. To improve attention, we used the attention process training (ATP) that includes task targeting for the sustained, selective, split and alternating attention. The memory enhancement goal was achieved by working on recognition and remembrance tasks with verbal and nonverbal material, reminiscence and validation therapy, mnemonic techniques and strategic skills. The training of the executive function was reached by working on categorization, planning, association and analogical reasoning (Table 3). The PT was provided according to Bobath approach, aimed at improving balance, reducing spasticity and increasing left side muscle force.
Table 3.
Cognitive rehabilitative program, including the standard and the experimental (VRRS) one.

2.4. Combined neurorehabilitation approach
The combined neurorehabilitation approach consisted of the virtual reality (VR) treatments, in addition to the conventional rehabilitation approach. This kind of approach was provided by the virtual reality rehabilitation system (VRRS), one of the most advanced comprehensive and clinically proven VR system for rehabilitation[19,23] and telerehabilitation.[24] Extremely easy to use, high customization capacity, complete automated reporting, functional telerehabilitation, are some of the guiding principles of continuous system development. The VRRS, in fact, is conceived as a “central HUB” to which you can connect via USB a series of specialized peripherals, fully synchronized and integrated with the system. The VRRS can be used in many neurological disorders, thanks to the different modules for cognitive, language, postural, and motor rehabilitation.
During the cognitive training, the patient was sitting in front of the device, actively interacting with the platform. The VRRS cognitive module consists in a large set of activities for rehabilitation, with more than 50 exercises already available and many others under development. All activities are organized by cognitive function: memory, attention, language, spatial-temporal orientation, planning, reasoning and other executive function, calculation, and praxis. Cognitive exercises provided through the VRRS can be classified in 2 main categories. The 1st category includes 2D exercises where the patient interacts with objects and scenarios through the touch screen or through a particular magnetic tracking sensor coupled with a squeezable object, thus emulating mouse-like interaction capabilities. The 2nd category consists of 3D exercises, where the patients interacts with 3D on immersive virtual scenarios and objects through a magnetic tracking sensor generally placed over the hand (that permits a 3D position tracking of the end effector). Furthermore, cognitive tasks are generally coded as pick and place activities, ordering activities, selection activities, and sequential selection activities (Table 3).
The VRRS motor program includes specific tasks of the virtual sensory motor to stimulate muscle strengthening, strengthen leg tendons and ligaments, improve posture, pelvis movements, and balance reactions. All virtual exercises have been planned and organized by the therapist (after consultation with the neurologist), with increasing difficulty in relation to the time of execution and the category of activity. In fact, in the 1st training phase, the therapist used the stabilometric platform to increase the static balance. Supine mobilization and assisted and free exercises for trunk control and balance in a sitting position, along with exercises with weight shift and walking, were carried out. At a later stage, the therapist used a proprioceptive/dynamic platform, characterized by greater instability and greater executive difficulty, with training of the trunk control, in static and dynamic equilibrium and weight displacement (Figure 1).
Figure 1.
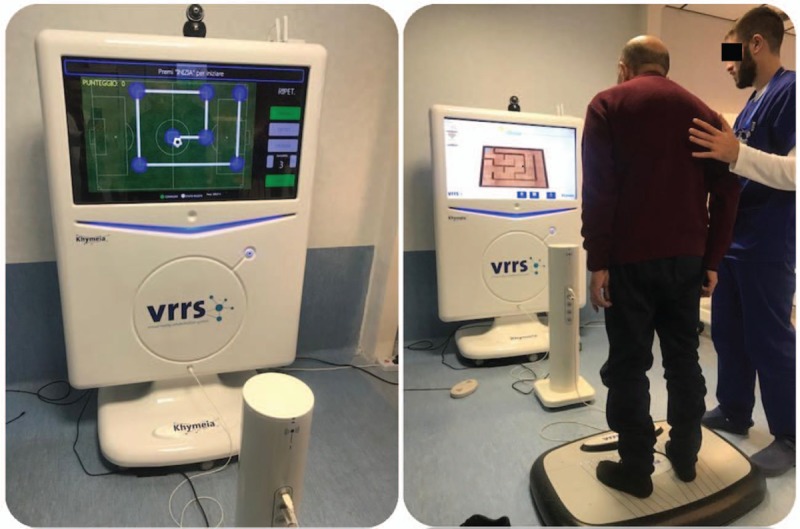
A: the patient is located on a dynamic platform shifting the weight thought right, left, forward, and back and following the indicated path so to reach different points in the football field. The score is calculated according to the symmetry between the required trajectory and the trajectory performed. B: (Virtual labyrinth) The patient, situated on a static footboard, is trained by moving a ball inside the labyrinth to reach a specific destination; scoring is calculated in relation to the time of execution and the number of errors.
At the end of the traditional treatment (T1) using standard techniques, the patient presented a mild improvement only in orientation and mood, with a reduction of negative thoughts, besides a better postural control. Only at the end of combined approach using VRRS, we observed a significant improvement in different cognitive domains such as executive process, selective attention, memory abilities, and spatial cognition; moreover, we have seen a substantial reduction in anxiety's level and depressive symptoms (Table 4), with the optimization of global motor performance and balance improvement, in both static and dynamic control, as per Berg balance scale (BBS) score (Table 5).
Table 4.
Cognitive outcome evaluated at baseline (T0), after the standard training (T1), at the beginning of the combined experimental approach (T2), and after such approach (T3).
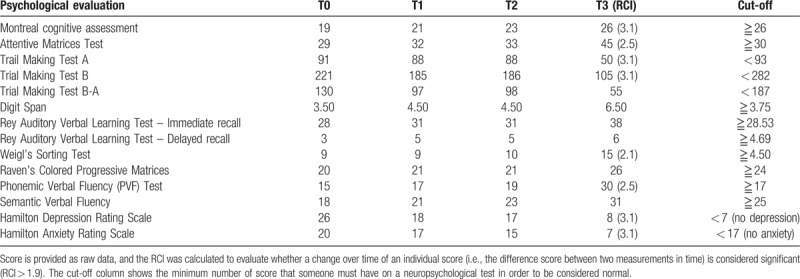
Table 5.
Motor outcome evaluated at baseline (T0), after the standard training (T1), at the beginning of the combined experimental approach (T2), and after such approach (T3).

3. Discussion
Spinal cord injury is a condition that causes total or partial tetra or paraplegia and alteration of the muscle tone[2] with possible sensory deficit, genito-urinary, neurovegetative, and respiratory symptoms. Generally speaking, no cognitive impairment is found[2] although, as described by Hartmann et al,[25] our case shows that the evaluation and treatment of neuropsychological and motor deficits may eventually contribute to a better functional outcome. We are not completely able to state if cognitive deficit pre-existed the accident or total anaesthesia; however, the impairment appeared after this event, and it was neglected during the 1st rehabilitative intervention. The accurate neuropsychological evaluation we carried out led us to the detection of the cognitive decline for which allowed us proper management of the disorder. Therefore, the rehabilitative team should consider applied neuropsychology as an integral part of the rehabilitation process, even when no cognitive deficits are referred[26] in patients including those with spinal cord injury (SCI).
In this report, we want to underline the important role of innovative devices using virtual reality (VR) in improving both motor and cognitive performances.
In the last decades, in the field of rehabilitation, we have witnessed the development of innovative tools that make use of VR. This consists of a set of computing technologies that can create interactive environments involving the user in simulating real world activities. Several clinical and experimental applications demonstrated the effectiveness of these technologies on people of all ages, affected by neurological and motor disorders and spasticity of different etiologies.[11,14] In addition, VR may promote psychological wellbeing, participation and autonomy of a person with a disability, including SCI, avoiding monotone activities, to have a positive effect on the self-esteem, the motivation and mood.
Shin et al[26] has shown that VR has a positive impact on cognitive functions, and literature confirms that the use of new technologies in cognitive rehabilitation is an important resource.
The VR is an efficient approach to neurological rehabilitation that creates more stimulating environments, is more effective in improving problem solving skills and performing functional tasks.[27] In fact, VR allows the calibrating of the difficulty of proposed activities based on the actual capabilities and the potential of the patient which therapist is dealing with. In addition, it is possible to constantly measure and monitor performance by providing a wide range of responses. It has been demonstrated that VR technology, providing a higher feedback on the characteristics of motion, can improve motor learning and performance in both healthy and post-stroke subjects compared to traditional training.[27]
The VR can also be a “afe” tool to simulate everyday life activities that are not always accessible to a person with disability (i.e. simulating alpine skiing or playing musical instruments). One last advantage is the possibility that some VR systems pursue home-based rehabilitation[15] by prolonging the time spent to perform the exercises in terms of cost and effectiveness of the intervention itself. Among the several existing tools, virtual reality rehabilitation system (VRRS) allows the creation of different simulated scenarios so that the therapist can prepare a patient tailored rehabilitation. Indeed, VRRS is designed to put the patient in a situation that generates increased feedback to his central nervous system (augmented feedback) through exercises performed in a virtual environment helping to develop the knowledge of the results of movements (knowledge of the results) and the knowledge of the quality of movements (knowledge of performance), leading to a training-specific motor learning. In this way, the central nervous system can activate a physiological learning mechanism called “reinforcement learning” which implies an increase in the specific information of a movement to produce an effective improvement in performance quality. Indeed, the patient treated with the VRRS device was submitted to an intensive stimulation as visual and auditory feedbacks given by both the therapist and the device may have boosted neuroplasticity and thus functional recovery.[19,28–29] This can partly explain the reason why our patient had clearly better outcomes after the experimental training with VR, as demonstrated both by the scales administered to the patient and by the patient himself, who rated his improvement in quality of life as 7 in a 0 to 10 Likert scale. Our case-report suggests that VRRS may be a valuable tool in promoting better functional outcomes, also in patients with SCI, when coupled to traditional rehabilitation. However, our conclusions need confirmation as our findings comes from a single case report, which represents the main limitation of this study. Further studies are needed to confirm what extent cognitive deficits in SCI individuals are underestimated.
4. Conclusions
With this case-report we want underline the possibility that our combined standard cognitive training-virtual reality rehabilitation system (SCT-VRRS) approach could be effective in improving motor and cognitive recovery in patients with spinal cord injury (SCI). However, a complete neuropsychological evaluation should be performed in all the patients attending a neurorehabilitation ward, in order to treat properly possible and underestimated cognitive deficits with better functional outcomes.
Acknowledgment
The authors wish to thank Dr Antonina Donato for English editing.
Author contributions
Conceptualization: Giuseppa Maresca, Maria Grazia Maggio, Rosaria De Luca, Rocco Salvatore Calabrò.
Data curation: Giuseppa Maresca, Maria Grazia Maggio, Antonio Buda, Gianluca La Rosa, Rocco Salvatore Calabrò.
Investigation: Antonio Buda, Gianluca La Rosa, Alfredo Manuli, Placido Bramanti, Rosaria De Luca.
Methodology: Maria Grazia Maggio, Alfredo Manuli, Placido Bramanti, Rosaria De Luca, Rocco Salvatore Calabrò.
Supervision: Placido Bramanti, Rocco Salvatore Calabrò.
Validation: Gianluca La Rosa, Placido Bramanti, Rocco Salvatore Calabrò.
Visualization: Giuseppa Maresca, Antonio Buda, Alfredo Manuli.
Writing – original draft: Giuseppa Maresca, Maria Grazia Maggio.
Writing – review & editing: Giuseppa Maresca, Maria Grazia Maggio, Rosaria De Luca, Rocco Salvatore Calabrò.
Footnotes
Abbreviations: ATP = attention process training, BBS = Berg balance scale, CAM = attentive matrices test, FIM = functional independence measure, HRS-A = Hamilton rating scale for anxiety, HRS-D = Hamilton rating scale for depression, MoCA = Montreal cognitive assessment, MSCIS = the model spinal cord injury system, PT = physiotherapy, RAVLI = Rey auditory verbal learning immediate, RAVLR = Rey auditory verbal learning recall, RCI = reliable change index, ROT = reality orientation therapy, SCI = spinal cord injury, SCT = standard cognitive training, SD = standard deviation, SFT = semantic fluency test, TMT = trail making test, VFT = verbal fluency test, VR = virtual reality, VRRS = virtual reality rehabilitation system.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Pagliacci MC, Celani MG, Spizzichino L, et al. Spinal cord lesion management in Italy: a 2-year survey. Spinal Cord 2003;41:620–8. [DOI] [PubMed] [Google Scholar]
- [2].Sabharwal S. Frontera WR, Silver JK, Rizzo TD., Jr Spinal cord injury (Cervical). Elsevier Health Sciences, Essentials of physical medicine and rehabilitation. Berlin: 2014. [Google Scholar]
- [3].Lin VW, Cardenas DD, Cutter NC, et al. Spinal cord medicine: principles and practice. New York: Demos Medical Publishing; 2002. [Google Scholar]
- [4].Go BK, De Vivo MJ, Richard JS. Stover SL, DeLisa JA, Whiteneck GG. The epidemiology of spinal cord injury. Spinal cord injury: clinical outcomes from the model systems. Gaithersburg: Aspen; 1995. 21–51. [Google Scholar]
- [5].Kreutzer JS, Barth J, Ellwood MS, et al. Occult neuropsychological impairments in spinal cord injured patients. Arch Phys Med Rehabil 1988;69:764–5. [Google Scholar]
- [6].Craig A, Guest R, Tran Y, et al. Depressive mood in adults with spinal cord injury as they transition from an inpatient to a community setting: secondary analyses from a clinical trial. Spinal Cord 2017;55:926–34. [DOI] [PubMed] [Google Scholar]
- [7].Davidoff G, Thomas P, Johnson M, et al. Closed head injury in acute traumatic spinal cord injury: incidence and risk factors. Arch Phys Med Rehabil 1988;69:869–72. [PubMed] [Google Scholar]
- [8].Davidoff G, Morris J, Roth E, et al. Closed head injury in spinal cord injured patients: retrospective study of loss of consciousness and post-traumatic amnesia. Arch Phys Med Rehabil 1985;66:41–3. [PubMed] [Google Scholar]
- [9].Merritt VC, Rabinowitz AR, Arnett PA. Injury-related predictors of symptom severity following sports-related concussion. J Clin Exp Neuropsychol 2015;37:265–75. [DOI] [PubMed] [Google Scholar]
- [10].Meehan WP, Mannix R, Monuteaux MC, et al. Early symptom burden predicts recovery after sport-related concussion. Neurology 2014;83:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 2011;92:519–30. [DOI] [PubMed] [Google Scholar]
- [12].De Luca R, Calabrò RS, Gervasi G, et al. Is computer-assisted training effective in improving rehabilitative outcomes after brain injury? A case-control hospital-based study. Disabil Health J 2014;7:356–60. [DOI] [PubMed] [Google Scholar]
- [13].Martinez Moreno JM, Solana Sánchez J, Sanchez Carrion R, et al. Monitoring visual attention on a neurorehabilitation environment based on Interactive Video. International Conference on Recent Advances in Neurorehabilitation 2013. 182–4. [Google Scholar]
- [14].De Luca R, Calabrò RS, Bramanti P. Cognitive rehabilitation after severe acquired brain injury: current evidence and future directions. Neuropsychol Rehabil 2016. [DOI] [PubMed] [Google Scholar]
- [15].Larson E, Feigon M, Gagliardo P, et al. Virtual reality and cognitive rehabilitation: a review of current outcome research. Neuro Rehabil 2014;34:759–72. [DOI] [PubMed] [Google Scholar]
- [16].Subramanian S, Prasanna S. Virtual reality and non-invasive brain stimulation in stroke: How effective is their combination for upper limb motor improvement? International Conference on Virtual Rehabilitation (ICVR);2017:1–8. [DOI] [PubMed] [Google Scholar]
- [17].Laver K, George S, Thomas S, et al. Virtual reality for stroke rehabilitation: an abridged version of a Cochrane review. Eur J Phys Rehabil Med 2015;51:497–506. [PubMed] [Google Scholar]
- [18].Laver KE, George S, Thomas S, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2011;9:CD008349. [DOI] [PubMed] [Google Scholar]
- [19].Turolla A, Dam M, Ventura L, et al. Virtual reality for the rehabilitation of the upper limb motor function after stroke: a prospective controlled trial. J Neuroeng Rehabil 2013;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–9. [DOI] [PubMed] [Google Scholar]
- [21].Bauer S, Lambert MJ, Nielsen SL. Clinical significance methods: a comparison of statistical techniques. J Pers Assess 2014;82:60–70. [DOI] [PubMed] [Google Scholar]
- [22].Gavett BE, Ashendorf L, Gurnani AS. Reliable change on neuropsychological tests in the uniform data set. J Int Neuropsychol Soc 2015;21:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carpinella I, Jonsdottir J, Lencioni T, et al. Planar robotic rehabilitation of upper limb in post-stroke subjects: transfer of training effects to a non-trained 3D functional task. Gait Posture 2016;49:23–4. [Google Scholar]
- [24].Portaro S, Calabrò RS, Bramanti P, et al. Telemedicine for facio-scapulo-humeral muscular dystrophy: a multidisciplinary approach to improve quality of life and reduce hospitalization rate? Disabil Health J 2017;pii: S1936-6574(17)30168-1. [DOI] [PubMed] [Google Scholar]
- [25].Hartmann A, Kegelmeyer D, Kloos A. Use of an errorless learning approach in a person with concomitant traumatic spinal cord injury and brain injury: a case report. J Neurol Phys Ther 2018;42:102–9. [DOI] [PubMed] [Google Scholar]
- [26].Harvey PD. Clinical applications of neuropsychological assessment. Dialogues Clin Neurosci 2012;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shin H, Kim K. Virtual reality for cognitive rehabilitation after brain injury: a systematic review. J Phys Ther Sci 2015;27:2999–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rose FD, Brooks BM, Rizzo AA. Virtual Reality in brain demage rehabilitation: review. CyberPsichol Behav 2005;8:3. [DOI] [PubMed] [Google Scholar]
- [29].Cheung VC, Turolla A, Agostini M, et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proceedings of the National Academy of Sciences 2012;109:14652–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


