Abstract
Background:
The argument on whether extracorporeal shock-wave therapy (ESWT) and corticosteroid injections (CSIs) exert an equivalent pain control or which is the better treatment for plantar fasciitis (PF) in adults remains to be resolved. It is important and necessary to conduct a meta-analysis to make a relatively more credible and overall assessment about which treatment method performs better pain control in treatment of PF in adults.
Methods:
From the inception to July 2018, the Embase, PubMed, Web of Science, and Cochrane Library electronic databases were searched for all relevant studies. Only randomized controlled trials (RCTs) focusing on comparing ESWT and CSI therapies in PF cases in adults were included. The primary outcome measure was visual analog scale (VAS) reduction, whereas the secondary outcomes included treatment success rate, recurrence rate, function scores, and adverse events.
Results:
Nine RCTs involving 658 cases were included in this meta-analysis. In the present study, meta-analysis showed that high-intensity ESWT had superior pain relief and success rates relative to the CSI group within 3 months, but the ESWT with low intensity was slightly inferior to CSI for efficacy within 3 months. In addition, patients with CSI may tend to increase the need for the analgesic and more adverse events may be associated with the ESWT. However, the ESWT and CSI present similar recurrent rate and functional outcomes.
Conclusion:
Our analysis showed that the pain relief and success rates were related to energy intensity levels, with the high-intensity ESWT had the highest probability of being the best treatment within 3 months, followed by CSI, and low-intensity ESWT. More high-quality RCTs with long-term follow-up time are needed to further compare the differences of CSI and ESWT for adults with PF.
Keywords: corticosteroid injection, extracorporeal shock-wave therapy, meta-analysis, plantar fasciitis
1. Introduction
Plantar fasciitis (PF), as a musculoskeletal problem, is a common cause of chronic plantar heel pain in the adult population,[1,2] with accounting for approximately 11% to 15% of all foot symptoms.[3,4] It is manifested as pain caused by insertion of plantar fascia near the medial calcaneal tubercle.[5] The pathophysiology of PF still remains unknown and may be caused by biomechanical overstress of the calcaneal tuberosity or poor foot biomechanics.[6–10] The possible risk factors consist of increasing age, higher body mass index (BMI), walking and standing too long, and some anatomic factors such as foot deformities, leg length discrepancy, and intrinsic muscle weakness.[11–13]
Conservative treatment measures of PF include bed rest, reduce weight, nonsteroidal anti-inflammatory drugs, and physiotherapies such as ultrasound, stretching of the plantar fascia, and laser therapy.[14,15] For patients who are refractory to conservative treatment methods, corticosteroid injection (CSI) is an effective method to treat this condition. It has been used for treating PF since 1950s and is still widely used because of its rapid pain relief, easy availability, and low cost.[16] However, serious side effects of CSI such as PF rupture have been reported.[17,18] Recently, many other treatment modalities consisting of extracorporeal shock-wave therapy (ESWT), intralesional botulinum toxin A, and platelet-rich plasma have been developed to treat patients who are refractory to conservative treatment.[19–21] ESWT is a pulsed sound wave, characterized by short duration, high-pressure amplitude and relatively low tensile wave component. The mechanism of ESWT is not completely clear. However, it is speculated that ESWT may produce a reflexive analgesic effect by inducing excitability of the axon and destroying unmyelinated sensory fibers.[22] Several recent trials have suggested that ESWT may induce nitric oxide production, which plays a critical role in suppressing the inflammatory process.[23] It has been reported that ESWT is safe and effective therapy for patients with chronic musculoskeletal diseases such as chordae tendinitis, plantar fasciitis, and tennis elbow.[24,25] The argument on whether ESWT and CSI exert an equivalent pain control or which is the better treatment remains to be resolved.
To our knowledge, some randomized controlled trials (RCTs) have compared the effectiveness of these 2 treatments in PF, but drawn different conclusions.[22,26–33] Limited by small number of studies and insufficient outcomes, previous meta-analyses also failed to draw a consistent conclusion of clear superiority of 1 therapy over the other.[34,35] Thus, it is important and necessary to conduct a meta-analysis to make a relatively more credible and overall assessment about which treatment method performs better pain control in treatment of PF in adults.
2. Materials and methods
2.1. Search strategy
Two independent investigators followed The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines[36] and the recommendations of the Cochrane Collaboration[37] to conduct the present meta-analysis. From the inception to July 2018, the Embase, PubMed, Web of Science, and Cochrane Library electronic databases were searched using the key phrases “shock wave,” “corticosteroid,” “plantar fasciitis,” “plantar fasciopathy,” “glucocorticoid,” and “steroid” for all relevant studies. Moreover, references cited by the relevant sources were also hand searched to identify any additional articles that could not be found in our database query. There were no language restrictions. Ethical approval was not necessary because the present meta-analysis was performed on the basis of previous published studies.
2.2. Inclusion and exclusion criteria
Study included in our meta-analysis had to meet the following criteria: patients with PF and without injection history; RCTs focusing on comparing ESWT and CSI therapies in PF cases for adults; at least one of the following outcome measures was reported: numerical rating scale or visual analog scale (VAS) reduction, function scores, treatment success rate, recurrence rate, and adverse events. Articles with no assessment of outcomes mentioned above or no comparison of ESWT and CSI therapies were not included into meta-analysis. Duplicate reports and conference abstracts were excluded. Case reports, retrospective studies, biochemical trials, letters, and reviews were also eliminated.
2.3. Data extraction
Two independent authors extracted the following descriptive raw information from the selected studies: study characteristics such as author, study design, study language, publication year, mean follow-up period; patient demographic details such as number of foots and patients (only adults), average age, BMI, and gender ratio; ESWT intensity, details of interventions, and outcome measures. The primary outcome measure was VAS reduction, whereas the secondary outcomes included treatment success rate, recurrence rate, function scores, and adverse events. The data would be collected into different subgroups of similar periods when a study reported outcomes at multiple follow-up time. If the data were missing or could not be extracted directly, we contacted the corresponding authors to ensure that the information integrated. Otherwise, we calculated them with the guideline of Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. If necessary, we would abandon the extraction of incomplete data.
2.4. Data analysis
The present study was performed by Review Manager Software (RevMan Version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). Odds ratios (OR) with a 95% confidence interval (CI) or mean difference (MD) with 95% CI were assessed for dichotomous outcomes or continuous outcomes, respectively. P < .05 was set as the significance level. The heterogeneity was assessed by using the Q test and I2 statistic. When I2 ≥ 50%, it was considered to represent significant heterogeneity, then the outcome was pooled on random-effect model. If I2 < 50%, a fixed-effect model was used. We also conducted the sensitivity analysis to evaluate whether any single study had the weight to skew on the overall estimate and data. The Z test was used to assess the overall effect.
2.5. Risk of bias and quality assessments
The Cochrane risk of bias tool was independently used to evaluate the risk of bias of included RCTs by 2 reviewers. The quality of RCTs was assessed by using following 7 items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Any controversy was resolved by discussing with a 3rd author to reach a final consensus.
The publication bias was assessed by using funnel plots diagram. As many outcome measures were investigated, we only evaluated publication bias of VAS reduction. The funnel plot asymmetry was evaluated by an Egger linear regression test to reveal any possible publication bias.[38]
3. Results
3.1. Search results
On the basis of the key words mentioned above, a total of 1437 potentially relevant citations were initially identified from the 4 electronic databases: 668 from Embase, 328 from PubMed, 428 from Web of science, and 13 from Cochrane library. After excluding 669 duplicate studies, 728 irrelevant articles were deleted based on a title and abstract review. We reviewed full-text of the remaining 40 studies, 31 citations were ruled out for reasons such as systematic reviews, nonrandomized clinical studies, conference abstract, no comparison of ESWT and CSI, and lack of useful outcomes. Ultimately, of the 1437 studies, 9 RCTs fulfilled the selection criteria and were included in the present study (Fig. 1).[22,26–33]
Figure 1.
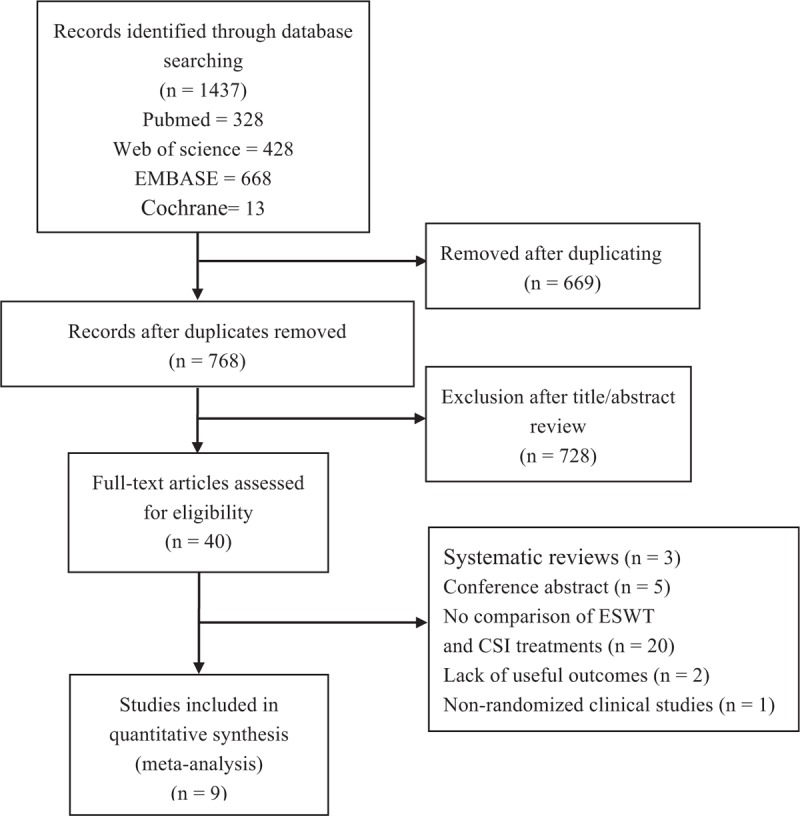
Flow diagram showing details of literature search.
3.2. Characteristics and quality assessment of included RCTs
Tables 1 and 2 summarize the study baseline characteristics and patient demographic details. A total of 9 RCTs directly comparing ESWT and CSI therapies in PF were included, with 8 English articles[22,26,28–33] and 1 Spanish article.[27] A total of 658 cases were enrolled in this meta-analysis, with 330 undergoing ESWT and 328 undergoing CSI. The mean age between the groups were similar, as ESWT group was 40.19 years (34.27–54.53) and 40.18 years in CSI group (P = .98). The rates of male in ESWT group was 31.4% (11.1–80.8%), while in CSI group was 22.6% (12.5–87.5%; P = .13). Only 4 studies provided BMI and mean BMI ranged from 26.8 to 30.21 kg/m2.[22,26,28,31] Four studies[26,28,30,33] compared low-intensity ESWT with CSI, whereas the other 5 studies[22,27,29,31,32] only included high-intensity ESWT. The minimum follow-up period was 6 weeks and the maximum follow-up time was 1 year.
Table 1.
Study characteristics and patient demographic details (part 1).
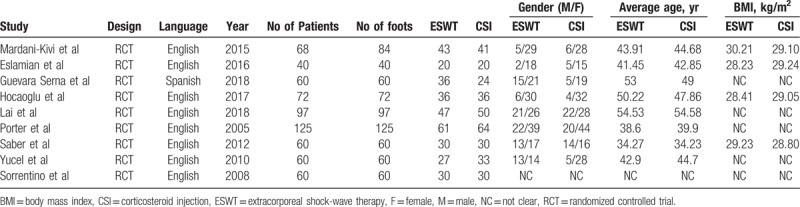
Table 2.
Study characteristics and patient demographic details (part 2).
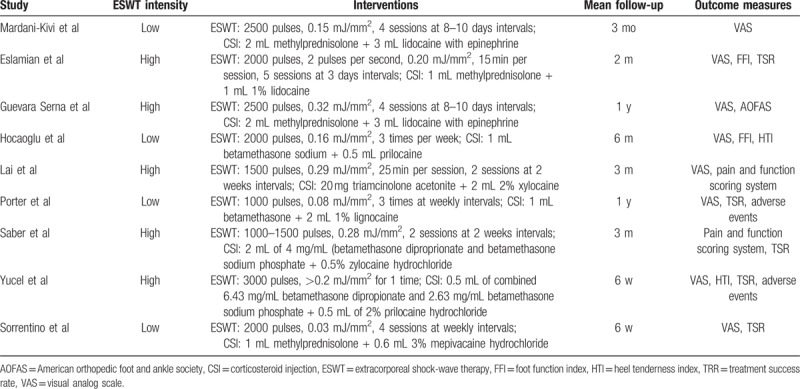
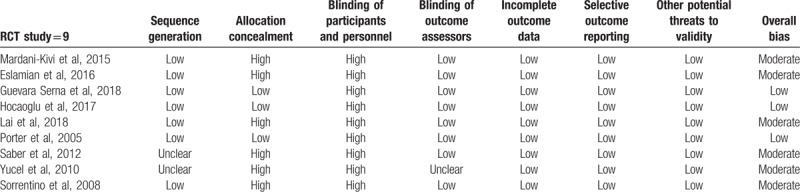
Seven of 9 studies described the methods of randomization, but 2 studies[31,32] failed to provide sufficient details about randomization. Only 3 studies[27,28,30] used appropriate and feasible methods to describe concealment of allocation. There was no blinding of the participants and personnel in all 9 studies. Blinding of outcome assessors was mentioned in all studies. The overall risk of bias from the evidence in our meta-analysis is moderate, with low bias for 3 studies,[27,28,30] and moderate for 6 studies.[22,26,29,31,33] The study qualities are shown in Table 3.
Table 3.
Methodological assessment according to 6 domains of potential biases (cochrane risk of bias tool).
3.3. Synthesis of results and meta-analysis
3.3.1. VAS reduction
Eight trials[22,26–30,32,33] reported on VAS reduction in different follow-up periods. According to the different intensity levels, we divided the studies into 2 subgroups: low intensity (energy < 0.20 mJ/mm2) and high intensity (energy ≥ 0.2 mJ/mm2).
For the low-intensity group (4 studies),[26,28,30,33] the difference of VAS reduction between the 2 groups at 1 to 1.5 months in favor of the CSI group (MD, −2.04; 95% CI, −3.30 to −0.78; P = .002; I2 = 81%, random effect model was used) (Fig. 2A). At 3 months, the pooled data showed that there was also a significant difference in favor of CSI (MD, −1 .67; 95% CI, −3.31 to −0.04; P = .04; I2 = 85%, random effect model was used) (Fig. 2A).
Figure 2.
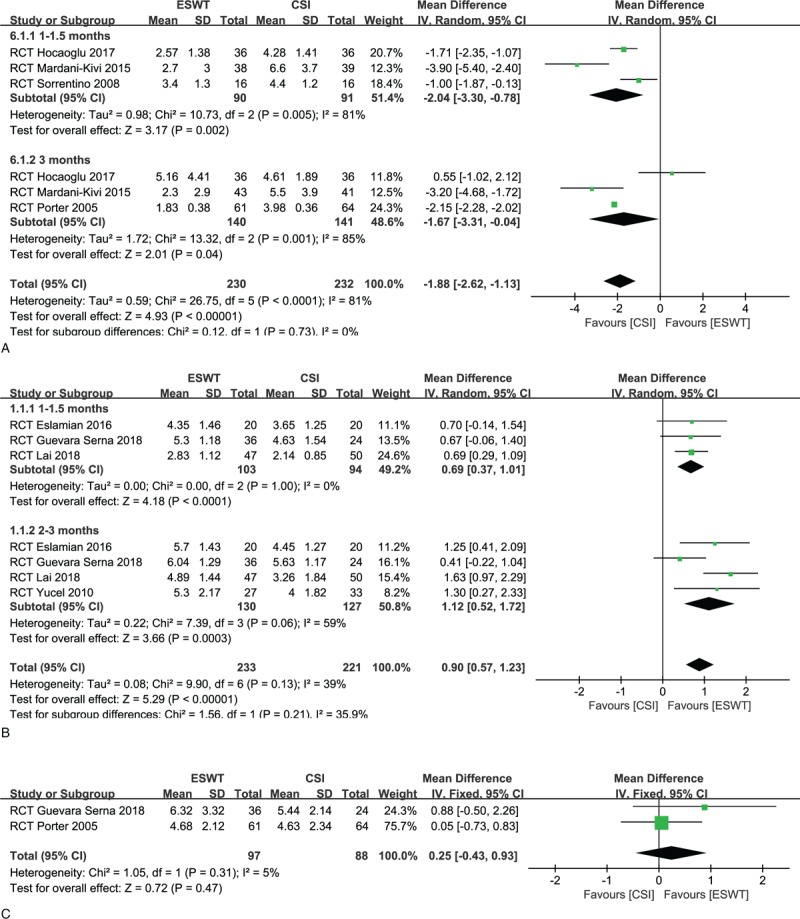
(A) Forest plots of visual analog scale (VAS) reduction in low-intensity extracorporeal shock-wave therapy (ESWT) group and corticosteroid injection (CSI) groups within 3 months. (B) Forest plots of VAS reduction in high-intensity ESWT group and CSI groups within 3 months. (C) Forest plots of VAS reduction in ESWT group and CSI groups at 12 months.
For the high-intensity group (5 studies),[22,27,29,31,32] however, a significant difference between the 2 groups in favor of ESWT (MD, 0.69; 95% CI, 0.37–1.01; P < .0001; I2 = 0%) (Fig. 2B). Also, the ESWT group had superior pain relief relative to the CSI group at 2 to 3 months (MD, 1.12; 95% CI, 0.52–1.72; P = .0003; I2 = 59%, random effect model was used) (Fig. 2B).
Only 1 study[30] with low intensity and 1 study[27] with high intensity compared the pain relief between the CSI and ESWT groups at 12 months. Both of studies showed similar pain reductions in the 2 groups. After pooling data, the differences between the CSI and ESWT groups were not statistically significant at 12 months regardless of energy intensity (MD, 0.26; 95% CI, −0.45 to 0.97; P = .47; I2 = 5%) (Fig. 2C).
3.3.2. Treatment success rate and recurrence rate
Five included trials[22,26,31–33] reported a treatment success rate that was defined as loss of heel tenderness with a decrease in the VAS score of at least 50% from baseline.[22]
For success rate in the low-intensity group (2 studies),[26,33] 21 of 50 patients (42.0%) in the ESWT group and 43 of 50 patients (86.0%) in the CSI group did respond to treatment. The CSI tended to present a high success rate compared to the ESWT with a statistically significant difference (OR, 0.12; 95% CI, 0.04–0.31; P < .0001; I2 = 0%) (Fig. 3A).
Figure 3.
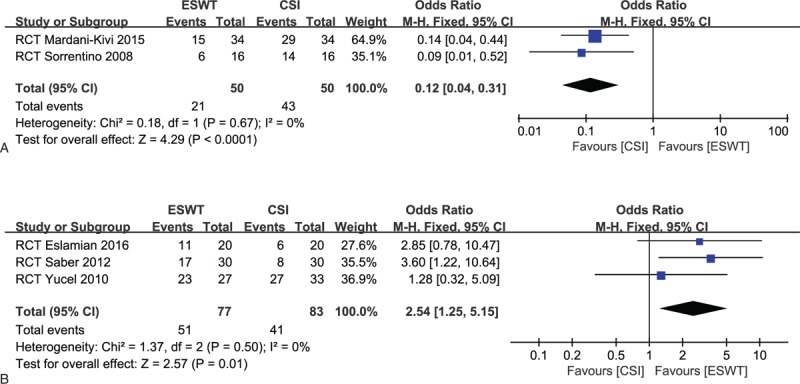
(A) Forest plots of treatment success rate in low-intensity extracorporeal shock-wave therapy (ESWT) group and corticosteroid injection (CSI) groups. (B) Forest plots of treatment success rate in high-intensity ESWT group and CSI groups.
For success rate in the high-intensity group (3 studies),[22,31,32] 51 of 77 patients (66.2%) in the ESWT group and 41 of 83 patients (49.4%) in the CSI group did respond to treatment. The ESWT tended to present a high success rate, compared to the CSI, with a statistically significant pooled result (OR, 2.54; 95% CI, 1.25–5.15; P = .01; I2 = 0%) (Fig. 3B).
Recurrence was defined as an increase of 2 points in the VAS score after recovery.[26] It was only reported in 2 studies.[26,31] Recurrent rate was found in 10 of the 45 cases (22.2%) in ESWT group and in 12 of the 59 patients (20.3%) in CIS group. The meta-analysis showed no significant difference between both groups (OR, 1.52; 95% CI, 0.55–4.20; P = .42; I2 = 0%) (Fig. 4).
Figure 4.
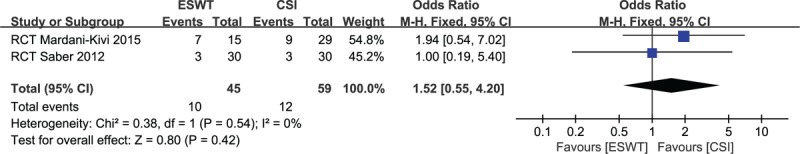
Forest plot of recurrent rate in extracorporeal shock-wave therapy group and corticosteroid injection groups.
3.3.3. Functional outcomes
Five trials[22,27,28,29,31] measured foot functional outcomes using different scoring systems such as Foot Function Index score, American Orthopedic Foot and Ankle Society score, and Mayo Clinic Scoring System score. On the whole, the functional scores of the 2 groups were similar in different follow-up time.
3.3.4. Adverse events
Only 3 of 9 studies reported adverse events.[30–32] No infections or plantar fascia rupture occurred in both groups. One trial showed no complications in either group.[31] Zero of 88 cases (0%) in the ESWT group and 12 of 97 patients (12.4%) in the CSI group required analgesia, with a significant difference in favor of the ESWT group (OR, 0.08; 95% CI, 0.01–0.59; P = .01; I2 = 0%) (Fig. 5A). Two studies reported throbbing pain and erythema.[30,32] It occurred in 10 of 88 subjects (11.4%) in the ESWT group and 0 of 97 subjects (0%) in the CSI group. There was a significant difference in favor of the CSI group (OR, 14.05; 95% CI, 1.76–112.20; P = .01; I2 = 0%) (Fig. 5B). One trial reported that 4 participants had severe headache or migraine in the ESWT group.[30] However, no severe adverse events occurred in the CSI group.
Figure 5.
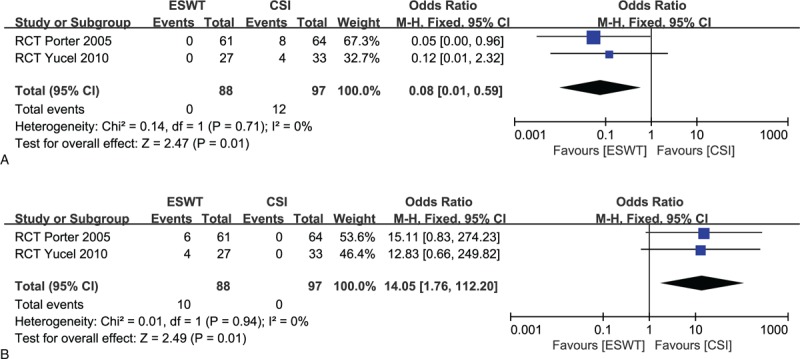
(A) Forest plot of required analgesia in extracorporeal shock-wave therapy (ESWT) group and corticosteroid injection (CSI) groups. (B) Forest plot of throbbing pain and erythema in ESWT group and CSI groups.
3.3.5. Sensitivity analysis and publication bias
Most of the pooled analyses revealed low or no heterogeneity, except for VAS reduction for the high-intensity group at 2 to 3 months (I2 = 59%), VAS reduction for the low-intensity group at 1 to 1.5 months (I2 = 81%) and 3 months (I2 = 85%). In terms of these outcomes, the sensitivity analysis showed that excluding any single study did not change the statistical results, meaning reliable outcomes.
The funnel plots of VAS reduction for the high-intensity group were symmetrical, indicating a low risk of publication bias (Fig. 6).
Figure 6.
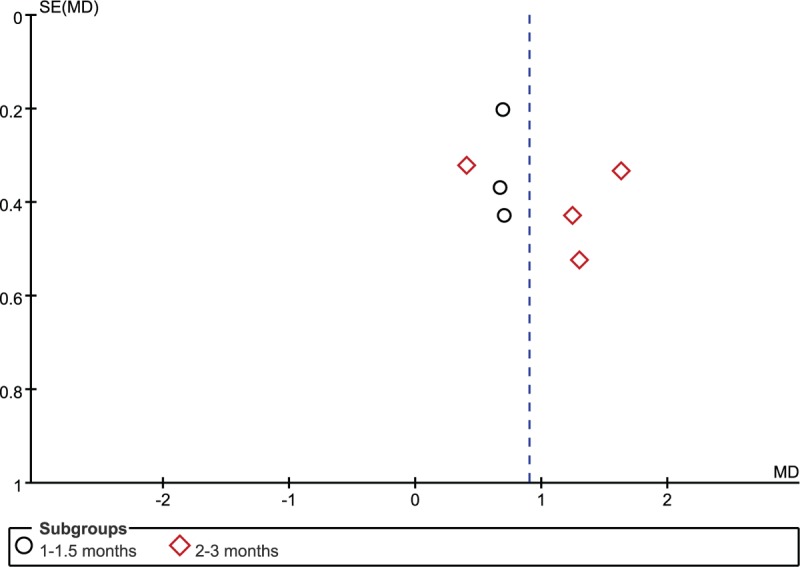
Funnel plots of visual analog scale reduction in high-intensity extracorporeal shock-wave therapy group and corticosteroid injection groups.
4. Discussion
The PF is the most common cause of chronic pain in the heel and is considered to be self-limiting.[30] ESWT and CSI are 2 popular methods with proven efficacy in pain relief and functional improvement in patients who has no response to conservative treatment methods. However, surgeons hold positive attitudes toward CSI because of its advantage in easy availability and low cost.[16] To our knowledge, there remains a controversy regarding the clinical effects of ESWT and CSI methods in treatment of PF. The present meta-analysis was conducted to make a relatively more credible and overall assessment about which treatment method in PF patients has better clinical effects.
In the present study, meta-analysis showed that high-intensity ESWT therapy had reliably highest success rates of treatments and magnitudes of pain reduction against plantar fasciitis, followed by CSI therapy, and low-intensity ESWT. In addition, patients with CSI may tend to increase the need for the analgesic and more adverse events may be associated with the ESWT. However, the ESWT and CSI present similar recurrent rate and functional outcomes.
In our analysis of pain relief, some included studies[27,29,33] preferred the terminal value of the VAS score to compare the pain controls of the 2 groups; however, this measure only reflects the pain status after intervention and cannot reflect the role of intervention in the healing process. Thus we used VAS reduction to compare the value of CSI and ESWT for pain relief before and after treatment. Intensity assessment is crucial for analysis of outcomes following ESWT. It has been reported in previous studies that low-dose ESWT regimen might be less efficacious than higher doses.[22,30] Marks et al carried out a RCT with follow-up of 24 months and found that the effect of placebo treatment was similar to that of low energy ESWT in treating patients with painful heels.[39] Therefore, we conducted a subgroup analysis according to the different intensity levels in ESWT and firstly found that the high-intensity ESWT had the highest probability of being the best treatment within 3 months, but the ESWT with low-intensity was slightly inferior to CSI for VAS reduction within 3 months. The sensitivity analysis indicated reliable outcomes. The differences for pain control between the CSI and ESWT groups were not statistically significant at 12 months. However, owing to the lack of studies in long-term follow-up, the subgroup analysis based on intensity levels could not be carried out, thus conclusion for long-term efficacy remains unknown. In addition, a previous review indicated that ESWT appeared to be dose dependent in the treatment of PF, with greater success seen with higher dose regimes, which was consistent with our analysis.[40]
Except for intensity levels, several other crucial factors such as intervention frequency, total energy, and other related parameters in ESWT should be taken into consideration for further researches. However, many key information were not fully reported in our included trials. We are unable to provide further optimal and practical information for clinicians in this regard.
Treatment success rate was defined as loss of heel tenderness with a decrease in the VAS score of at least 50% from baseline.[22] One study considered the treatment successful with the improvement heel tenderness index score by >50%.[32] Such nonuniform evaluation criteria may cause bias during evaluation. Similar to the investigation for VAS reduction, we also conducted a subgroup analysis based on intensity levels when assessing treatment success rate. Interestingly, our finding showed that the high-intensity ESWT, followed by the CSI, presented the higher success rate, which further strengthens the results of VAS reduction. In addition, the meta-analysis showed a similar recurrence rate between the 2 treatments.
For function outcomes, we could not carry out a meta-analysis because different studies used different functional assessment scales. On the whole, the functional scores of the 2 groups were similar in different follow-up time. We acknowledged that using some functional assessment scales was more clinically useful than reporting success rates of pain reduction. Therefore, unified and effective scoring system should be used in future studies.
Previous reviews that investigated the effects of CSI and ESWT led to inconclusive results.[34,35] A previous network meta-analysis which showed that CSI provided better pain relief at 3 months than ESWT.[34] Only 2 studies[30,32] were included in the quantitative analysis of their study, so their conclusion was debated. Another meta-analysis conducted by Chen et al[35] included 5 RCTs[22,26,30,32,33] and showed that CSI and ESWT were comparable in VAS score reduction at 1 to 3 months follow-up, but with I2 = 95% in their result. However, many defects were existed in their analysis. Firstly, a relatively small sample size (293 cases) was included in their meta-analysis, with a follow-up period of only 3 months; secondly, no sufficient subgroup analysis was performed on the basis of types of ESWT; thirdly, no sensitivity analysis was conducted for high heterogeneity in their results; fourthly, their analysis only discussed the VAS score for 3 months, without fully summarizing the other clinical outcomes.
Our analysis showed that ESWT tend to have less need for additional analgesia compared to CSI in the early stages of the treatments. In general, many patients suffered from postinjection pain after receiving CSI, and the pain lasts an average of a week.[32] On the contrary, patients experienced less pain during the ESWT protocol and had more rapid return to full activities after treatment, which make it an attractive alternative to surgery.[30] Severe headache and throbbing sensation were reported in Roerdink et al[41] that included 39 studies and concluded that ESWT was likely a safe treatment for PF. In the present study, ESWT also seemed to present more adverse events (such as throbbing pain, erythema, and serious headache). To our knowledge, the most feared complication in CSI is the iatrogenic rupture of PF, and risk of other complications such as fat pad atrophy and osteomyelitis of the calcaneus were also associated with this relatively invasive treatment. Although no any complication was reported for CSI in our included studies, every surgeon, however, should be aware of the importance of precise injection, aseptic operation and avoidance of impact activities postinjection when implementing this treatment. More high-quality RCTs with long-term follow-up time are needed to report adverse events following CSI and ESWT.
The main strengths of our study includes the following: all available RCTs directly comparing CSI and ESWT were searched; we extracted and quantified the largest number of clinical outcome variables as far as possible; sufficient subgroup analysis and sensitivity analysis were conducted for high heterogeneity in our results. Many limitations were also included in present meta-analysis as following: only 9 RCTs with 658 cases were included in this meta-analysis, so the outcomes should be treated cautiously; only 2 trials[27,30] had 1 year follow-up, it is difficult to draw convincing conclusions due to the lack of long-term follow-up periods; the analyses of some outcome measures such as VAS reduction at 12 months, recurrent rate, and adverse events were based on a relatively small sample size, so firm conclusions cannot be derived; types and doses of CSI were various, which might generate heterogeneity.
5. Conclusion
The present meta-analysis compared the efficacy of ESWT and CSI therapies in the treatment of PF in adult. Our analysis showed that the pain relief and success rates were related to energy intensity levels, with the high-intensity ESWT had the highest probability of being the best treatment within 3 months, followed by CSI, and low-intensity ESWT. However, owing to the lack of studies in long-term follow-up, the subgroup analysis based on intensity levels could not be carried out, thus conclusion for long-term efficacy remains unknown. More high-quality RCTs with long-term follow-up time are needed to further compare the differences of CSI and ESWT for adults with PF.
Author contributions
Conceptualization: Shuxiang Li, Xiaoliang Sun.
Data curation: Kun Wang.
Formal analysis: Peng Wang.
Investigation: Xiaomin Luo.
Methodology: Han Sun.
Project administration: Sheng Fang.
Resources: Xiaomin Luo.
Software: Han Sun.
Supervision: Xiaoliang Sun.
Validation: Sheng Fang.
Visualization: Haifeng Chen.
Writing – original draft: Shuxiang Li.
Writing – review & editing: Shuxiang Li, Kun Wang, Xiaoliang Sun.
Footnotes
Abbreviations: CSI = corticosteroid injection, ESWT = extracorporeal shock-wave therapy, PF = plantar fasciitis, RCTs = randomized controlled trials, VAS = visual analog scale.
The authors report no conflicts of interest.
References
- [1].Gerdesmeyer L, Frey C, Vester J, et al. Radial extracorporeal shock wave therapy is safe and effective in the treatment of chronic recalcitrant plantar fasciitis: results of a confirmatory randomized placebo-controlled multicenter study. Am J Sports Med 2008;36:2100–9. [DOI] [PubMed] [Google Scholar]
- [2].Ibrahim MI, Donatelli RA, Schmitz C, et al. Chronic plantar fasciitis treated with two sessions of radial extracorporeal shock wave therapy. Foot Ankle Int 2010;31:391–7. [DOI] [PubMed] [Google Scholar]
- [3].Rompe JD, Furia J, Weil L, et al. Shock wave therapy for chronic plantar fasciopathy. Br Med Bull 2007;81-82:183–208. [DOI] [PubMed] [Google Scholar]
- [4].Dizon JN, Gonzalez-Suarez C, Zamora MT, et al. Effectiveness of extracorporeal shock wave therapy in chronic plantar fasciitis: a meta-analysis. Am J Phys Med Rehabil 2013;92:606–20. [DOI] [PubMed] [Google Scholar]
- [5].Tu P, Bytomski JR. Diagnosis of heel pain. Am Fam Physician 2011;84:909–16. [PubMed] [Google Scholar]
- [6].Bergmann JN. History and mechanical control of heel spur pain. Clin Podiatr Med Surg 1990;7:243–59. [PubMed] [Google Scholar]
- [7].Contompasis JP. Surgical treatment of calcaneal spurs. A three-year post-surgical study. J Am Podiatry Assoc 1974;64:987–99. [DOI] [PubMed] [Google Scholar]
- [8].McCarthy DJ, Gorecki GE. The anatomical basis of inferior calcaneal lesions. A cryomicrotomy study. J Am Podiatry Assoc 1979;69:527–36. [DOI] [PubMed] [Google Scholar]
- [9].Mitchell IR, Meyer C, Krueger WA. Deep fascia of the foot. Anatomical and clinical considerations. J Am Podiatr Med Assoc 1991;81:373–8. [DOI] [PubMed] [Google Scholar]
- [10].Kogler GF, Solomonidis SE, Paul JP. Biomechanics of longitudinal arch support mechanisms in foot orthoses and their effect on plantar aponeurosis strain. Clin Biomech (Bristol, Avon) 1996;11:243–52. [DOI] [PubMed] [Google Scholar]
- [11].Buchbinder R, Ptasznik R, Gordon J, et al. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA 2002;288:1364–72. [DOI] [PubMed] [Google Scholar]
- [12].Ogden JA. Extracorporeal shock wave therapy for plantar fasciitis: randomised controlled multicentre trial. Br J Sports Med 2004;38:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Williams SK, Brage M. Heel pain-plantar fasciitis and Achilles enthesopathy. Clin Sports Med 2004;23:123–44. [DOI] [PubMed] [Google Scholar]
- [14].Hammer DS, Adam F, Kreutz A, et al. Extracorporeal shock wave therapy (ESWT) in patients with chronic proximal plantar fasciitis: a 2-year follow-up. Foot Ankle Int 2003;24:823–8. [DOI] [PubMed] [Google Scholar]
- [15].Hammer DS, Adam F, Kreutz A, et al. Ultrasonographic evaluation at 6-month follow-up of plantar fasciitis after extracorporeal shock wave therapy. Arch Orthop Trauma Surg 2005;125:6–9. [DOI] [PubMed] [Google Scholar]
- [16].Lapidus PW, Guidotti FP. Local injections of hydrocortisone in 495 orthopedic patients. Ind Med Surg 1957;26:234–44. [PubMed] [Google Scholar]
- [17].Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int 1998;19:91–7. [DOI] [PubMed] [Google Scholar]
- [18].Sellman JR. Plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int 1994;15:376–81. [DOI] [PubMed] [Google Scholar]
- [19].Schwartz EN, Su J. Plantar fasciitis: a concise review. Perm J 2014;18:105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Goff JD, Crawford R. Diagnosis and treatment of plantar fasciitis. Am Fam Physician 2011;84:676–82. [PubMed] [Google Scholar]
- [21].Placzek R, Deuretzbacher G, Meiss AL. Treatment of chronic plantar fasciitis with botulinum toxin A: preliminary clinical results. Clin J Pain 2006;22:190–2. [DOI] [PubMed] [Google Scholar]
- [22].Eslamian F, Shakouri SK, Jahanjoo F, et al. Extra corporeal shock wave therapy versus local corticosteroid injection in the treatment of chronic plantar fasciitis, a single blinded randomized clinical trial. Pain Med 2016;17:1722–31. [DOI] [PubMed] [Google Scholar]
- [23].Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: A randomized controlled trial. Am J Phys Med Rehabil 2013;92:327–34. [DOI] [PubMed] [Google Scholar]
- [24].Moghtaderi A, Khosrawi S, Dehghan F. Extracorporeal shock wave therapy of gastrocsoleus trigger points in patients with plantar fasciitis: a randomized, placebo-controlled trial. Adv Biomed Res 2014;3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aqil A, Siddiqui MR, Solan M, et al. Extracorporeal shock wave therapy is effective in treating chronic plantar fasciitis: a meta-analysis of RCTs. Clin Orthop Relat Res 2013;471:3645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mardani-Kivi M, Karimi Mobarakeh M, Hassanzadeh Z, et al. Treatment outcomes of corticosteroid injection and extracorporeal shock wave therapy as two primary therapeutic methods for acute plantar fasciitis: a prospective randomized clinical trial. J Foot Ankle Surg 2015;54:1047–52. [DOI] [PubMed] [Google Scholar]
- [27].Guevara Serna JA, Acosta Morón JA. Extracorporeal shockwave therapy versus corticosteroid injection in chronic plantar fasciitis. Rev Colomb Ortop Traumatol 2018;32:43–9. [Google Scholar]
- [28].Hocaoglu S, Vurdem UE, Cebicci MA, et al. Comparative effectiveness of radial extracorporeal shockwave therapy and ultrasound-guided local corticosteroid injection treatment for plantar fasciitis. J Am Podiatr Med Assoc 2017;107:192–9. [DOI] [PubMed] [Google Scholar]
- [29].Lai TW, Ma HL, Lee MS, et al. Ultrasonography and clinical outcome comparison of extracorporeal shock wave therapy and corticosteroid injections for chronic plantar fasciitis: a randomized controlled trial. J Musculoskelet Neuronal Interact 2018;18:47–54. [PMC free article] [PubMed] [Google Scholar]
- [30].Porter MD, Shadbolt B. Intralesional corticosteroid injection versus extracorporeal shock wave therapy for plantar fasciopathy. Clin J Sport Med 2005;15:119–24. [DOI] [PubMed] [Google Scholar]
- [31].Saber N, Diab H, Nassar W, et al. Ultrasound guided local steroid injection versus extracorporeal shockwave therapy in the treatment of plantar fasciitis. Alex J Med 2012;48:35–42. [Google Scholar]
- [32].Yucel I, Ozturan KE, Demiraran Y, et al. Comparison of high-dose extracorporeal shockwave therapy and intralesional corticosteroid injection in the treatment of plantar fasciitis. J Am Podiatr Med Assoc 2010;100:105–10. [DOI] [PubMed] [Google Scholar]
- [33].Sorrentino F, Iovane A, Vetro A, et al. Role of high-resolution ultrasound in guiding treatment of idiopathic plantar fasciitis with minimally invasive techniques. Radiol Med 2008;113:486–95. [DOI] [PubMed] [Google Scholar]
- [34].Hsiao MY, Hung CY, Chang KV, et al. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: a network meta-analysis. Rheumatology 2015;54:1735–43. [DOI] [PubMed] [Google Scholar]
- [35].Chen CM, Lee M, Lin CH, et al. Comparative efficacy of corticosteroid injection and non-invasive treatments for plantar fasciitis: a systematic review and meta-analysis. Sci Rep 2018;8:4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bero L, Rennie D. The Cochrane Collaboration Preparing, maintaining, and disseminating systematic reviews of the effects of health care. JAMA 1995;274:1935e8. [DOI] [PubMed] [Google Scholar]
- [38].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Marks W, Jackiewicz A, Witkowski Z, et al. Extracorporeal shock-wave therapy (ESWT) with a new-generation pneumatic device in the treatment of heel pain. A double blind randomised controlled trial. Acta Orthop Belg 2008;74:98–101. [PubMed] [Google Scholar]
- [40].Speed C. A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med 2014;48:1538–42. [DOI] [PubMed] [Google Scholar]
- [41].Roerdink RL, Dietvorst M, van der Zwaard B, et al. Complications of extracorporeal shockwave therapy in plantar fasciitis: systematic review. Int J Surg 2017;46:133–45. [DOI] [PubMed] [Google Scholar]


