Abstract
The aim of the present study was to evaluate the efficacy and safety of preoperative celecoxib administration in alleviating postoperative pain in knee osteoarthritis (OA) patients undergoing total knee arthroplasty (TKA).
A total of 226 knee OA patients underwent TKA were consecutively recruited and randomized into preoperative analgesia group and postoperative analgesia group as 1:1 ratio. Preoperative analgesia group received celecoxib before and post operation; postoperative analgesia group received celecoxib post operation, all patients received TKA and intravenous patient-controlled analgesia (PCA) post operation. Pain visual analog scale (VAS), patient's global assessment (PGA), flexional angles, PCA consumption, percentage of patients receiving pethidine, pethidine consumption, and adverse events were assessed.
Pain VAS scores at rest and at flexion were both lower in preoperative analgesia group compared to postoperative analgesia group at 2 hours, 6 hours, 12 hours, and 24 hours post operation. Preoperative analgesia group also exhibited decreased PGA score compared to postoperative analgesia group at 2 hours, 6 hours, 12 hours, 24 hours, and 48 hours post operation. Meanwhile, active flexional angle and passive flexional angle in preoperative analgesia group were larger than that in postoperative analgesia group at 72 hours post operation. More interestingly, preoperative analgesia group patients consumed less PCA compared to postoperative analgesia group patients at 72 hours post operation. No difference of adverse event incidences between 2 groups was observed.
Preoperative administration of celecoxib exhibits better efficacy and equal safety profiles compared to postoperative administration of celecoxib in knee OA patients undergoing TKA.
Keywords: celecoxib, efficacy, knee osteoarthritis, preoperative analgesia, safety, total knee arthroplasty
1. Introduction
Knee osteoarthritis (OA) is one of the most common degeneration diseases which affects 10% of population worldwide, while in athletes and elderly populations, the prevalence of knee OA is even higher.[1–4] The swelling and painful knees make numerous knee OA patients difficult in moving, consequently, bring in great burdens to both families and societies.[5] Fortunately, total knee arthroplasty (TKA) is able to help knee OA patients alleviate pain and improve their ambulation in a long-term duration.[6] However, the acute pain post operation of TKA suffers a lot of knee OA patients, which decreases these patients’ quality of life and willingness to do rehabilitative exercise post operation, thus prolongs their recovery time and hospital stays.[7,8] Therefore, controlling post-operation pain of TKA is pivotal for early recovery of knee OA patients undergoing TKA.
Preemptive analgesia is an analgesia strategy that uses analgesics before operation, which has been demonstrated to decrease postoperative analgesic consumptions while exhibit better analgesia effect compared to post-operation analgesia.[9–11] As one of the selective inhibitors of cyclooxygenase (COX)-2, celecoxib is widely used as a preemptive analgesic during perioperative period of many surgeries such as maxillomandibular advancement surgery, hand surgery, and testicular surgery.[12–14] Although a few studies have reported that preoperative celecoxib administration is effective in decreasing acute postoperative pain and consumption of other analgesics in knee OA patients undergoing TKA, these studies either use celecoxib in combination with other analgesics such as tramadol in experimental group, or use placebo in control group, and they don’t compare the efficacy between preoperative celecoxib analgesia and postoperative celecoxib analgesia directly.[15–17] Thus, whether preoperative celecoxib analgesia has an advantage over postoperative celecoxib analgesia in knee OA patients who receive TKA remains unknown. Therefore, the aim of the present study was to evaluate the efficacy and safety of preoperative celecoxib administration versus postoperative celecoxib administration in alleviating postoperative pain in knee OA patients undergoing TKA.
2. Materials and methods
2.1. Patients
226 knee OA patients underwent TKA in The Third Hospital of Hebei Medical University between Jul 2014 and Jun 2017 were consecutively recruited in this randomized, controlled study. The inclusion criteria were:
-
(1)
Diagnosed as single-side knee OA by clinical and imaging findings;
-
(2)
About to receive TKA treatment;
-
(3)
Age above 18 years;
-
(4)
American Society of Anesthesiology (ASA) physical status as I-II.
The exclusive criteria were:
-
(1)
Received analgesic drugs within 7 days before enrollment;
-
(2)
Received intra-articular treatment including hyaluronic acid injection or corticosteroid within 3 months before the enrollment;
-
(3)
Two-side knee OA;
-
(4)
History of knee surgery or interventional examination/therapy;
-
(5)
History of coagulopathy or thromboembolic diseases.
-
(6)
History with gastrointestinal ulceration, perforation, obstruction or bleeding;
-
(7)
History with severe heart, kidney or liver dysfunction;
-
(8)
Known to be allergy to COX-2 selective inhibitors or pethidine.
-
(9)
Women with lactating or pregnancy.
2.2. Ethics and informed consents
The Human Ethics Review Board of The Third Hospital of Hebei Medical University approved this protocol, and this study was conducted according to the Declaration of Helsinki. In addition, all the patients signed the informed consents before initiation.
2.3. Randomization
Design and execution of randomization were delegated to Shanghai Qeejen Institution (Shanghai, China) as follows:
-
(1)
randomization code was generated by an individual statistician using blocked randomization method with block length 6 and ratio 1:1 by SAS 9.0 software (Statistical Analysis System) and relevant treatment protocols were allocated;
-
(2)
The randomization documents were then kept in Shanghai Qeejen Institution (Shanghai, China) as code acquisition center;
-
(3)
When a patient was eligible for inclusion, a call was made to Shanghai Qeejen Institution (Shanghai, China) and a unique subject identification number was provided, subsequently, the patient was assigned to the corresponding treatment.
2.4. Treatment
Patients were randomized into preoperative analgesia group (N = 113) and postoperative analgesia group (N = 113) as 1:1 ratio. In preoperative analgesia group, patients received celecoxib 400 mg at 24 hours before the operation, and then 200 mg every 12 hours until 72 hours post operation; while in the postoperative analgesia group patients received celecoxib 400 mg at 2 hours after the operation and then 200 mg every 12 hours until 72 hours post operation. All patients received TKA operation and initiated intravenous patient-controlled analgesia (PCA) post operation for 48 hours as routine, and the PCA consisted of 1 mg fentanyl, 50 mg tramadol and 5 mg granisetron diluted in 100 mL, the parameters of PCA was as follows: basal rate 1 mL/h, bolus 0.5 mL, and lock-out time 10 minutes. If intolerant pain occurred, patients were allowed to receive rescue treatment with 50 mg pethidine injection.
2.5. Assessments
Pain visual analog scale (VAS) score at rest (range 0–10), pain VAS score at flexion (range 0–10) and patient's global assessment (PGA) score (range 0–10) were assessed at preoperation, 2 hours, 6 hours, 12 hours, 24 hours, 48 hours, and 72 hours post operation. Active and passive flexional angles were evaluated at 72 hours post operation. Consumption of PCA, percentage of patients receiving rescue use of pethidine and consumption of pethidine were also recorded. In addition, adverse events were documented.
2.6. Statistics
SPSS 21.0 software (IBM) was used for statistical analysis, and GraphPad Prism 5.01 (GraphPad Int) was used for drawing graphs. Data were presented as mean ± standard deviation or count (with or without percentage). Comparison between 2 groups was determined by t test or Chi-square test. P <.05 was considered as significant.
3. Results
3.1. Study flow
In this study, 371 knee OA patients were invited, among whom 38 patients missed invitation and 42 patients declined to attend prescreening procedure. Thus, 291 patients in total were screened for eligibility, during the screening procedure, 43 patients were excluded, and another 22 patients disagreed to sign informed consents. The remaining 226 patients underwent TKA operation were recruited and randomly allocated into preoperative analgesia group (n = 113) and postoperative analgesia group (n = 113) with a ratio of 1:1. In preoperative analgesia group, 8 patients withdrew during the study for the following reasons: 5 patients violated the protocol, 2 patients lacked efficacy and 1 patient decided to quit; in postoperative analgesia group, 10 patients withdrew for the following reasons: 5 patients violated the protocol, 4 patients lacked efficacy and 1 patient decided to quit. Therefore, a total of 105 patients in preoperative analgesia group and 103 patients in postoperative analgesia group completed the study. All analyses were performed based on the intention-to-treat (ITT) principles with the last observation carried forward (LOCF) method from any of the 3 post-baseline measures. (Fig. 1).
Figure 1.
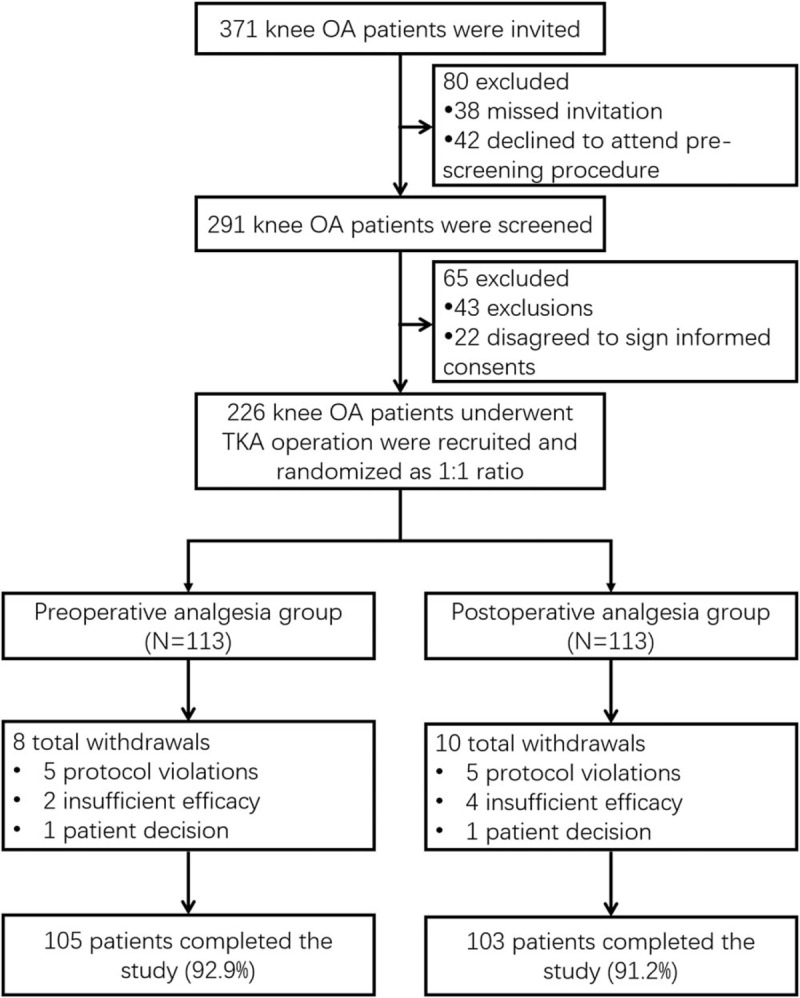
Study flow.
3.2. Characteristics of patients
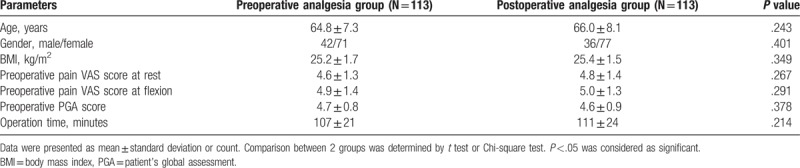
As depicted in Table 1, no difference of characteristics between preoperative analgesia group and postoperative analgesia group was observed. The mean age was 64.8 ± 7.3 years in preoperative analgesia group and 66.0 ± 8.1 years in postoperative analgesia group (P = .243). There were 71 and 77 females in preoperative analgesia group and postoperative analgesia group, respectively (P = .401). Meanwhile, preoperative analgesia group patients exhibited a mean body mass index (BMI) of 25.2 ± 1.7 kg/m2, postoperative analgesia group patients presented with a mean BMI of 25.4 ± 1.5 kg/m2 (P = .349). In preoperative analgesia group, preoperative pain VAS score at rest (P = .267), at flexion (P = .291) and preoperative PGA score (P = .378) was 4.6 ± 1.3, 4.9 ± 1.4, and 4.7 ± 0.8, respectively; in postoperative analgesia group, these mean scores were 4.8 ± 1.4, 5.0 ± 1.3, and 4.6 ± 0.9, respectively. Additionally, mean operation time in preoperative analgesia group was 107 ± 21 minutes, and in postoperative analgesia group, it was 111 ± 24 minutes (P = .214).
Table 1.
Patients’ characteristics.
3.3. Comparison of pain VAS score at rest and at flexion between 2 groups
Pain VAS score at rest between preoperative analgesia group and postoperative analgesia group was of no difference at preoperation (P >.05), 48 hours (P >.05), or 72 hours (P >.05) post-operation, while it was lower in preoperative analgesia group compared to postoperative analgesia group at 2 hours (P <.05), 6 hours (P <.05), 12 hours (P <.05), and 24 hours (P <.05) post-operation (Fig. 2A). Meanwhile, preoperative analgesia group exhibited similar pain VAS score at flexion preoperation (P >.05), 48 hours (P >.05), and 72 hours (P >.05) post-operation, whereas decreased pain VAS score at flexion 2 hours (P <.05), 6 hours (P <.05), 12 hours (P <.05), and 24 hours (P <.05) post-operation compared to postoperative analgesia group (Fig. 2B).
Figure 2.
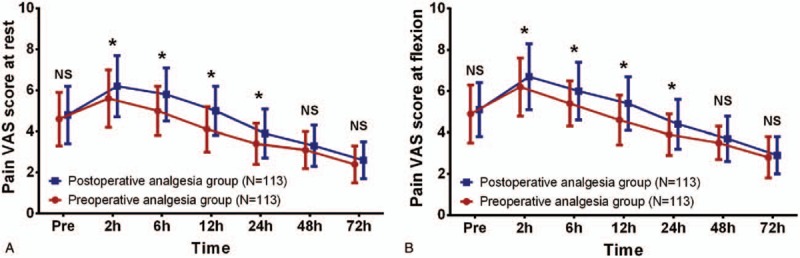
Pain VAS score at rest and at flexion between 2 groups. Pain VAS score at rest (A) and at flexion (B) were similar between preoperative analgesia group and postoperative analgesia group at preoperation, 48 hours and 72 hours post operation. However, at 2 hours, 6 hours, 12 hours, and 24 hours post operation, the preoperative analgesia group exhibited lower pain VAS score at rest and at flexion compared to postoperative analgesia group. Comparison between 2 groups was determined by t test. P <.05 was considered as significant. ∗P < .05. Pain VAS score, pain visual analogue scale score.
3.4. Comparison of PGA score between 2 groups
As shown in Fig. 3, no difference of PGA score between preoperative analgesia group and postoperative analgesia group was discovered at preoperation (P >.05) or 72 hours (P >.05) post operation, while it was decreased in preoperative analgesia group compared to postoperative analgesia group at 2 hours (P <.05), 6 hours (P <.05), 12 hours (P <.05), 24 hours (P <.05), and 48 hours (P <.05) post operation.
Figure 3.
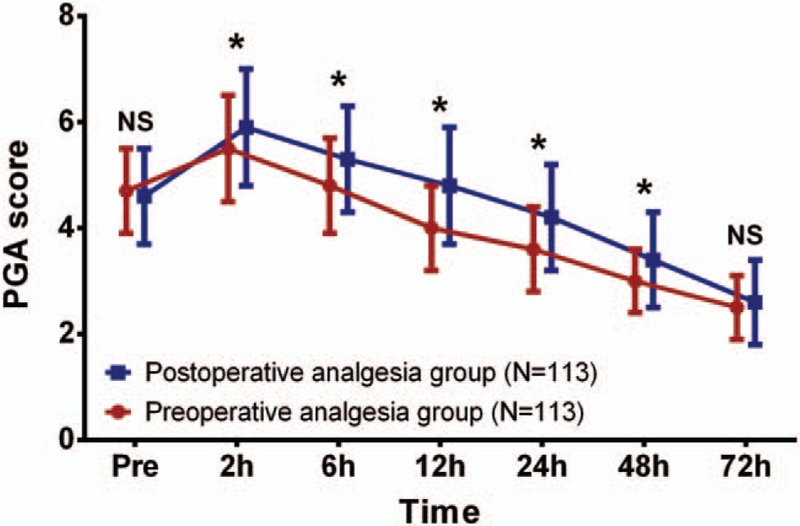
PGA score between 2 groups. No difference of PGA score was found between preoperative analgesia group and postoperative analgesia group at preoperation or 72 hours post operation. However, at 2 hours, 6 hours, 12 hours, 24 hours, and 48 hours post operation, preoperative analgesia group presented with lower PGA scores compared to postoperative analgesia group. Comparison between 2 groups was determined by t test. P <.05 was considered as significant. ∗P <.05. PGA score, patient's global assessment score.
3.5. Comparison of active flexional angle and passive flexional angle at 72 hours post operation
Both active flexional angle (P <.05, Fig. 4A) and passive flexional angle (P <.05, Fig. 4B) in preoperative analgesia group were increased compared to postoperative analgesia group at 72 hours post operation.
Figure 4.
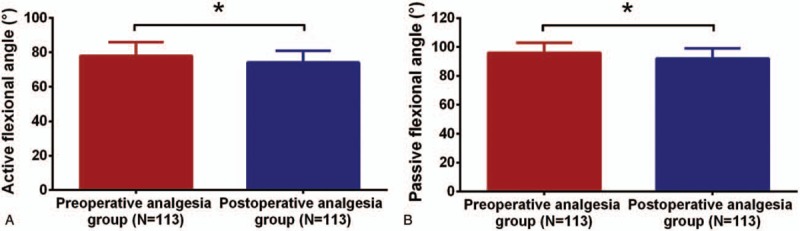
Active flexional angle and passive flexional angle between 2 groups at 72 hours post operation. Preoperative analgesia group displayed larger active flexional angle (A) and passive flexional angle (B) than that in postoperative analgesia group at 72 hours post operation. Comparison between 2 groups was determined by t test. P <.05 was considered as significant. ∗P <.05.
3.6. Comparison of PCA consumption, percentage of patients using pethidine and pethidine consumption between 2 groups at 72 hours post operation
PCA consumption in preoperative analgesia group was less compared to postoperative analgesia group (P <.05, Fig. 5A). However, neither percentage of patients using pethidine (P >.05, Fig. 5B) nor pethidine consumption (P >.05, Fig. 5C) was different between 2 groups.
Figure 5.
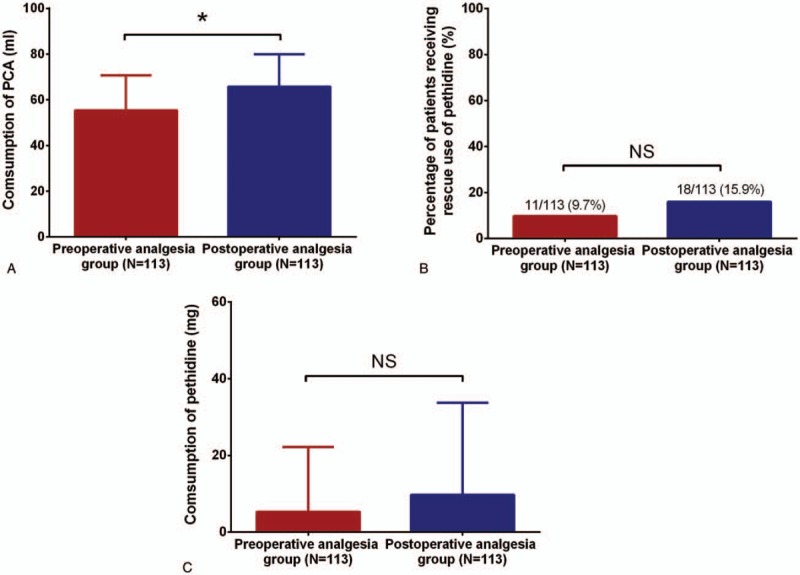
PCA consumption, percentage of patients using pethidine and pethidine consumption between 2 groups at 72 hours post operation. Consumption of PCA in preoperative analgesia group was declined compared to postoperative analgesia group (A), whereas percentage of patients receiving rescue use of pethidine (B) and consumption of pethidine (C) between the two groups were similar at 72 hours post operation. Comparison between 2 groups was determined by t test and Chi-square test. P <.05 was considered as significant. ∗P <.05. PCA = patient-controlled analgesia.
3.7. Comparison of adverse events between 2 groups
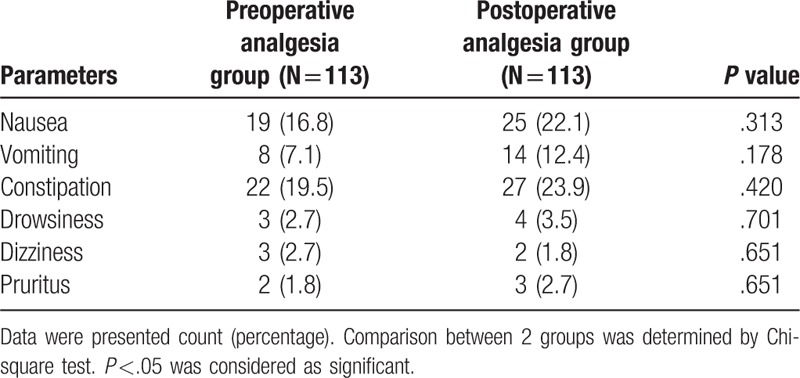
The numbers of nausea, vomiting, constipation, drowsiness, dizziness and pruritus in preoperative analgesia group were 19 (16.8%), 8 (7.1%), 22 (19.5%), 3 (2.7%), 3 (2.7%), and 2 (1.8%), respectively, while in postoperative analgesia group, they were 25 (22.1%), 14 (12.4%), 27 (23.9%), 4 (3.5%), 2 (1.8%), and 3 (2.7%), respectively. All of these adverse event incidences between 2 groups were similar (all P >.05, Table 2).
Table 2.
Adverse events.
4. Discussion
The present study discovered that:
-
(1)
Compared to postoperative analgesia group, the preoperative analgesia group exhibited decreased pain VAS score at rest and at flexion, as well as decreased PGA score during the hospitalization. In addition, preoperative analgesia group also presented with higher active flexional angle and passive flexional angle.
-
(2)
PCA consumption in preoperative analgesia group was lower than that in postoperative analgesia group.
-
(3)
Adverse event incidences between 2 groups were similar.
Compared to non-selective inhibitors of COX, the selective COX 2 inhibitor celecoxib exhibits same efficient analgesic activity while less gastrointestinal side effects.[18–20] In knee OA patients undergoing unilateral TKA, preoperative analgesia by using celecoxib in combination with low-dose tramadol/acetaminophen is more superior in decreasing resting pain and movement pain compared to control group without preoperative analgesia.[17] In another study conducted on knee OA patients undergoing TKA, patients who receive preoperative analgesia by celecoxib present with reduced movement pain intensity, larger range of motion and earlier achievement of 90 degrees knee flexion compared to patients who receive placebo.[16] What is more, preoperative administration of celecoxib exhibits lower pain scores and earlier achievement of 90 degrees knee flexion than postoperative administration of celecoxib in OA or rheumatoid arthritis patients undergoing TKA.[20] However, these studies either lack direct comparison of efficacy between preoperative administration of celecoxib and postoperative administration of celecoxib, or lack a large sample of knee OA patients undergoing TKA, which makes the result less reliable. In the present study, the efficacy between preoperative analgesia group and postoperative analgesia group was compared in a larger sample than that in previous studies, which showed that preoperative analgesia group patients achieved lower pain VAS score both at rest and at flexion before 24 hours post operation and lower PGA score before 48 hours post operation; they also achieved larger active flexional angle and larger passive flexional angle at 72 hours post-operation compared to postoperative analgesia group patients, indicating that preoperative celecoxib analgesia not only exhibited better analgesia efficacy in the early stage of postoperative period, but also improved knee function and promoted rehabilitation of patients. The explanation might be that: Since preoperative analgesia group patients received celecoxib at 24 hours preoperation, they achieved steady-state plasma concentration (Css) earlier than postoperative analgesia group patients, meanwhile, celecoxib is a concentration-dependent analgesic drug, thus, preoperative analgesia group presented with better analgesia efficacy at the early stage of postoperative period.[21,22] Subsequently, better analgesia efficacy in preoperative analgesia group led to better recovery of knee function compared to postoperative analgesia group.
Besides pain VAS score, PGA score and flexional angles, other indicators such as PCA consumption, percentage of patients using rescue drugs, and rescue drugs consumption are also applied for evaluation of analgesia efficacy indirectly. It is reported that the consumption of rescue drug post TKA is decreased by 40% in preoperative celecoxib analgesia patients compared to patients only receive PCA.[15] Similarly, another study discloses that in knee OA patients undergoing TKA, preoperative celecoxib analgesia group requires less consumption of patient-controlled epidural analgesia and rescue drug compared to placebo group.[16] Additionally, Shen Bin et al illustrate that preoperative analgesia of celecoxib decreases the PCA consumption compared to postoperative analgesia of celecoxib in OA or rheumatoid arthritis patients undergoing TKA in a small-sample-size population.[20] These studies suggest that preoperative analgesia by celecoxib may be able to reduce the consumption of PCA and rescue drug. In this study, we found that PCA consumption in preoperative analgesia group was lower than that in postoperative analgesia group. The possible explanation was that: As previously described, celecoxib is a concentration-dependent analgesic drug, and preoperative analgesia group patients exhibited larger area under the plasma concentration-time curve in a few days post operation, which made them consume less PCA compared to postoperative analgesia group patients at 72 hours post operation. For other results in our study, the percentage of patients using pethidine and its consumption between the 2 groups in our study were similar, which may be due to the powerful analgesia efficacy of PCA plus celecoxib was able to address most of the acute pain a few days post TKA; besides, the relative short time of evaluation might also account for the result.
A number of studies have proved the safety of celecoxib in treating a variety of postoperative pains.[23–25] Nausea, vomiting, constipation, drowsiness, dizziness, and pruritus were common adverse events in the present study, and none of these adverse events were severe or deadly. What is more, no difference of the adverse event incidences between the 2 groups was found, indicating that the preoperative celecoxib analgesia did not aggravate the adverse events in knee OA patients undergoing TKA compared with postoperative celecoxib analgesia.
There were some limitations in this study. First, the study was not a multicenter, double-blinded study, which might cause selection and assessment bias. Second, the evaluation time was relative short, thus, a longer evaluation time would be better. Lastly, there might exist other confounding factors such as the distinction in the operating skill of surgeons.
In summary, preoperative administration of celecoxib exhibits better efficacy and equal safety profiles compared to postoperative administration of celecoxib in knee OA patients undergoing TKA.
Author contributions
Conceptualization: Fei Wang.
Data curation: Jiangfeng Liu.
Formal analysis: Jiangfeng Liu.
Methodology: Jiangfeng Liu.
Writing – original draft: Jiangfeng Liu.
Writing – review & editing: Fei Wang.
Fei Wang orcid: 0000-0001-8856-8853.
Footnotes
Abbreviations: BMI = body mass index, COX = cyclooxygenase, OA = osteoarthritis, PCA = patient-controlled analgesia, PGA = patient's global assessment, TKA = total knee arthroplasty, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Madaleno FO, Santos BA, Araujo VL, et al. Prevalence of knee osteoarthritis in former athletes: a systematic review with meta-analysis. Braz J Phys Ther 2018;22:437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mills K, Hubscher M, Leary HO, et al. Current concepts in joint pain in knee osteoarthritis. Schmerz 1–7. DOI: 10.1007/s00482-018-0275-9. [DOI] [PubMed] [Google Scholar]
- [3].Nelson FRT. The value of phenotypes in knee osteoarthritis research. Open Orthop J 2018;12:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clarke H, Pereira S, Kennedy D, et al. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Res Manag 2009;14:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Canovas F, Dagneaux L. Quality of life after total knee arthroplasty. Orthop Traumatol Surg Res 2018;104:S41–6. [DOI] [PubMed] [Google Scholar]
- [7].Meftah M, Wong AC, Nawabi DH, et al. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics 2012;35:e660–4. [DOI] [PubMed] [Google Scholar]
- [8].Sporer SM, Rogers T. Postoperative pain management after primary total knee arthroplasty: the value of liposomal bupivacaine. J Arthroplasty 2016;31:2603–7. [DOI] [PubMed] [Google Scholar]
- [9].Katz J, Kavanagh BP, Sandler AN, et al. Preemptive analgesia. Clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology 1992;77:439–46. [DOI] [PubMed] [Google Scholar]
- [10].Bridenbaugh PO. Preemptive analgesia—is it clinically relevant? Anesth Analg 1994;78:203–4. [DOI] [PubMed] [Google Scholar]
- [11].Lee JK, Chung KS, Choi CH. The effect of a single dose of preemptive pregabalin administered with COX-2 inhibitor: a trial in total knee arthroplasty. J Arthroplasty 2015;30:38–42. [DOI] [PubMed] [Google Scholar]
- [12].Cillo JE, Jr, Dattilo DJ. Pre-emptive analgesia with pregabalin and celecoxib decreases postsurgical pain following maxillomandibular advancement surgery: a randomized controlled clinical trial. J Oral Maxillofac Surg 2014;72:1909–14. [DOI] [PubMed] [Google Scholar]
- [13].Stepan JG, London DA, Osei DA, et al. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg Am 2018;43:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mehta A, Hsiao W, King P, et al. Perioperative celecoxib decreases opioid use in patients undergoing testicular surgery: a randomized, double-blind, placebo controlled trial. J Urol 2013;190:1834–8. [DOI] [PubMed] [Google Scholar]
- [15].Huang YM, Wang CM, Wang CT, et al. Perioperative celecoxib administration for pain management after total knee arthroplasty - a randomized, controlled study. BMC Musculoskelet Disord 2008;9:77DOI: 10.1186/1471-2474-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reuben SS, Buvenandran A, Katz B, et al. A prospective randomized trial on the role of perioperative celecoxib administration for total knee arthroplasty: improving clinical outcomes. Anesth Analg 2008;106:1258–64. [DOI] [PubMed] [Google Scholar]
- [17].Xu Z, Zhang H, Luo J, et al. Preemptive analgesia by using celecoxib combined with tramadol/APAP alleviates post-operative pain of patients undergoing total knee arthroplasty. Phys Sportsmed 2017;45:316–22. [DOI] [PubMed] [Google Scholar]
- [18].Ekman EF, Wahba M, Ancona F. Analgesic efficacy of perioperative celecoxib in ambulatory arthroscopic knee surgery: a double-blind, placebo-controlled study. Arthroscopy 2006;22:635–42. [DOI] [PubMed] [Google Scholar]
- [19].Sun T, Sacan O, White PF, et al. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesth Analg 2008;106:950–8. [DOI] [PubMed] [Google Scholar]
- [20].Shen B, Tang X, Yang J, et al. Effects of perioperative administration of celecoxib on pain management and recovery of function after total knee replacement. Zhonghua Wai Ke Za Zhi 2009;47:116–9. [PubMed] [Google Scholar]
- [21].Dutta N, Sarotra P, Gupta S, et al. Mechanism of action of celecoxib on normal and acid-challenged gastric mucosa. Exp Toxicol Pathol 2009;61:353–61. [DOI] [PubMed] [Google Scholar]
- [22].Dabhi JK, Solanki JK, Mehta A. Antiatherosclerotic activity of ibuprofen, a non-selective COX inhibitor—an animal study. Indian J Exp Biol 2008;46:476–81. [PubMed] [Google Scholar]
- [23].Emery P, Koncz T, Pan S, et al. Analgesic effectiveness of celecoxib and diclofenac in patients with osteoarthritis of the hip requiring joint replacement surgery: a 12-week, multicenter, randomized, double-blind, parallel-group, double-dummy, noninferiority study. Clin Ther 2008;30:70–83. [DOI] [PubMed] [Google Scholar]
- [24].Gong L, Dong JY, Li ZR. Effects of combined application of muscle relaxants and celecoxib administration after total knee arthroplasty (TKA) on early recovery: a randomized, double-blind, controlled study. J Arthroplasty 2013;28:1301–5. [DOI] [PubMed] [Google Scholar]
- [25].Mammoto T, Fujie K, Mamizuka N, et al. Effects of postoperative administration of celecoxib on pain management in patients after total knee arthroplasty: study protocol for an open-label randomized controlled trial. Trials 2016;17:45DOI: 10.1186/s13063-015-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


