Abstract
The association between tamoxifen use and risk of deep vein thrombosis or pulmonary embolism in women with breast cancer has been reported in the Western population. The study aimed to evaluate the association between tamoxifen use and deep vein thrombosis or pulmonary embolism in older women with breast cancer in Taiwan.
We conducted a retrospective case–control study using the database of the Taiwan National Health Insurance Program. A total of 281 women subjects with breast cancer aged ≥65 years with newly diagnosed deep vein thrombosis/or pulmonary embolism from 2000 to 2011 were identified as the cases. Additionally, 907 women subjects with breast cancer aged ≥65 years without deep vein thrombosis or pulmonary embolism were randomly selected as the controls. The cases and the controls were matched with age and comorbidities. Ever use of tamoxifen was defined as subjects who had at least a prescription for tamoxifen before index date. Never use of tamoxifen was defined as subjects who never had a prescription for tamoxifen before index date. We used the multivariable logistic regression model to calculate the odds ratio (OR) and the 95% confidence interval (CI) of deep vein thrombosis or pulmonary embolism associated with tamoxifen use.
After adjustment for confounding variables, the adjusted OR of deep vein thrombosis or pulmonary embolism was 1.95 for subjects with ever use of tamoxifen (95% CI 1.45, 2.62), as compared with never use of tamoxifen. In addition, atrial fibrillation (adjusted OR 3.73, 95% CI 1.89, 7.35) and chronic kidney disease (adjusted OR 1.72, 95% CI 1.06, 2.80) were also associated with deep vein thrombosis or pulmonary embolism.
Tamoxifen use is associated with 1.95-fold increased odds of deep vein thrombosis or pulmonary embolism among older women with breast cancer in Taiwan.
Keywords: deep vein thrombosis, National Health Insurance Program, older people, pulmonary embolism, Taiwan, tamoxifen
1. Introduction
Venous thromboembolism is a primary and leading preventable cause of death worldwide that comprises both deep vein thrombosis and pulmonary embolism.[1] A venous thrombus predominately comprises erythrocytes, platelets, and leukocytes bound together by fibrin and is formed in sites of vessel damage and areas of stagnant blood flow, such as the valve pockets of the deep veins of the calf, or extends proximally.[2] The invasion of a thrombus into the pulmonary arteries that obstructs the vessels might lead to pulmonary embolism. Per Rudolph Virchow, 3 conditions that predispose to thrombus are endothelial vessel injury, stasis or turbulence of the blood flow, and enhanced activation of clotting factors.[3] In addition, the traditional risk factors for venous thromboembolism include major trauma, major surgery, increasing age, cancer and its treatment, prior venous thromboembolism, prolonged immobility, oral contraceptives, and pregnancy.[4–6] The correlation between cancer and venous thrombosis has been established for nearly 150 years,[7] and, reportedly, breast cancer has the lowest rate of developing venous thromboembolism.[8]
Tamoxifen is an anti-estrogen drug, belonging to the class of selective estrogen receptor modulators, which can act on estrogen receptors to prevent estrogen binding. Owing to its anti-estrogenic properties, such as well-tolerated in chronic use, ready availability, relatively low cost, and well-demonstrated efficacy, tamoxifen has rapidly gained prominence in the treatment of breast cancer.[9,10] Studies have proven that tamoxifen decreases mortality and recurrence rates in patients with breast cancer and is recommended as a prophylactic agent in pre-menopausal women at the risk of breast cancer.[11,12] However, despite being a well-tolerated drug, some studies have revealed increased adverse effects, such as venous thromboembolism, related to tamoxifen.[13–15] In addition, the prophylaxis with tamoxifen in patients without breast cancer is also correlated with an increased risk of venous thromboembolism.[16] Although the risk of thromboembolic disease in Asians has been reported as lower than that in the Western population,[17,18] little data are available on the relationship between the tamoxifen use and the risk of deep vein thrombosis or pulmonary embolism in Asian women with breast cancer. Chen et al[19] reported that the risks of developing deep vein thrombosis and pulmonary embolism are not elevated in Asian patients with early breast cancer receiving adjuvant tamoxifen and that ethnic differences should be considered when planning optimal endocrine treatments for patients with early breast cancer. To the best of our knowledge, the report of Chen et al[19] is the first study to investigate the absolute and relative risk of deep vein thrombosis and pulmonary embolism in patients with early breast cancer receiving the adjuvant tamoxifen treatment by using an East Asian population database; however, the study of Chen et al[19] reached a different conclusion than studies conducted in the Western countries.
Owing to limited research on the correlation between the tamoxifen use and the risk of deep vein thrombosis or pulmonary embolism in Asian women with breast cancer, this study aims to investigate a possible association between the tamoxifen use and deep vein thrombosis or pulmonary embolism in elderly women with breast cancer in Taiwan.
2. Methods
2.1. Study design and data source
We conducted a retrospective nationwide case–control study in Taiwan to analyze the database of the Taiwan National Health Insurance Program, which began in March 1995 and had covered 99.6% of the entire population (23 million people) of Taiwan at the end of 2015.[20–30] The details of the program are described previously.[31–34]
2.2. Sampled subjects
In this study, we considered cases of female patients with breast cancer, aged ≥65 years, who were newly diagnosed with deep vein thrombosis or pulmonary embolism (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9] codes 453.8 and 415.1) from 2000 to 2011. We defined the date of diagnosing deep vein thrombosis or pulmonary embolism as the index date. In addition, for every 1 patient with deep vein thrombosis or pulmonary embolism, nearly 3 female patients with breast cancer, aged ≥65 years, who had never been diagnosed with deep vein thrombosis or pulmonary embolism were identified from the same database as controls. We matched cases and controls with age (5-year interval), comorbidities, and the index year of diagnosing deep vein thrombosis or pulmonary embolism.
2.3. Comorbidities
We included the following comorbidities that could be potentially associated with deep vein thrombosis or pulmonary embolism before the index date: alcohol-related disease, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease, coronary artery disease, diabetes mellitus, heart failure, hyperlipidemia, hypertension, and fracture of lower limbs with/without operation. Based on the ICD-9 codes, the diagnostic precision of comorbidities has been illustrated previously.[35–39]
2.4. Assessment of tamoxifen and aromatase inhibitors use
We included the prescription histories of tamoxifen and aromatase inhibitors in this study. Ever use of medications was defined as subjects who, at least, had a prescription for medications before the index date. In contrast, never use of medications was defined as subjects who never had a prescription for medications before the index date. We adapted these definitions from previous studies.[32,33,40–43]
2.5. Statistical analysis
In this study, we compared the distribution of the demographic status, tamoxifen use, aromatase inhibitors use, and comorbidities between cases and controls using the χ2 test for categorized variables and the t-test for continuous variables. In addition, the univariate and multivariate logistic regression analyses were used to calculate the odds ratio (OR) and the 95% confidence interval (CI) of deep vein thrombosis or pulmonary embolism related to the tamoxifen use. Variables that significantly correlated with deep vein thrombosis or pulmonary embolism in the univariate logistic regression model were further assessed by the multivariate logistic regression model. Furthermore, we analyzed the dose-dependent effect of the tamoxifen use on the risk of deep vein thrombosis or pulmonary embolism. The average daily dose of tamoxifen was evaluated by using the total quantity of tamoxifen divided by the total number of days supplied. We divided the average daily dose into 2 levels based on the median dose, <20 mg and ≥20 mg. All analyses were performed using the SAS statistical software (version 9.2; SAS Institute, Inc., Cary, NC). Finally, we considered the results as statistically significant when two-tailed P values were <.05.
3. Results
3.1. Characteristics of the study population
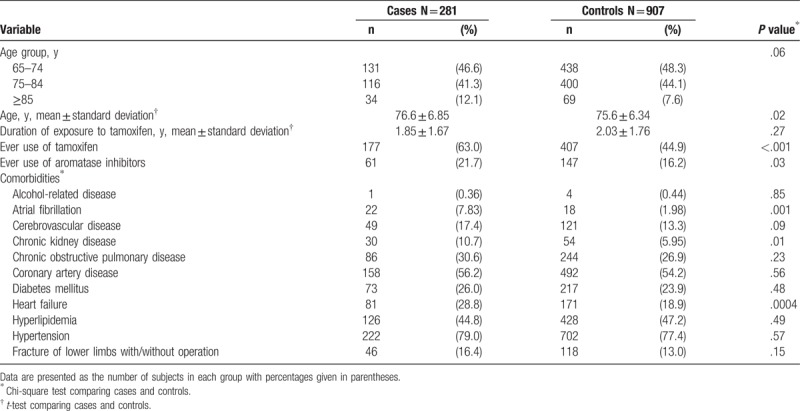
Table 1 summarizes the characteristics of the study population. We recognized 281 cases of newly diagnosed deep vein thrombosis or pulmonary embolism and 907 controls in 2000 to 2011. The mean ages (standard deviation) of our study population were 76.6 (6.85) years in cases and 75.6 (6.34) years in controls, with statistical significance (t test, P = .02). In addition, the mean duration of exposure to tamoxifen (standard deviation) was 1.85 (1.67) years in cases and 2.03 (1.76) years in controls, without statistical significance (t test, P = .27). The results revealed that cases with deep vein thrombosis or pulmonary embolism were more likely to demonstrate a higher proportion of ever use of tamoxifen than controls (63% vs 44.9%; χ2 test, P < .001). In addition, cases demonstrated a higher tendency to exhibit greater proportions of ever use of aromatase inhibitors, atrial fibrillation, chronic kidney disease, and heart failure than controls (χ2 test, P < .05 for all).
Table 1.
Characteristics between cases with deep vein thrombosis or pulmonary embolism and controls.
3.2. Correlation of the risk of deep vein thrombosis or pulmonary embolism with the tamoxifen use, aromatase inhibitors use, and comorbidities
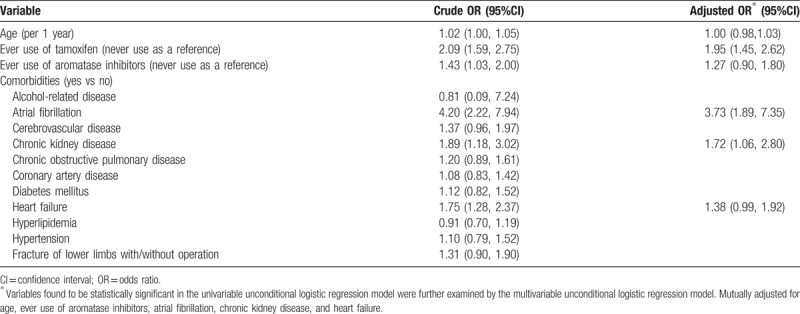
Table 2 summarizes the risk of deep vein thrombosis or pulmonary embolism associated with the tamoxifen use, aromatase inhibitors use, and comorbidities. After adjusting confounding variables in this study, the multivariate unconditional logistic regression model revealed that the adjusted OR of deep vein thrombosis or pulmonary embolism was 1.95 for subjects with ever use of tamoxifen (95% CI: 1.45–2.62), compared with never use of tamoxifen. Furthermore, atrial fibrillation (adjusted OR, 3.73; 95% CI: 1.89–7.35) and chronic kidney disease (adjusted OR, 1.72; 95% CI: 1.06–2.80) correlated with deep vein thrombosis or pulmonary embolism.
Table 2.
Crude and adjusted odds ratio and 95% confidence interval of deep vein thrombosis or pulmonary embolism associated with tamoxifen use, aromatase inhibitors use, and comorbidities by logistical regression model.
3.3. Risk of deep vein thrombosis or pulmonary embolism associated with the dosage of tamoxifen
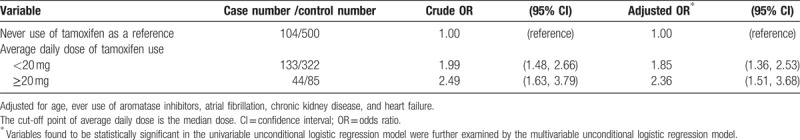
Table 3 presents a sub-analysis on the risk of deep vein thrombosis or pulmonary embolism related to the dosage of the tamoxifen use. The adjusted ORs of deep vein thrombosis or pulmonary embolism were 1.85 for patients with average daily dose of tamoxifen use <20 mg (95% CI: 1.36–2.53) and 2.36 for patients with average daily dose of tamoxifen use ≥20 mg (95% CI: 1.51–3.68), compared with never use of tamoxifen. The findings of this study suggested a dose-dependent effect of the tamoxifen use on the risk of deep vein thrombosis or pulmonary embolism.
Table 3.
Average daily dose of tamoxifen use and risk of deep vein thrombosis or pulmonary embolism.
4. Discussion
Venous thromboembolic events are the leading severe complications associated with adjuvant hormonal therapy for breast cancer. Several studies have established a correlation between the use of tamoxifen in breast cancer and an increased incidence of venous thromboembolism. However, Chen et al[19] reported that the risk of developing deep vein thrombosis and pulmonary embolism is not increased in Asian patients with early breast cancer receiving adjuvant tamoxifen. This case–control study established a correlation between the tamoxifen use and 1.95-fold increased odds of developing deep vein thrombosis or pulmonary embolism among older female patients with breast cancer in Taiwan. In a sub-analysis on the risk of deep vein thrombosis or pulmonary embolism associated with the dosage of tamoxifen use, the adjusted ORs of deep vein thrombosis or pulmonary embolism were 1.85 and 2.36 for patients with an average daily dose of tamoxifen use <20 mg and ≥20 mg, compared with never use of tamoxifen. Based on these findings, a dose-dependent effect of tamoxifen use on the risk of deep vein thrombosis or pulmonary embolism could be assumed. Of note, the findings of this study corroborate with some previous studies. A Danish population study revealed that women treated with tamoxifen were at a higher risk for developing deep vein thrombosis and pulmonary embolism during the first 2 years after the exposure (RR, 3.5; 95% CI: 2.1–6.0).[13] In addition, Onitilo et al[15] demonstrated that the tamoxifen use seemingly led to a clustering phenomenon of venous thromboembolism events at the start of therapy, and they observed a persistent effect on thromboembolism events for female patients with sustained exposure to tamoxifen over time. Likewise, Decensi et al[44] revealed a hazards ratio of 1.63 for venous thromboembolism among patients receiving tamoxifen for breast cancer prevention, compared with those not receiving tamoxifen. This study deduces that the tamoxifen use is associated with increased odds of deep vein thrombosis or pulmonary embolism, corroborate with some previous studies in the Western population.
Reportedly, estrogens enhance the risk of developing venous thromboembolism.[45,46] In some studies, women during pregnancy and while receiving estrogen have demonstrated increased levels of von Willebrand factor, factor VIII, fibrinogen, and decreased levels of anti-thrombin, protein S.[47–49] Tamoxifen is an anti-estrogen drug, belonging to the class of selective estrogen receptor modulators, which also exerts partial estrogen-agonistic effects on particular receptor subtypes. Assumedly, the estrogenic activity of tamoxifen might elevate the risk of venous thromboembolism, and its effects on anticoagulant proteins seem to be similar to the effects of postmenopausal estrogen. The pathological mechanism underlying the association of tamoxifen with venous thromboembolism is not entirely understood. Hemostatic risk factors for venous thromboembolism include defects of the anticoagulant system, namely activated protein C resistance and deficiencies of anti-thrombin, protein C, and protein S.[50–54] Cushman et al[55] established a correlation between the tamoxifen treatment and reduction in anti-thrombin and protein S levels. Some studies have reported postmenopausal estrogen reductions in anti-thrombin, which correlated with increased prothrombin fragment 1 to 2.[56,57] Prothrombin fragment 1 to 2 is a marker of thrombin action that might enhance the risk of venous thromboembolism. In addition, the reduced level of protein S by tamoxifen might reflect the reduced anticoagulant function,[58,59] and increase the risk of venous thromboembolism.
In contrast, an in vitro study demonstrated that tamoxifen increased the Ca2+ influx and led to an increased platelet activity by the PI3 and NADPH oxidase pathways.[60] In addition, it revealed that thrombotic effects of tamoxifen are associated with the oxidative mechanisms in thrombocytes. In addition, an experimental study in rats revealed that the chronic tamoxifen use increased the intimal thickness in arteries.[61] Another experimental study in rats revealed that the chronic tamoxifen consumption in the presence of anastomosis led to a prominent endothelial proliferation in rat femoral veins.[62] Rudolph Virchow has described the correlation of vascular endothelial injury with thrombus, suggesting that an increased intimal thickness and prominent endothelial proliferation might also predispose to thrombus formation, partially explaining a venous thrombogenic activity of tamoxifen.
5. Limitations
This study has some limitations. First, whether patients actually used prescribed tamoxifen could not be ascertained in an observational study. Hence, the prescription history was used as a proxy for the tamoxifen usage. Besides, there was no reason to suspect a difference in the noncompliance of the tamoxifen usage between case and control groups. Second, we did not record some risk factors for venous thromboembolism, such as obesity, cigarette smoking, congenital thrombophilia, recent surgery or trauma, the stage of cancer disease, chemotherapy, and radiotherapy, in this database because of inherent limitations. Reportedly, women with the body mass index >29 kg/m2 have a 3-fold increase in the pulmonary embolism risk, which, in turn, significantly increases over the baseline among women who smoked, at least, 25 cigarettes per day.[63] In addition, chemotherapy and radiotherapy increase the risk of deep vein thrombosis or pulmonary embolism. Hence, further studies are required to illustrate the association of these potential confounding variables and tamoxifen and the risk of venous thromboembolism. However, we used chronic obstructive pulmonary disease rather than cigarette smoking. These points have been explained previously.[64,65] Third, this study is a retrospective analysis since correlation does not infer causality. Finally, we did not record the breast cancer histology or estrogen receptor status in this database because of inherent limitations. Perhaps, these parameters could be associated with both the outcome and the exposure, thereby confounding the results to some extent.
6. Strengths
Of note, this study also has some strengths. First, we used a well-organized database to provide complete information. Second, the diagnoses of deep vein thrombosis or pulmonary embolism and comorbidities were based on ICD-9 codes, the diagnostic accuracy of which has been thoroughly investigated in previous studies.[35–39,66–70] Finally, we used an appropriate statistical methodology and reviewed the literature thoroughly.
7. Conclusion
This study deduces that the tamoxifen use is associated with 1.95-fold increased odds of deep vein thrombosis or pulmonary embolism among older women with breast cancer in Taiwan, with a likely dose-dependent effect of the tamoxifen use on the risk of deep vein thrombosis or pulmonary embolism. As deep vein thrombosis and pulmonary embolism are primary and common preventable causes of death worldwide, physicians prescribing tamoxifen as either primary or adjuvant cancer therapy should investigate cases comprehensively and closely monitor thromboembolic events during tamoxifen therapy in breast cancer.
Author contributions
Specific author contributions: Hsien-Feng Lin, Kuan-Fu Liao, and Ching-Mei Chang participated in data interpretation, revised the article, and contributed equally to the article.
Cheng-Li Lin and Chung-Y. Hsu conducted data analysis.
Shih-Wei Lai contributed to the conception of the article, initiated the draft of the article, and revised the article.
Data curation: Cheng-Li Lin, Chung-Y Hsu.
Formal analysis: Cheng-Li Lin.
Investigation: Kuan-Fu Liao.
Supervision: Shih-Wei Lai.
Visualization: Ching-Mei Chang.
Writing – original draft: Hsien-Feng Lin.
Writing – review & editing: Hsien-Feng Lin, Kuan-Fu Liao, Ching-Mei Chang, Shih-Wei Lai.
Footnotes
Abbreviation: ICD-9 code = International Classification of Diseases, 9th Revision, Clinical Modification.
Ethical Statement: Insurance reimbursement claims data used in this study were available for public access. Patient identification numbers were scrambled to ensure confidentiality. Patient informed consent was not required. The study was approved by the Research Ethics Committee of China Medical University and Hospital in Taiwan (CMUH-104-REC2-115).
This study was supported in part by the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-113004), China Medical University Hospital, Taiwan (DMR-107-192), Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-003), Tseng-Lien Lin Foundation at Taichung in Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors disclose no conflicts of interest.
References
- [1].Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med 2013;126:832.e13–21. [DOI] [PubMed] [Google Scholar]
- [2].Kesieme E, Kesieme C, Jebbin N, et al. Deep vein thrombosis: a clinical review. J Blood Med 2011;2:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rosendaal FR. Risk factors for venous thrombosis: prevalence, risk, and interaction. Semin Hematol 1997;34:171–87. [PubMed] [Google Scholar]
- [4].Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 suppl 1):9–16. [DOI] [PubMed] [Google Scholar]
- [5].Chung WS, Lin CL, Chang SN, et al. Increased risk of deep vein thrombosis and pulmonary thromboembolism in patients with spinal cord injury: a nationwide cohort prospective study. Thromb Res 2014;133:579–84. [DOI] [PubMed] [Google Scholar]
- [6].Chung WS, Lin CL, Hsu WH, et al. Idiopathic venous thromboembolism: a potential surrogate for occult cancer. QJM 2014;107:529–36. [DOI] [PubMed] [Google Scholar]
- [7].Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol 2001;106:6–12. [DOI] [PubMed] [Google Scholar]
- [8].Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007;110:2339–46. [DOI] [PubMed] [Google Scholar]
- [9].Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol 2007;25:5815–24. [DOI] [PubMed] [Google Scholar]
- [10].Jordan VC, Gapstur S, Morrow M. Selective estrogen receptor modulation and reduction in risk of breast cancer, osteoporosis, and coronary heart disease. J Natl Cancer Inst 2001;93:1449–57. [DOI] [PubMed] [Google Scholar]
- [11].Swaby RF, Sharma CG, Jordan VC. SERMs for the treatment and prevention of breast cancer. Rev Endocr Metab Disord 2007;8:229–39. [DOI] [PubMed] [Google Scholar]
- [12].Jordan VC, Morrow M. Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr Rev 1999;20:253–78. [DOI] [PubMed] [Google Scholar]
- [13].Hernandez RK, Sorensen HT, Pedersen L, et al. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: a Danish population-based cohort study. Cancer 2009;115:4442–9. [DOI] [PubMed] [Google Scholar]
- [14].Onder HI, Kilic AC, Kose SA, et al. Branch retinal vein occlusion associated with tamoxifen use. Semin Ophthalmol 2013;28:88–90. [DOI] [PubMed] [Google Scholar]
- [15].Onitilo AA, Doi SA, Engel JM, et al. Clustering of venous thrombosis events at the start of tamoxifen therapy in breast cancer: a population-based experience. Thromb Res 2012;130:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 2005;97:1652–62. [DOI] [PubMed] [Google Scholar]
- [17].White RH, Zhou H, Murin S, et al. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost 2005;93:298–305. [DOI] [PubMed] [Google Scholar]
- [18].Cheuk BL, Cheung GC, Cheng SW. Epidemiology of venous thromboembolism in a Chinese population. Br J Surg 2004;91:424–8. [DOI] [PubMed] [Google Scholar]
- [19].Chen TW, Chen HM, Lin CH, et al. No increased venous thromboembolism risk in Asian breast cancer patients receiving adjuvant tamoxifen. Breast Cancer Res Treat 2014;148:135–42. [DOI] [PubMed] [Google Scholar]
- [20].Ministry of Health and Welfare Taiwan. 2016 Taiwan Health and Welfare Report. Available at: http://www.mohw.gov.tw [access date June 1, 2018, English version]. [Google Scholar]
- [21].Yang MD, Lin KC, Lu MC, et al. Contribution of matrix metalloproteinases-1 genotypes to gastric cancer susceptibility in Taiwan. Biomedicine (Taipei) 2017;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang JS, Lu CC, Kuo SC, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) 2017;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liao KF, Huang PT, Lin CC, et al. Fluvastatin use and risk of acute pancreatitis:a population-based case-control study in Taiwan. Biomedicine (Taipei) 2017;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang JS, Peng YR, Tsai SC, et al. The molecular mechanism of contrast-induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM). Biomedicine 2018;8:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Sheu JJ, Lai MT, et al. RSF-1 overexpression determines cancer progression and drug resistance in cervical cancer. Biomedicine (Taipei) 2018;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu MH, Lee TH, Lee HP, et al. Kuei-Lu-Er-Xian-Jiao extract enhances BMP-2 production in osteoblasts. Biomedicine (Taipei) 2017;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yu CC, Chien CT, Chang TC. M2 macrophage polarization modulates epithelial-mesenchymal transition in cisplatin-induced tubulointerstitial fibrosis. Biomedicine (Taipei) 2016;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ooi H. Bedside pleuroscopy in Taiwan: a great vision for critically-ill patients and intensivists. Biomedicine (Taipei) 2016;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Maa MC, Leu TH. Src is required for migration, phagocytosis, and interferon beta production in Toll-like receptor-engaged macrophages. Biomedicine (Taipei) 2016;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lai SW, Lin CL, Liao KF. Population-based cohort study investigating the association between weight loss and pyogenic liver abscesses. Biomedicine (Taipei) 2017;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hung SC, Liao KF, Hung HC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract 2018;35:166–71. [DOI] [PubMed] [Google Scholar]
- [32].Liao KF, Lin CL, Lai SW. Nationwide case-control study examining the association between Tamoxifen Use and Alzheimer's Disease in aged women with breast cancer in Taiwan. Front Pharmacol 2017;8:612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cheng KC, Liao KF, Lin CL, et al. Correlation of proton pump inhibitors with pulmonary tuberculosis: a case-control study in Taiwan. Front Pharmacol 2017;8:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lin HF, Liao KF, Chang CM, et al. Tamoxifen usage correlates with increased risk of Parkinson's disease in older women with breast cancer: a case-control study in Taiwan. Eur J Clin Pharmacol 2018;74:99–107. [DOI] [PubMed] [Google Scholar]
- [35].Liao KF, Cheng KC, Lin CL, et al. Etodolac and the risk of acute pancreatitis. Biomedicine (Taipei) 2017;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shen ML, Liao KF, Tsai SM, et al. Herpes zoster correlates with pyogenic liver abscesses in Taiwan. Biomedicine (Taipei) 2016;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liao KF, Chuang HY, Lai SW. Metformin use correlates with reduced risk of gallstones in diabetic patients: a 12-year follow-up study. Front Pharmacol 2017;8:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin HF, Liao KF, Chang CM, et al. Population-based cohort study examining the association between splenectomy and empyema in adults in Taiwan. BMJ Open 2017;7:e015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lin HF, Liao KF, Chang CM, et al. Use of thiazolidinediones and risk of hip fracture in old people in a case-control study in Taiwan. Medicine (Baltimore) 2017;96:e7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lai SW, Lin CL, Liao KF. Tamoxifen use correlates with increased risk of the first episode of ischemic cerebrovascular disease in older women with breast cancer: a case-control study in Taiwan. Front Pharmacol 2017;8:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lai SW, Lin CL, Liao KF. Use of oral corticosteroids and risk of hip fracture in the elderly in a case-control study. Front Pharmacol 2017;8:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lai SW, Lin CL, Liao KF. Zolpidem administration and risk of hepatocellular carcinoma: a case-control study in Taiwan. Front Pharmacol 2017;8:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liao KF, Lin CL, Lai SW. Population-based case-control study assessing the association between statins use and pulmonary tuberculosis in Taiwan. Front Pharmacol 2017;8:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Decensi A, Maisonneuve P, Rotmensz N, et al. Effect of tamoxifen on venous thromboembolic events in a breast cancer prevention trial. Circulation 2005;111:650–6. [DOI] [PubMed] [Google Scholar]
- [45].Jick H, Derby LE, Myers MW, et al. Risk of hospital admission for idiopathic venous thromboembolism among users of postmenopausal oestrogens. Lancet 1996;348:981–3. [DOI] [PubMed] [Google Scholar]
- [46].Grodstein F, Stampfer MJ, Goldhaber SZ, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet 1996;348:983–7. [DOI] [PubMed] [Google Scholar]
- [47].Bleyer WA, Breckenridge RT. Studies on the detection of adverse drug reactions in the newborn. II. The effects of prenatal aspirin on newborn hemostasis. JAMA 1970;213:2049–53. [PubMed] [Google Scholar]
- [48].Stirling Y, Woolf L, North WR, et al. Haemostasis in normal pregnancy. Thromb Haemost 1984;52:176–82. [PubMed] [Google Scholar]
- [49].Sakkinen PA, Cushman M, Psaty BM, et al. Correlates of antithrombin, protein C, protein S, and TFPI in a healthy elderly cohort. Thromb Haemost 1998;80:134–9. [PubMed] [Google Scholar]
- [50].Koster T, Rosendaal FR, de Ronde H, et al. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet 1993;342:1503–6. [DOI] [PubMed] [Google Scholar]
- [51].van Boven HH, Vandenbroucke JP, Briet E, et al. Gene-gene and gene-environment interactions determine risk of thrombosis in families with inherited antithrombin deficiency. Blood 1999;94:2590–4. [PubMed] [Google Scholar]
- [52].Koster T, Rosendaal FR, Briet E, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study). Blood 1995;85:2756–61. [PubMed] [Google Scholar]
- [53].Folsom AR, Aleksic N, Wang L, et al. Protein C, antithrombin, and venous thromboembolism incidence: a prospective population-based study. Arterioscler Thromb Vasc Biol 2002;22:1018–22. [DOI] [PubMed] [Google Scholar]
- [54].Liberti G, Bertina RM, Rosendaal FR. Hormonal state rather than age influences cut-off values of protein S: reevaluation of the thrombotic risk associated with protein S deficiency. Thromb Haemost 1999;82:1093–6. [PubMed] [Google Scholar]
- [55].Cushman M, Costantino JP, Bovill EG, et al. Effect of tamoxifen on venous thrombosis risk factors in women without cancer: the Breast Cancer Prevention Trial. Br J Haematol 2003;120:109–16. [DOI] [PubMed] [Google Scholar]
- [56].Caine YG, Bauer KA, Barzegar S, et al. Coagulation activation following estrogen administration to postmenopausal women. Thromb Haemost 1992;68:392–5. [PubMed] [Google Scholar]
- [57].de Valk-de Roo GW, Stehouwer CD, Meijer P, et al. Both raloxifene and estrogen reduce major cardiovascular risk factors in healthy postmenopausal women: a 2-year, placebo-controlled study. Arterioscler Thromb Vasc Biol 1999;19:2993–3000. [DOI] [PubMed] [Google Scholar]
- [58].Kessler CM, Szymanski LM, Shamsipour Z, et al. Estrogen replacement therapy and coagulation: relationship to lipid and lipoprotein changes. Obstet Gynecol 1997;89:326–31. [DOI] [PubMed] [Google Scholar]
- [59].Zoller B, Garcia de Frutos P, Dahlback B. Evaluation of the relationship between protein S and C4b-binding protein isoforms in hereditary protein S deficiency demonstrating type I and type III deficiencies to be phenotypic variants of the same genetic disease. Blood 1995;85:3524–31. [PubMed] [Google Scholar]
- [60].Shah VP, Chegini HA, Vishneski SR, et al. Tamoxifen promotes superoxide production in platelets by activation of PI3-kinase and NADPH oxidase pathways. Thromb Res 2012;129:36–42. [DOI] [PubMed] [Google Scholar]
- [61].De Pinho Pessoa BB, Menezes Cavalcante BB, Maia MP, et al. Effect of tamoxifen on arterial microvascular anastomosis. Microsurgery 2007;27:286–8. [DOI] [PubMed] [Google Scholar]
- [62].Ceran C, Aksam E, Aksam B, et al. Tamoxifen-related thrombosis: an experimental study in rat venous microvascular anastomosis model. Ann Plast Surg 2017;78:213–6. [DOI] [PubMed] [Google Scholar]
- [63].Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997;277:642–5. [PubMed] [Google Scholar]
- [64].Liao KF, Lin CL, Lai SW, et al. Sitagliptin use and risk of acute pancreatitis in type 2 diabetes mellitus: a population-based case-control study in Taiwan. Eur J Intern Med 2016;27:76–9. [DOI] [PubMed] [Google Scholar]
- [65].Lai SW, Lin CL, Liao KF. Digoxin use may increase the relative risk of acute pancreatitis: a population-based case-control study in Taiwan. Int J Cardiol 2015;181:235–8. [DOI] [PubMed] [Google Scholar]
- [66].Haynes AB, Edmondson L, Lipsitz SR, et al. Mortality trends after a voluntary checklist-based surgical safety collaborative. Ann Surg 2017;266:923–9. [DOI] [PubMed] [Google Scholar]
- [67].Lin HF, Liao KF, Chang CM, et al. Correlation between proton pump inhibitors and risk of pyogenic liver abscess. Eur J Clin Pharmacol 2017;73:1019–25. [DOI] [PubMed] [Google Scholar]
- [68].Liao KF, Lin CL, Lai SW. Parkinson's disease and risk of colorectal cancer: a population-based case-control study in Taiwan. Neurology Asia 2017;22:133–8. [Google Scholar]
- [69].Lai SW, Lin HF, Lin CL, et al. No association between losartan use and acute pancreatitis in hypertensive patients. Eur J Hosp Pharm 2017;24:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lai SW, Lin HF, Lin CL, et al. Immune thrombocytopenic purpura might be an early hematologic manifestation of undiagnosed human immunodeficiency virus infection. Intern Emerg Med 2017;12:157–62. [DOI] [PubMed] [Google Scholar]


