Abstract
Distinguishing lung adenocarcinoma from squamous cell carcinoma (SCC) is clinically important. Computed tomography (CT) scan is an economical, effective, noninvasive, commonly available, and quick diagnostic way for lung cancer. In this study, we aim to compare the CT characteristics in adenocarcinoma and SCC.
Data from 275 cases (259 adenocarcinoma and 16 SCC) were retrospectively compared. CT characteristics, including lesion size and shape, single/multifocal lesions, location of the tumor, the margin of lobes, whether the lesion had deep lobulated margin, bronchial cut-off sign, signs of dilated bronchial arteries, signs of vascular bundle thickening, signs of short burrs, spinous processes, and pleural indentation, were compared in 148 cases (137 adenocarcinoma and 11 SCC).
Patients with adenocarcinoma were more likely to be female (44.2% vs 25.0%, P = .017). Compared with SCC, adenocarcinomas were more likely to have deep lobulated margin (81.0% vs 54.5%, P = .038), less likely to have smooth lobes margin (2.7% vs 83.3%, P < .001), more likely to have vascular bundle thickening (37.2% vs 0, P = .016) and pleural indentation (59.9% vs 18.2%, P = .01), and marginally less likely to have dilated bronchial arteries (17.5% vs 45.5%, P = .064). No significant difference was observed regarding to characteristics, including tumor size, location of the tumor, signs of bronchial cut-off, dilated bronchial arteries, short burrs, or spinous processes.
CT scan has the potential to help to distinguish lung adenocarcinoma and SCC in a fast and commonly available way. CT could be a rough but fast way to diagnosis, and may thus shorten the waiting time to treatment and allow more time for clinicians, patients, and their families to prepare for future treatment.
Keywords: adenocarcinoma, CT scan, lung cancer, squamous cell carcinoma
1. Introduction
Lung cancer remains the main contributor to cancer-related mortality, with 224,210 new cases and 159,260 deaths in the United States in 2014.[1] Squamous cell carcinoma (SCC) and adenocarcinoma are the 2 major subtypes of nonsmall cell lung carcinoma. Differential diagnosis between adenocarcinoma (AC) and SCC is of clinical significance. Compared with squamous lung cancer, adenocarcinoma was associated with better prognosis.[2] Chemotherapy regimens for AC and SCC are also different according to the guidelines of National Comprehensive Cancer Network (NCCN) for NSCLC.[3] Major advances in thoracic medical oncology brought new treatments such as epidermal growth factor receptor (EGFR)-targeted therapies, which are currently recommended as the first-line treatment for nonsmall cell lung carcinoma with EGFR mutations, and these mutations primarily occur in adenocarcinoma.[4] Other new treatments such as Pemetrexed is contraindicated in SCC due to the lack of effectiveness.[5] A “histology specific” therapy is expected, as more important molecular differences between adenocarcinoma and SCC are identified. What is more, the cost for treatment varied by histologic types. Cipriano et al found that in USA, a 72-year-old diagnosed with lung cancer in 2000, the monthly costs in the first 6 months of care ranged from $2687 (no active treatment) to $9360 (chemoradiotherapy), and varied by stage at diagnosis and histologic type.[6]
Standard morphologic criteria can distinguish the adenocarcinoma and SCC in most cases,[7] which takes a period of time. Serial imaging studies suggested that nonsmall-cell lung cancer (NSCLC) may progress rapidly between presentation and initiation of treatment.[1] It is necessary for clinicians to identify between these 2 diseases in a convenient and faster way. Computed tomography (CT) scan, which is the most economical and effective noninvasive diagnostic way for lung cancer, is available in most hospitals in China. If CT could provide some clues to distinguish the 2 cancer, clinicians could provide patients and their families more information about diagnosis, choices of therapies, prognosis and cost in shorter time, and allow more time for patients and their families to be prepared.
In the study, we retrospectively checked all lung adenocarcinoma and SCC cases in the pulmonary department in our hospital from December 2014 to June 2017, to compare their CT characteristics and the pathology, in an attempt to provide a basis for the CT to distinguish adenocarcinoma from SCC.
2. Materials and methods
2.1. Study population
The retrospective database was from the Pulmonary Department of the First Affiliated Hospital of Xinxiang Medical University. All patients provided signed informed consent. We selected lung adenocarcinoma and SCC cases that were confirmed by pathological diagnosis from December 2014 to June 2014. Two hundred seventy-five cases (259 adenocarcinoma and 16 SCC) were detected. Patients with preoperative CT scans of the chest were included if the images were available for study. Exclusion criteria were other types of lung cancer, and patients who were previously diagnosed with other cancers.
2.2. Acquisition of CT images
All CTs were performed with CT scanners (Toshiba Aquilion 64-slice spiral CT or Toshiba Aquilion ONE 320-slice spiral CT scanner; Toshiba, Tokyo, Japan) in helical mode from the apex to the lung base. The patient was positioned in the supine position. Technical parameters were X-ray tube current 196 to 405 mA; tube voltage 120 kV; collimation 5 mm; rotation speed 0.5 s; matrix 512 × 512. Iopromide (370 g/L) was used to perform the contrast-enhanced scanning. A high-pressure syringe was used to inject 70 to 90 mL of contrast agent from the right elbow vein with an injection rate of 4.0 mL/s. Twenty milliliter saline were later injected at the same rate. Scanning range was set to from the upper chest to the costal angle level.
All image data were transmitted directly to our picture archiving and communication system. Monitors were used to view both mediastinal and lung window images
2.3. CT image analysis
Descriptions of the imaging features were made according to the Nomenclature Committee of the Fleischner Society (NCFS) chest image definition.[8] All CT images were reviewed by the same 2 senior radiologists who were blinded from patients’ pathologic diagnosis. The radiologists assessed and recorded lesion size and shape, single/multifocal lesions, location of the tumor, whether the lesion had deep lobulated margin, the margin of lobes, bronchial cut-off sign, signs of dilated bronchial arteries, signs of vascular bundle thickening, signs of short burrs, spinous processes, and pleural indentation (Fig. 1).
Figure 1.
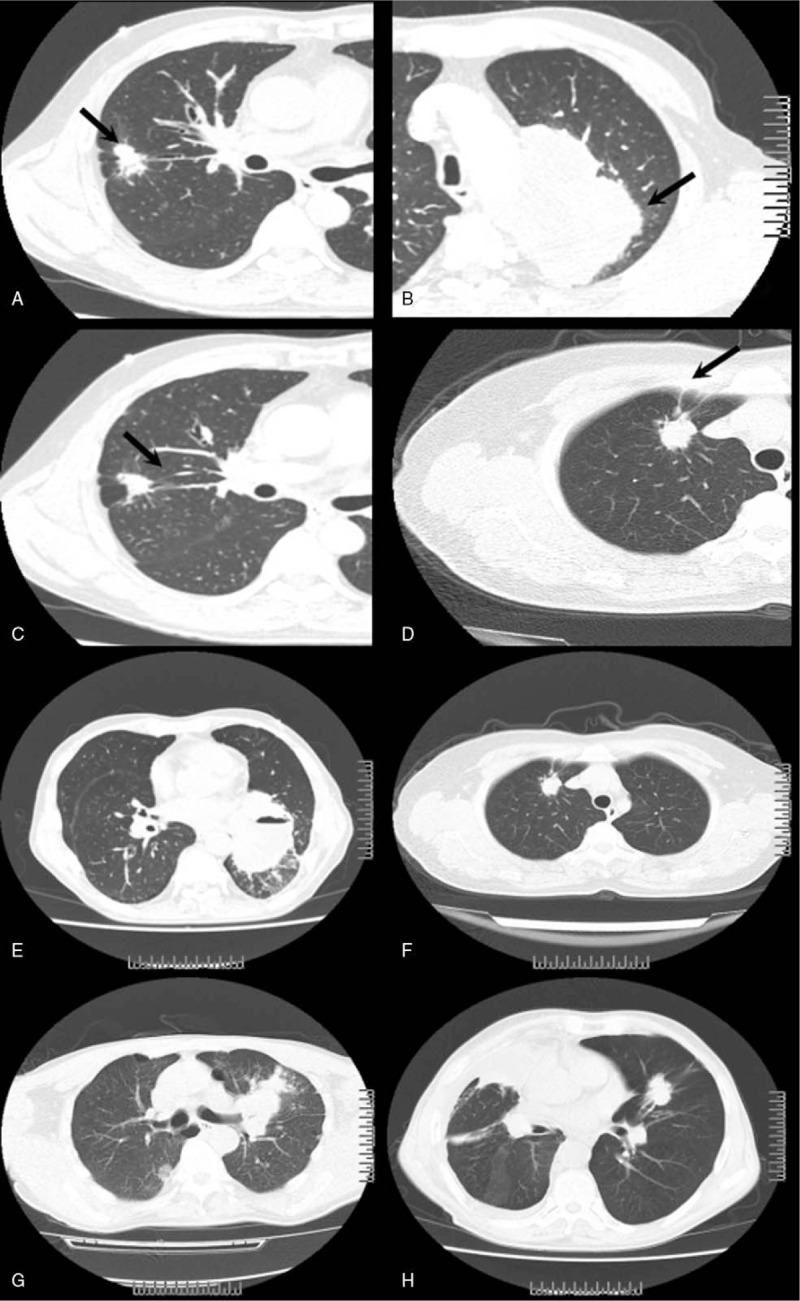
Some CT features of the tumors. Both smooth tumor lobes margin and nonsmooth lobes margin were observed. In a case of adenocarcinoma with nonsmooth lobes margin, the tumor was lobulated and an irregular shape (arrowhead, A). In a case of squamous cell carcinoma with smooth lobes margin, the shape of tumor was regular (arrowhead, B). Sign of vascular bundle thickening in a case of adenocarcinoma. Several small pulmonary vessels were pulled and displaced to the lesion (arrowhead, C). Sign of pleural indentation was shown in a case of squamous cell carcinoma, showing a taped extension of the lesion to pleura and adjacent pleural retraction (arrowhead, D). Sign of short burrs and spinous processes of tumor margin was shown in a case of squamous cell carcinoma (arrowhead, E) and in a case of adenocarcinoma (arrowhead, F). Bronchial cut-off sign was shown in a case of squamous cell carcinoma (arrowhead, G) and in a case of adenocarcinoma (arrowhead, H). Abrupt truncation of a bronchus from obstruction was shown in both cases.
3. Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 13.0 (Statistical Package for the Social Sciences Inc, Chicago, IL). Comparisons of continuous variables between adenocarcinoma and SCC were carried out with the Mann–Whitney Wilcoxon test. Chi-squared test was used for categorical data. A 2-tail P value less than .05 was considered statistically significant.
4. Results
4.1. Patient characteristics
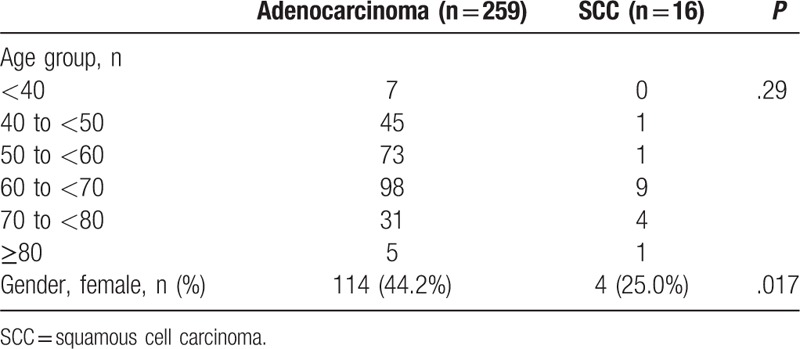
In the study, a total of 275 patients (259 adenocarcinoma and 16 SCC) were detected. There were significantly more females in the adenocarcinoma group than that in the SCC group (44.2% vs 25.0%, P = .017, Table 1). The mean overall age was 59.9 ± 11.4 years, with most cases aged 50 to 70 years. There is no significant difference in the age distribution.
Table 1.
Baseline characteristics of lung adenocarcinoma and SCC patients.
4.2. Imaging findings
Among the 275 cases, CT-enhanced scan data were available in 148 cases, including 137 adenocarcinoma and 11 SCC. On average, the diameters of adenocarcinoma were marginally larger than those of SCC (52.6 ± 27.5 vs 40.2 ± 20.3 mm, P = .09, Table 2). A single lesion was observed in most cases. There were 111 cases (81.0%) with single lesion in the adenocarcinoma group, and 10 cases (90.9%) with single lesion in the SCC group. Most of the adenocarcinomas were with peripheral lesions (101 cases, 73.7%), while nearly half of the SCCs were with peripheral lesions. Significantly higher proportion of cases in the adenocarcinoma group had tumor with deep lobulated margin on CT scans (81.0%), and non-smooth margin (97.3%) than those in the SCC group (P < .05 for both). Most of the cases had short burrs in the lesion boundary, and no significant difference was detected in the adenocarcinoma and the SCC groups. Forty-six adenocarcinoma cases (33.6%) and 6 SCC cases (54.5%) had lesions with spinous processes and the difference was not statistically significant.
Table 2.
Comparison of the CT features between lung adenocarcinoma and SCC.
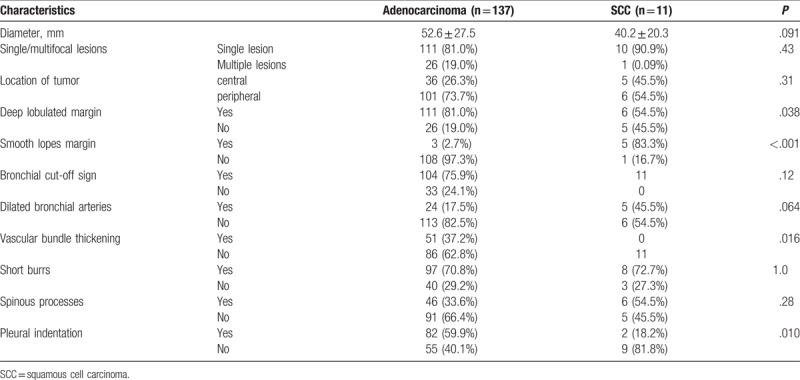
All SCC cases and most adenocarcinoma cases (75.9%) had bronchial cut-off sign on CT scans. Marginally significantly more dilated bronchial arteries (45.5%) were observed than in the adenocarcinoma group (17.5%, P = .06). About the vascular bundle thickening, no case in the SCC group was observed to have the vascular bundle thickening, while 51 adenocarcinoma cases (37.2%) were observed to have the vascular bundle thickening. Significantly more signs of pleural indentation were shown in the adenocarcinoma group (82, 59.9%) than that in the SCC group (2, 18.2%).
5. Discussion
In this study, compared with SCC, adenocarcinoma is more common in female cases. And significantly less tumor with smooth lobes margin, more vascular bundle thickening, and more pleural indentation were observed in the adenocarcinoma tumors.
In our study, there were significantly more adenocarcinoma cases than SCC cases. And in the adenocarcinoma group, more female cases were observed. Our results were consistent with previous work. Lortet et al[9] found that in Europe, North America, and Oceania, the adenocarcinoma incidence rates have risen and surpassed those of SCC (historically the most frequent subtype) in the majority of these populations. Devesa et al[10] and Janssen-Heijnen and Coebergh[11] had shown that adenocarcinoma is consistently more frequent in women than in men (in both smokers and nonsmokers). The high prevalence of adenocarcinoma in women, especially in Asian women, can be associated with their exposure to tobacco smoke from husbands and to cooking fume.[12,13]
Eleven characteristics of pulmonary nodules or masses were compared in the analysis. We observed significantly different characteristics in adenocarcinoma and SCC, including the surface of tumor lobes, vascular bundle thickening, and pleural indentation, which were consistent with pathological features of the cancer. The adenocarcinoma's histologic growth patterns were mostly acinar, papillary, and solid.[14] Therefore, the surface of the lobes in adenocarcinoma often has small differentiated leaves and spinous processes. SCC often invades in a tuft of tumor cells[15] and the surfaces are relatively smooth. The vascular bundle sign and the pleural indentation both are associated with internal tissue fibrosis traction on the neighboring tissue structure,[16] which are more common in adenocarcinoma.[17] The difference in vascular bundle sing was also consistent with a previous study. Kuriyama et al[18] observed more vascular bundle sign in adenocarcinoma than in SCC.
Tumor progression is rapid in patients with untreated NSCLC.[19] Bozcuk and Martin [20] found the median time to treatment in NSCLC patients was 48 days in a retrospective study. Everitt et al[19] found in untreated, predominantly stage III, NSCLC patients, the probability of upstaging within 24 days was 32%. Work to promote early diagnosis and treatment may improve the prognosis in NSCLC patients.
While comprehensive pathologic and molecular diagnosis is advocated for patients with advanced lung cancers, both radiologists and clinicians should be aware of the radiological appearance of adenocarcinoma and SCC. CT scan is the most inexpensive, noninvasive, and available diagnostic way for lung cancer. As shown in our study, characteristics shown by CT such as vascular bundle thickening and pleural indentation have the potential to help to distinguish lung adenocarcinoma from SCC, and due to the small sample size of our study, especially the small number of SCC cases, further studies with more SCC cases are in need. Using CT scan to have a rough but fast preliminary diagnosis has the potential to shorten the waiting time to treatment, and could help the clinicians and patients to know more about the diagnosis, and allow more time for clinicians, patients, and their families to prepare for future treatments.
Author contributions
Conceptualization: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Ying Hu, Dong-Ming Han.
Data curation: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Dong-Ming Han.
Formal analysis: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Dong-Ming Han.
Funding acquisition: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Qing-Wu Wu, Dong-Ming Han.
Investigation: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Ying Hu, Mei-Xia Li, Dong-Ming Han.
Methodology: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Ying Hu, Mei-Xia Li, Qing-Wu Wu, Dong-Ming Han.
Project administration: Jun-Yan Yue, Jie Chen, Feng-Mei Zhou, Mei-Xia Li, Dong-Ming Han.
Resources: Jun-Yan Yue, Dong-Ming Han.
Software: Jun-Yan Yue, Dong-Ming Han.
Supervision: Jun-Yan Yue, Mei-Xia Li, Qing-Wu Wu, Dong-Ming Han.
Validation: Jun-Yan Yue, Ying Hu, Mei-Xia Li, Dong-Ming Han.
Visualization: Jun-Yan Yue, Ying Hu, Mei-Xia Li, Dong-Ming Han.
Writing – original draft: Jun-Yan Yue, Ying Hu, Mei-Xia Li, Qing-Wu Wu, Dong-Ming Han.
Writing – review & editing: Jun-Yan Yue, Qing-Wu Wu, Dong-Ming Han.
Footnotes
Abbreviations: NCCN = National Comprehensive Cancer Network, NSCLC = nonsmall-cell lung cancer, SCC = squamous cell carcinoma.
Funding/support: This work was supported by grants from the Henan Province science and technology key projects (Funding no. 172102310503).
The author(s) of this work have nothing to disclose and have no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- [2].Asamura H, Goya T, Koshiishi Y, et al. A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 2008;3:46–52. [DOI] [PubMed] [Google Scholar]
- [3].Kim HL, Puymon MR, Qin M, et al. NCCN clinical practice guidelines in oncology™, 2013. [Google Scholar]
- [4].Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005;97:339–46. [DOI] [PubMed] [Google Scholar]
- [5].Scagliotti G, Brodowicz T, Shepherd FA, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 2011;6:64–70. [DOI] [PubMed] [Google Scholar]
- [6].Cipriano LE, Romanus D, Earle CC, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health 2011;14:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Müller-Hermelink HK, Engel P, Kuo TT, et al. Tumours of the thymus. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. World Health Organization Classification of Tumours: Pathology and Genetics Tumours of the Lung, Pleura, Thymus and Heart; 2004. [Google Scholar]
- [8].Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- [9].Lortet-Tieulent J, Soerjomataram I, Ferlay J, et al. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 2014;84:13–22. [DOI] [PubMed] [Google Scholar]
- [10].Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294–9. [DOI] [PubMed] [Google Scholar]
- [11].Janssen-Heijnen ML, Coebergh J-WW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41:245–58. [DOI] [PubMed] [Google Scholar]
- [12].Wen W, Shu XO, Gao Y-T, et al. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. BMJ 2006;333:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ignatius T, Chiu Y-l, Au JS, et al. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res 2006;66:4961–7. [DOI] [PubMed] [Google Scholar]
- [14].Solis LM, Behrens C, Raso MG, et al. Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer 2012;118:2889–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yousem SA. Peripheral squamous cell carcinoma of lung: patterns of growth with particular focus on airspace filling. Hum Pathol 2009;40:861–7. [DOI] [PubMed] [Google Scholar]
- [16].Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. Am J Roentgenol 2002;178:1053–7. [DOI] [PubMed] [Google Scholar]
- [17].Fraire AE, Greenberg SD. Carcinoma and diffuse interstitial fibrosis of lung. Cancer 1973;31:1078–86. [DOI] [PubMed] [Google Scholar]
- [18].Kuriyama K, Tateishi R, Doi O, et al. CT-pathologic correlation in small peripheral lung cancers. Am J Roentgenol 1987;149:1139–43. [DOI] [PubMed] [Google Scholar]
- [19].Everitt S, Herschtal A, Callahan J, et al. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer 2010;116:5030–7. [DOI] [PubMed] [Google Scholar]
- [20].Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer 2001;34:243–52. [DOI] [PubMed] [Google Scholar]


