Supplemental Digital Content is available in the text
Keywords: antibiotics, correlation, Korea, resistance, stewardship
Abstract
The aim of this study was to evaluate the changing pattern of antibiotic usage and antimicrobial resistance of bacterial pathogens among hospitalized patients in Korea. We simultaneously investigated the correlation between antimicrobial resistance and antibiotic consumption.
Data on total antibiotic prescriptions, patient days, and antimicrobial sensitivity tests among inpatients from 6 university hospitals in Korea in 2004, 2008, and 2012 were collected. The consumption of each antibiotic class was converted to defined daily dose/1000 patient-days by using the anatomical therapeutic chemical classification system by the World Health Organization. We defined third-generation cephalosporins (3rd CEPs), fourth-generation cephalosporins, beta-lactam/beta-lactamase inhibitors, and fluoroquinolones (FQs) as broad-spectrum antibiotics and carbapenems, tigecycline, glycopeptides, oxazolidinone, and polymyxin as antibiotics against multidrug-resistant (MDR) pathogens.
A 15.1% decrease in total antibiotic consumption was observed in 2012 compared to that observed in 2004. In contrast, a 10.2% and 70.7% increase in broad-spectrum antibiotics and antibiotics against MDR pathogens were observed, respectively, in the same period. The resistance rate of Escherichia coli to 3rd CEPs (17.6% in 2004, 21.7% in 2008, and 33.8% in 2012, P <.001) and ciprofloxacin (37.5% in 2004, 38.7% in 2008, and 46.6% in 2012, P = .001) demonstrated a significantly increasing trend. Similarly, the resistance rate of Klebsiella pneumoniae to 3rd CEPs (34.3% in 2004, 33.7% in 2008, and 44.5% in 2012, P <.001) gradually increased. Resistance of Acinetobacter baumanii and Pseudomonas aeruginosa to imipenem significantly increased throughout the study period (A baumanii: 8.9% in 2004, 40.8% in 2008, and 65.3% in 2012, P <.001; P aeruginosa: 25.1% in 2004, 31.5% in 2008, and 29.7% in 2008, P = .050).
The consumption of carbapenems and FQs demonstrated significant positive correlation for resistance of E coli or K pneumoniae to 3rd CEPs as well as E coli or K pneumoniae to ciprofloxacin. Increasing resistance of A baumanii to ciprofloxacin was significantly correlated with increasing consumption of FQs; increasing resistance of A baumanii to imipenem was significantly correlated with increasing consumption of carbapenems.
In conclusion, overall antimicrobial resistance increased and consumption of broad-spectrum antibiotics and antibiotics against MDR pathogens subsequently increased in Korean hospitals.
1. Introduction
The discovery of antibiotics has offered mankind a dramatically new approach to infection control and is considered as one of the milestones in medical history.[1] A radical reduction in mortality and morbidity can be achieved in various infectious diseases. In the 1960s, some medical doctors including infectious disease specialists even declared “the end of the war against infectious diseases”.[2] However, bacteria have fought back aided by several evading strategies against antibiotics.[3] At present, growing resistance of microorganisms diminishes the efficacy of existing antibiotics.[4] Such ineffectiveness of antibiotics leads to increased mortality, morbidity, and medical costs.[5]
Increasing use of antibiotics is closely linked to the emergence of resistance due to enhancing selective pressure on bacteria.[6] The key strategy to overcome the current problems caused by antimicrobial-resistant pathogens is implementation of antimicrobial stewardship programs (ASPs): a set of multidisciplinary activities focusing on the proper use of antimicrobials.[7] In 2011, the World Health Organization (WHO) called on their national partners to take urgent action to realize ASPs,[8] and the Korean Ministry of Health and Welfare emphasized the importance of ASPs in the recently established Korean national action plan on antimicrobial resistance in 2016.[9]
Monitoring antibiotic use and resistance patterns is the first step and one of the “core elements” for successful ASPs.[10] Precisely measured data enables policy makers to establish proper measures for ASPs. The aim of this study is to evaluate the changing pattern of antibiotic usage and antimicrobial resistance of bacterial pathogens among hospitalized patients in Korea. We simultaneously investigated the correlation between antimicrobial resistance and antibiotic consumption.
2. Material and methods
2.1. Study design and setting
We conducted a multicenter retrospective study at six hospitals located throughout the Korean peninsula (Fig. 1):
Figure 1.
Geographic distribution of six hospitals in Korea.
-
1.
Hanyang University Seoul Hospital [858-bed, university-affiliated tertiary care hospital located in Seoul. It had 29-bed intensive care unit (ICU), 16-bed neonatal intensive care unit (NICU), and 10-bed bone marrow transplant unit (BMTU) in 2012. The patient-days for inpatients in 2012 was 237,805];
-
2.
Gyeongsang National University Hospital (889-bed, university-affiliated tertiary care hospital located in Jinju. It has 27-bed ICU, 25-bed NICU, and 2-bed BMTU in 2012. The patient-days for inpatients in 2012 was 282,548);
-
3.
Korea University Ansan Hospital (543-bed, university-affiliated secondary care hospital located in Ansan. It has 36-bed ICU, 20-bed NICU, no BMTU in 2012. The patient-days for inpatients in 2012 was 187,967);
-
4.
Hanyang University Guri Hospital (578-bed, university-affiliated secondary care hospital located in Guri. It has 26-bed ICU, 7-bed NICU, and no BMTU in 2012. The patient-days for inpatients in 2012 was 170,656);
-
5.
Chonnam National University hospital (970-bed, university-affiliated tertiary care hospital located in Gwangju. It has 113-bed ICU, 28-bed NICU, and no BMTU in 2012. The patient-days for inpatients in 2012 was 327,278);
-
6.
Chungbuk National University hospital (620-bed, university-affiliated tertiary care hospital located in Cheongju. It has 29-bed ICU, 25-bed NICU, and no BMTU in 2012. The patient-days for inpatients in 2012 was 199,601).
Data on total antibiotic prescriptions, patient days, and antimicrobial sensitivity tests were collected from inpatients at each hospital in 2004, 2008, and 2012. We blinded hospital's name in this article due to possibility of unintended blame on hospitals with higher rate of antibiotic use or antimicrobial resistance rate. The study protocol was approved by the Institutional Review Boards of the Hanyang University Hospital (2015-01-015), and the requirement for written informed consent from patients was waived due to the retrospective nature of the study, and its impracticability.
2.2. Definitions
2.2.1. Antibiotics
We defined antibiotics as medications with class J01 according to the Anatomical Therapeutic Chemical classification, which does not include antifungal or antituberculosis agents. Systemic agents with per oral or parenteral administration routes are included, while topical agents are excluded. We converted the amount of antibiotic consumption to defined daily dose (DDD) by using the Anatomical Therapeutic Chemical classification of WHO,[11] and then standardized it for 1000 patient-days.
We classified antibiotic agents into 19 classes: first-generation cephalosporins (1st CEPs), second-generation cephalosporins (2nd CEPs), third-generation cephalosporins (3rd CEPs), fourth-generation cephalosporins (4th CEPs), aminoglycosides (AGs), beta-lactam/beta-lactamase inhibitors (BL/BLIs), carbapenems, fluoroquinolones (FQs), glycopeptides, lincosamide, macrolides, monobactam, metronidazole, oxazolidinone, penicillins, polymyxin, tetracycline, tigecycline, and trimethoprim/sulfmonamide (SXT). Other antibiotics such as amphenicol, fosfomycin, and streptogramin were excluded because they are rarely used.
We defined 3rd CEPs, 4th CEPs, BL/BLIs, and FQs as broad-spectrum antibiotics, and carbapenems, tigecycline, glycopeptides, oxazolidinone, and polymyxin as antibiotics against multidrug-resistant (MDR) pathogens. The other antibiotic classes were defined as non-broad-spectrum antibiotics.
2.2.2. Major bacterial pathogens and antimicrobial resistance
We analyzed antimicrobial sensitivity tests for major bacterial pathogens: Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Staphylococcus aureus. The first isolate of these pathogens for each month per patient was included for analysis. If there were 2 or more pathogens isolated from different sites, we chose according to the priority order. The priority order was as follows: cerebrospinal fluid, joint fluid, pleural fluid, ascites, blood, closed pus, urine, and sputum. Susceptibilities to antibiotics were determined by means of semi-automated systems at each hospital (VITEK, bioMèrieux, Hazelwood, MO, or Microscan, Dade Behring, West Sacramento, CA). The breakpoints of each compound were defined in reference to the Clinical and Laboratory Standards Institute,[12] and R (resistance) or I (intermediate) were defined as resistance. We defined resistance against 3rd CEPs as resistance to at least one of the following antibiotics: cefotaxime, ceftriaxone, or ceftazidime.
2.3. Statistical analysis
The Jonckheere–Terpstra test using measures on a per-month basis was used to assess the trend of antibiotic consumption, proportion of pathogens, and antimicrobial resistance rate over time. We used the Kruskal–Wallis test to assess inter-hospital differences in antibiotic usage pattern and antimicrobial resistance. Pearson's correlation coefficient was used to describe the relationship between antibiotic consumption and bacterial resistance rates. Statistical significance was defined as P <.05. All analyses were performed using SPSS 24.0 (IBM Corporation, Armonk, NY).
3. Results
3.1. Overall consumption and trends of systemic antibiotic classes
The most commonly prescribed antibiotic subgroup was 3rd CEPs (24.8%, 213.82/862.94 DDD/1000 patient-days), followed by FQs (12.1%, 104.11/862.94 DDD/1000 patient-days), 2nd CEPs (11.4%, 98.17/862.94 DDD/1000 patient-days), 1st CEPs (10.6%, 91.84/862.94 DDD/1000 patient-days), and BL/BLIs (10.5%, 90.27/862.94 DDD/1000 patient-days) (Fig. 2). The proportion of broad-spectrum antibiotics, antibiotics against MDR pathogens, and non-broad-spectrum antibiotics use were 48.6% (419.88/862.93 DDD/1000 patient-days), 4.9% (41.95/862.93 DDD/1000 patient-days), and 46.5% (401.02/862.93 DDD/1000 patient-days), respectively.
Figure 2.
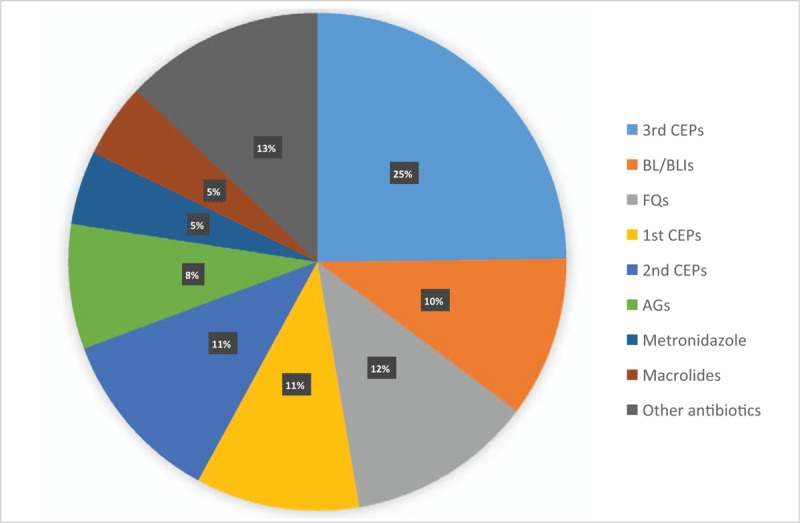
The proportion of consumption of antimicrobial agents for systemic use by subgroup at 6 hospitals in Korea, 2004 to 2012. 1st CEPs = first-generation cephalosporins, 2nd CEPs = second-generation cephalosporins, 3rd CEPs = third-generation cephalosporins, AGs = aminoglycosides, BL/BLIs = beta-lactam/beta-lactamase inhibitors, FQs = fluoroquinolones.
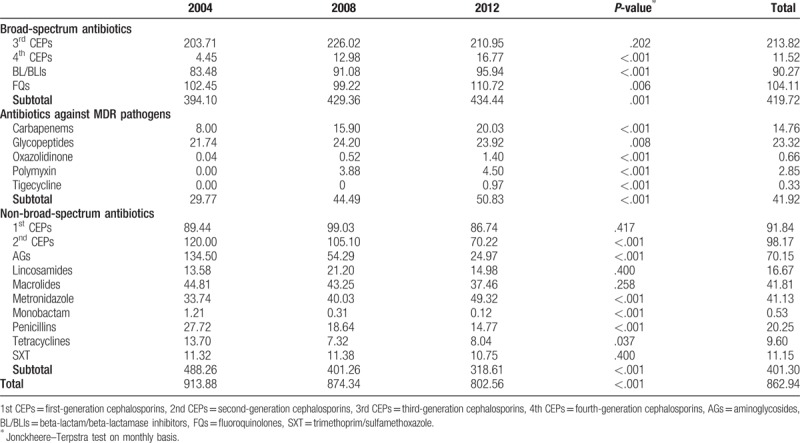
Table 1 presents the overall annual consumption of antimicrobial agents for systemic use. The mean antibiotic consumption was 862.93 DDD/1000 patient-days throughout the study period. Over the 9-year study period, a 15.1% decrease in total antibiotic consumption was observed in 2012 compared to that in 2004 (913.88 DDD/1000 patient-days in 2004; 874.34 DDD/1000 patient-days in 2008; 802.56 DDD/1000 patient-days in 2012, P <.001). On comparing antibiotic consumption in 2012 with that in 2004, a 10.2% (394.10 DDD/1000 patient-days in 2004; 429.36 DDD/1000 patient-days in 2008; 434.44 DDD/1000 patient-days in 2012, P = .001) and 70.7% (29.77 DDD/1000 patient-days in 2004; 44.49 DDD/1000 patient-days in 2008; 50.83 DDD/1000 patient-days in 2012, P <.001) increase in broad-spectrum antibiotics and antibiotics against MDR pathogens were observed, respectively. In comparison, a 34.7% decrease in non-broad-spectrum antibiotics (488.26 DDD/1000 patient-days in 2004; 401.26 DDD/1000 patient-days in 2008; 318.61 DDD/1000 patient-days in 2012, P <.001) was observed.
Table 1.
Annual consumption of antimicrobial agents for systemic use at 6 hospitals in Korea, 2004 to 2012 (unit: DDD/1000 patient-days).
3.2. Trend of antimicrobial resistance of bacterial pathogens
The proportion of bacterial pathogens commonly associated with antimicrobial resistance was 55.9%, and it had increased from 2004 to 2012 (P <.001). The most frequently isolated pathogen among them was S aureus (15.5%), followed by E coli (13.8%) and P aeruginosa (15.5%). The proportions of E coli (13.5% in 2004; 12.6% in 2008; 15.4% in 2012, P = .009) had significantly increased from 2004 to 2012. The proportions of K pneumoniae, A baumanii, P aeruginosa, and S aureus remained stable throughout the study period (Table 2).
Table 2.
Distribution of pathogens at 6 hospitals in Korea, 2004 to 2012 (unit: %).
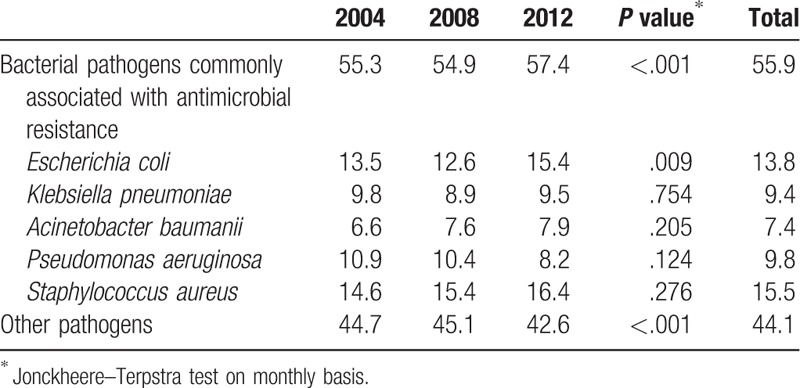
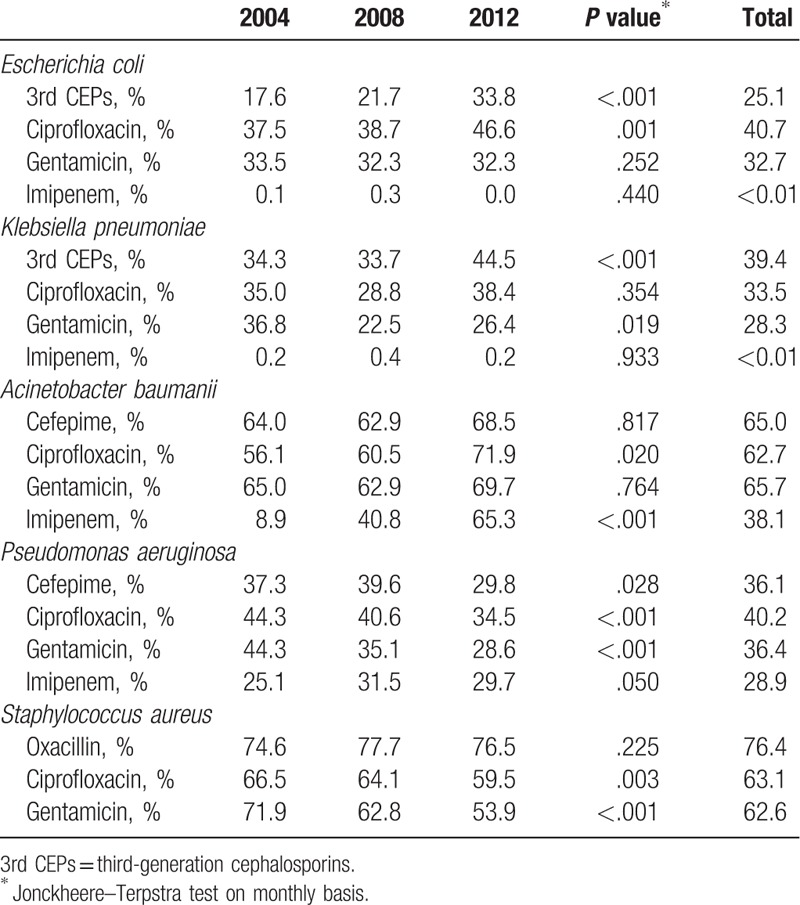
Table 3 presents the trend of antimicrobial resistance of bacterial pathogens. The resistance rate of E coli (from 17.6% in 2004 to 33.8% in 2012, P <.001) and K pneumoniae (from 34.3% in 2004 to 44.5% in 2012, P <.001) to 3rd CEPs showed a significantly increasing trend. Similarly, resistance of E coli to ciprofloxacin significantly increased (from 37.5% in 2004 to 46.6% in 2012, P = .001). In comparison, resistance of K pneumoniae to gentamicin significantly decreased throughout the study period (from 36.8% in 2004 to 26.4% in 2012, P = .019). The overall resistance rates of E coli and K pneumoniae to imipenem were lower than 0.01%. The resistance rate of A baumanii to ciprofloxacin (from 56.1% in 2004 to 71.9% in 2012, P = .020) and imipenem (from 8.9% in 2004 to 65.3% in 2012, P <.001) increased over the study period. Resistance of P aeruginosa to cefepime (from 37.3% in 2004 to 29.8% in 2012, P = .028), ciprofloxacin (from 44.3% in 2004 to 34.5% in 2012, P <.001), and gentamicin (from 44.3% in 2004 to 28.6% in 2012, P <.001) appeared to be significantly decreasing. In contrast, resistance to imipenem significantly increased (from 25.1% in 2004 to 29.7% in 2012, P =.050). We observed that 76.4% S aureus was resistant to oxacillin and remained stable throughout the study period (P = .225). The resistance of S aureus to ciprofloxacin (from 66.5% in 2004 to 59.5% in 2012, P = .003) and gentamicin (from 71.9% in 2004 to 53.9% in 2012, P <.001) demonstrated a significantly decreasing trend.
Table 3.
Trend of resistance to the indicated agent in bacterial pathogens commonly associated with antimicrobial resistance at 6 hospitals in Korea, 2004 to 2012.
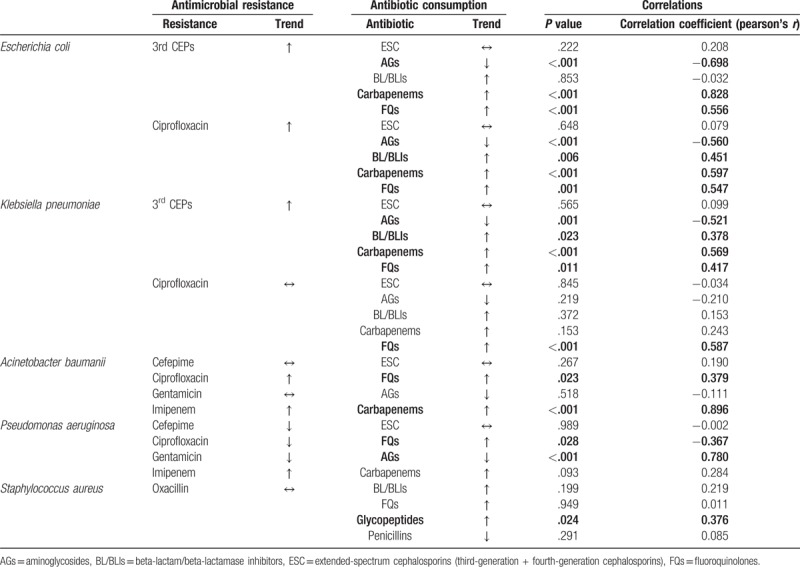
3.3. Correlations between antimicrobial resistance and antibiotic consumption
The correlations between antimicrobial resistance and antibiotic consumption are summarized in Table 4. The consumption of carbapenems and FQs demonstrated significant positive correlation for resistance of E coli or K pneumoniae to 3rd CEPs, while that to AGs demonstrated significant negative correlation. Similarly, for resistance of E coli or K pneumoniae to ciprofloxacin, the consumption of carbapenems and FQs demonstrated significant positive correlation, while that to AGs showed significant negative correlation. Increasing resistance of A baumanii to ciprofloxacin was observed during the study period and was significantly correlated with increasing consumption of FQs. Similarly, increasing resistance of A baumanii to imipenem was significantly correlated with increasing consumption of carbapenems. For P aeruginosa, the decreasing consumption of AGs significantly correlated with decreasing resistance rate to gentamicin. In comparison, the consumption of FQs demonstrated negative correlation with P aeruginosa resistance to ciprofloxacin. The resistance of S aureus to oxacillin demonstrated a significant positive correlation with the consumption of glycopeptides.
Table 4.
Correlations between antimicrobial resistance and antibiotic consumption at 6 hospitals in Korea.
3.4. Inter-hospital difference in antibiotic usage pattern and antimicrobial resistance
Hospital F consumed highest amount of broad-spectrum antibiotics, while hospital C consumed them least among the 6 hospitals (41.2% in hospital A; 54.1% in hospital B; 40.4% in hospital C; 42.6% in hospital D; 51.7% in hospital E; 58.7% in hospital F, P = .046). In comparison, there were no significant differences in the consumption of antibiotics against MDR pathogens (3.1% in hospital A; 5.2% in hospital B; 5.4% in hospital C; 3.5% in hospital D; 5.3% in hospital E; 7.0% in hospital F, P = .472) and non-broad-spectrum antibiotics (55.6% in hospital A; 40.7% in hospital B; 54.2% in hospital C; 53.9% in hospital D; 42.9% in hospital E; 34.3% in hospital F, P = .088) among the 6 hospitals. When compared according to antibiotic classes, 3rd CEPs (P = .020), FQs (P = .048), 1st CEPs (P = .010), lincosamide (P = .016), tetracycline (P = .029), and SXT (P = .050) demonstrated significant differences among the hospitals (Supplement 1).
The resistance rate of K pneumoniae to 3rd CEPs and ciprofloxacin demonstrated significant difference among hospitals, ranging from 16.7% to 50.0% and 19.4% to 48.0%, respectively (P = .038 and .035, respectively). Antimicrobial resistance of E coli, A baumanii, P aeruginosa, and S aureus did not significantly differ among hospitals (Supplement 2).
4. Discussion
The present study reflects the current status of antibiotic usage and antimicrobial resistance patterns at the hospital level in Korea. We believe that our findings can be useful for implementation of antimicrobial stewardship policies.
Most frequently prescribed antibiotics for inpatients were cephalosporins, FQs, and BL/BLIs, comprising 69.4% of the total antibiotic consumption. We noted that the antibiotic prescription patterns vary according to hospital type and size. Bitterman et al observed that antibiotic consumption was higher in teaching or public hospitals than other acute care hospitals.[13] Similarly, the antibiotic prescription patterns differ between referral hospitals and smaller hospitals in Korea.[14] Such differences may arise from differences in patient groups. Generally, patients in referral hospitals have more severe problems and/or underlying diseases compared those in smaller hospitals.
Although all 6 hospitals in our study are in similar settings, there were significant differences in the proportion of broad-spectrum antibiotics among hospitals, especially, 3rd CEPs and FQs. Interestingly, the inter-hospital differences in antimicrobial resistance may not influence the differences in antibiotic usage pattern. On examining the resistance of K pneumoniae to ciprofloxacin, which showed a statistically significant difference among hospitals, the resistance rate of hospital B was the lowest but the consumption of FQs was the highest. Similarly, resistance of K pneumoniae to 3rd CEPs was the second highest in hospital A but the consumption of 3rd CEPs was the second lowest. This indicates that other factors may influence antibiotic usage pattern in a hospital. We suggest that behavioral factors, such as physician's attitude and knowledge, may play an important role in influencing these patterns. A Korean study reported that lack of knowledge is one of the main factors responsible for inappropriate antibiotic use in university hospitals.[15] Therefore, the medical staff should be properly educated, and prompt feedback regarding inappropriate antibiotic usage should be mandatory to achieve antimicrobial stewardship. Some authors suggested that providing feedback letters or peer group interventions can also be useful strategies.[16,17]
In the present study, there was steady increase in the proportion of Enterobacteriaceae resistant to 3rd CEPs or ciprofloxacin. According to the Korean Antimicrobial Resistance Monitoring System (KARMS), involving a total of 35 secondary and tertiary hospitals, the resistance rate of E coli to cefotaxime and FQs increased from 10% in 2004 to 29% in 2013 and from 30% in 2004 to 42% in 2013, respectively.[18] Similarly, the resistance rate of K pneumoniae to cefotaxime and FQs increased from 30% in 2004 to 40% in 2013 and from 30% in 2004 to 34% in 2013, respectively.[18] Another notable finding is that the resistance rate of A baumanii to imipenem was the largest during the study period. Similar results were observed in the study by KARMS: the resistance increased from 18% in 2004 to 77% in 2013.[18] Another nationwide surveillance system, the Korean Nosocomial Infection Surveillance System, reported that imipenem-resistant A baumanii increased from 52.9% in 2006 to 89.8% in 2013 (P <.0001) in ICUs in Korea.[19] Interestingly, the resistance rate of P aeruginosa to gentamicin seems to be decreasing over time, which is concordant to the finding from KARMS.[18] Considering the resistance rate of E coli, K pneumoniae, and P aeruginosa to gentamicin was lower than to ciprofloxacin, AGs could be an option for the treatment of infections caused by MDR gram-negative pathogens in Korean hospitals.
The increasing antimicrobial resistance may partially explain the increasing consumption of broad-spectrum antibiotics and antibiotics against MDR pathogens. The result of the correlation between antibiotic consumption and antimicrobial resistance support this explanation. Increasing carbapenem consumption may have resulted from the increasing resistance of E coli to 3rd CEPs and ciprofloxacin, as well as increasing resistance of K pneumoniae to 3rd CEPs. In addition, an increasing proportion of oxacillin-resistant S aureus may have resulted in the increasing consumption of glycopeptide.
Antimicrobial resistance and antibiotic consumption influences each other. Antimicrobial resistance may influence the physician's antibiotic prescription, similar to how antibiotic consumption may increase selective pressure on certain classes of antibiotics in pathogens. Consistent with previous studies, the consumption of FQs may have caused the emergence of 3rd CEP-resistant E coli and K pneumoniae, as well as ciprofloxacin-resistant E coli and K pneumoniae.[20–22] Furthermore, the consumption of carbapenems may have increased resistance of A baumanii to imipenem.[22] However, we could not determine the protective effect of BL/BLIs against the emergence of 3rd CEPs-resistant E coli, as demonstrated in an Italian study.[23] Moreover, in contrast to a previous study, negative correlation between the consumption of FQs and ciprofloxacin-resistant P aeruginosa was demonstrated in our study.[22] The correlation between antibiotic prescription and the resistance rate in gram-negative bacteria has been reported as a non-uniform relationship in different studies.[20] We suggest that further studies are needed to investigate this in detail.
Our study has some limitations. First, the present study was conducted in university hospitals, and many of the patients had underlying illnesses. Therefore, the antimicrobial resistance rate and antibiotic consumption could be overestimated. Second, we analyzed the correlation between antibiotic consumption and antimicrobial resistance regardless of other factors. Even if statistically significant, the consumption of certain antibiotic drugs may not be directly correlated with antimicrobial resistance. Third, antibiotic susceptibility test was not conducted in a single center and the antibiotics tested for susceptibility varied among institutions. Therefore, we only could analyze resistance to a limited number of antibiotics. Finally, antibiotic consumption was measured by DDD instead of days of therapy (DOT). Although DDD has disadvantages in pediatrics and patients with chronic kidney disease,[24] we could not utilize DOT because only the total amount of antibiotic consumption for each identification number was available in most hospitals.
5. Conclusions
Nonetheless, the overall data in the present study may be a reasonable indicator of antibiotic usage and resistance pattern among hospitalized patients in Korea. In conclusion, overall antimicrobial resistance increased and consumption of broad-spectrum antibiotics and antibiotics against MDR pathogens subsequently increased in Korean hospitals. Proper ASPs in each hospital are mandatory to overcome the potential threats by MDR pathogens.
Author contributions
Conceptualization: Bongyoung Kim, Yeonjae Kim, Hyunjoo Pai.
Data curation: Bongyoung Kim, Yeonjae Kim, Hyeonjun Hwang.
Formal analysis: Bongyoung Kim, Hyeonjun Hwang.
Funding acquisition: Hyunjoo Pai.
Investigation: Bongyoung Kim, Yeonjae Kim, Hyeonjun Hwang, Jieun Kim, Shin-Woo Kim, In-Gyu Bae, Won Suk Choi, Sook In Jung, Hye Won Jeong.
Methodology: Bongyoung Kim, Hyeonjun Hwang.
Project administration: Bongyoung Kim.
Resources: Bongyoung Kim, Yeonjae Kim, Hyeonjun Hwang, Jieun Kim, Shin-Woo Kim, In-Gyu Bae, Won Suk Choi, Sook In Jung, Hye Won Jeong.
Software: Bongyoung Kim, Hyeonjun Hwang.
Supervision: Hyunjoo Pai.
Validation: Bongyoung Kim, Hyunjoo Pai.
Visualization: Bongyoung Kim.
Writing – original draft: Bongyoung Kim.
Writing – review & editing: Bongyoung Kim, Hyunjoo Pai.
Supplementary Material
Footnotes
Abbreviations: 1st CEPs = first-generation cephalosporins, 2nd CEPs = second-generation cephalosporins, 3rd CEPs = third-generation cephalosporins, 4th CEPs = fourth generation cephalosporins, AGs = aminoglycosides, ASP = antimicrobial stewardship program, BL/BLIs = beta-lactam/beta-lactamase inhibitors, BMTU = bone marrow transplant unit, DDD = defined daily dose, DOT = days of therapy, FQs = fluoroquinolones, ICU = intensive care unit, KARMS = Korean Antimicrobial Resistance Monitoring System, MDR = multidrug-resistant, NICU = neonatal intensive care unit, SXT = trimethoprim/sulfmonamide, WHO = World Health Organization.
The abstract was presented at the poster session of 60th Annual Meeting of the Korean Society for Chemotherapy, 2018, Seoul, Korea
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Nationwide surveillance system of multidrug-resistant pathogens for prevention and control of antimicrobial resistance in Korea (HI12C0756), Ministry of Health & Welfare, Republic of Korea.
Conflicts of interest: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
References
- [1].British Medical Journal (BMJ). Medical milestones: celebrating key advances since 1840. Available at: https://www.bmj.com/content/suppl/2007/01/18/334.suppl_1.DC2/milestones.pdf/ Accessed December 12, 2018 [Google Scholar]
- [2].Spellberg B, Dr. William H. Stewart: mistaken or maligned. Clin Infect Dis 2008;47:294. [DOI] [PubMed] [Google Scholar]
- [3].Fishman N, Patterson J, Saiman L, et al. Policy Statement on Antimicrobial Stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012;33:322–7. [DOI] [PubMed] [Google Scholar]
- [4].Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 2012;27:128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis 2008;47suppl 1:S3–13. [DOI] [PubMed] [Google Scholar]
- [6].Goossens H. Antibiotic consumption and link to resistance. Clin Microbiol Infect 2009;15suppl 3:12–5. [DOI] [PubMed] [Google Scholar]
- [7].Kim B, Kim J, Kim SW, et al. A survey of antimicrobial stewardship programs in Korea, 2015. J Korean Med Sci 2016;31:1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].World Health Organization (WHO). World Health Day 2011. Combat drug resistance: no action today means no cure tomorrow. Available at: http://www.who.int/dg/speeches/2011/WHD_20110407/en/ Accessed October 19, 2016. [Google Scholar]
- [9].Ryu S. The new Korean action plan for containment of antimicrobial resistance. J Glob Antimicrob Resist 2017;8:70–3. [DOI] [PubMed] [Google Scholar]
- [10].The Centers for Disease Control and Prevention (CDC). The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/getsmart/healthcare/pdfs/core-elements.pdf/ Accessed May 30, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].World Health Organization (WHO). Definition and general considerations of Defined Daily Dose (DDD). Available at: http://www.whocc.no/ddd/definition_and_general_considera/ Accessed October 28, 2016. [Google Scholar]
- [12].Performance standards for antimicrobial susceptibility testing; 15th informational supplement. CLSI document M100-S15. Wayne: Clinical and Laboratory Standards Institute (CLSI)I; 2007. [Google Scholar]
- [13].Bitterman R, Hussein K, Leibovici L, et al. Systematic review of antibiotic consumption in acute care hospitals. Clin Microbiol Infect 2016;22:561.e7–19. [DOI] [PubMed] [Google Scholar]
- [14].Yoon YK, Park GC, An H, et al. Trends of antibiotic consumption in Korea according to national reimbursement data (2008-2012): a population-based epidemiologic study. Medicine 2015;94:e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Song YJ, Kim M, Huh S, et al. Impact of an antimicrobial stewardship program on unnecessary double anaerobic coverage prescription. Infect Chemother 2015;47:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomized controlled trial. Lancet 2016;387:1743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wilf-Miron R, Ron N, Ishai S, et al. Reducing the volume of antibiotic prescriptions: a peer group intervention among physicians serving a community with special ethic characteristics. J Manag Care Pharm 2012;18:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim D, Ahn JY, Lee CH, et al. Increasing resistance to extended-spectrum cephalosporins, fluoroquinolone, and carbapenem in gram-negative bacilli and the emergence of carbapenem non-susceptibility in Klebsiella pneumoniae: analysis of Korean Antimicrobial Resistance Monitoring System (KARMS) data from 2013 to 2015. Ann Lab Med 2017;37:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Choi JY, Kwak YG, Yoo H, et al. Trends in the distribution and antimicrobial susceptibility of causative pathogens of device-associated infection in Korean intensive care units from 2006 to 2013: results from the Korean Nosocomial Infections Surveillance System (KONIS). J Hosp Infect 2016;92:363–71. [DOI] [PubMed] [Google Scholar]
- [20].Lai CC, Wang CY, Chu CC, et al. Correlation between antibiotic consumption and resistance of gram-negative bacterial causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J Antimicrob Chemother 2011;66:1374–82. [DOI] [PubMed] [Google Scholar]
- [21].Jacoby TS, Kuchenbecker RS, Dos Santos RP, et al. Impact of hospital-wide infection rate, invasive procedures use and antimicrobial consumption on bacterial resistance inside an intensive care unit. J Hosp Infect 2010;75:23–7. [DOI] [PubMed] [Google Scholar]
- [22].Hsu LY, Tan TY, Tam VH, et al. Surveillance and correlation of antibiotic prescription and resistance of gram-negative bacteria in Singaporean hospitals. Antimicrob Agents Chemother 2010;54:1173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mascarello M, Simonetti O, Knezevich A, et al. Correlation between antibiotic consumption and resistance of bloodstream bacteria in a university hospital in North Eastern Italy, 2008-2014. Infection 2017;45:459–67. [DOI] [PubMed] [Google Scholar]
- [24].Monnet DL. Measuring antimicrobial use: the way forward. Clin Infect Dis 2007;44:671–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


