Abstract
Objectives:
The present meta-analysis aimed to evaluate the short- and long-term outcomes of laparoscopic surgery (LS) versus open surgery (OS) for rectal cancer.
Methods:
PubMed, Web of Science, and Cochrane Library, were searched for eligible randomized controlled trials (RCTs) published up to June 2017. Operation related index, postoperative complication, and long-term survival rate and disease-free survival rate were evaluated by meta-analytical techniques.
Result:
Nine RCTs enrolling 4126 patients were included in the present meta-analysis. Compared to OS, LS had similar positive circumferential resection margin (CRM) and number of lymph nodes extracted (LNE) as well as long term 5 years survival rate and disease-free survival rate, but of which the risk tendency was higher in LS group. The short-term outcomes of major and total postoperative complication were lower in LS group.
Conclusions:
LS for rectal cancer was as safe and effective as OS in terms of long-term outcomes, but with lower postoperative complication.
Keywords: laparoscopic surgery, open surgery, randomized controlled trials, rectal cancer, systematic review
1. Introduction
Laparoscopic resection has been introduced to treat rectal cancer for decades.[1] Although some studies have demonstrated improved outcomes after laparoscopic-assisted resection of rectal cancer,[2–4] it is still controversial because of long skill learning, technical challenges related to the anatomical position of the rectum in the pelvis and lack of high quality published data regarding postoperative complications, oncologic safety, and long-term survival.
Recently, several randomized controlled research reported that laparoscopic resection for rectal show less blood loss, quicker recovery, shorter hospital stay, less complications, and a better quality of life compare with open surgery.[5–7] Meta-analysis can be used to evaluate the existing literature in a quantitative way by comparing and combining the results of different studies considering variations between studies.[4,8,9] In the present paper, we use meta-analytical techniques to compare the short- and long-term outcomes of LS and OS for rectal cancer from RCTs.
2. Material and methods
2.1. Study selection
A Web of Science, PubMed, and Cochrane library database search was performed on all studies between January 1986 and June 2017 to compare laparoscopic and open surgery for rectal cancer. We utilized the search terms in “PICOS” principle included the following: “colon neoplasm,” “rectum neoplasm,” “colonic,” “rectal,” “open surgery,” “laparoscopic surgery,” “laparoscopic operation,” “open operation,” “outcomes,” “complication,” “transfer,” “randomized,” “controlled,” and “randomly”. We used both free text and MeSH as searches keywords. The “related articles” function was used to broaden the search, and all abstracts, studies, and citations scanned were reviewed. Only full-text papers in English and the results of LS and OS resection compared were considered. If data sets overlapped, only the most recent information was included. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
2.2. Data extraction
Data were independently extracted by 2 investigators reviewed the titles, abstracts, and full texts of retrieved articles. Disagreements were resolved with a third reviewer. The following information was collected from each study: author, year, study design, characteristics of the study population, operation time, estimated blood loss, length of hospital stay, number of lymph nodes extracted, distance to distal circumferential resection margin, postoperative (1year) mortality, first time intake of solids, long-term mortality(5 year), long-term disease-free survival rate (5 year), etc.
2.3. Statistical analysis
RevMan version 5.3 and Stata version 12.0 were used to conduct the quality assessment and meta-analysis. Statistical analysis for categorical variables was performed by using the risk ratio (RR) or hazard ratio (HR) with 95% confidence interval (95% CI) and continuous variables were analyzed with standardized mean difference (SWD). If only the median, range, and size of the trial were reported in the literature, the means and standard deviations were calculated as described by Wan et al.[10] According to the Higgins’ I2 statistic, heterogeneity <25%, 25% to 50%, and ≥50% were defined as low, moderate and high, respectively.[11] Subgroup analyses would be taken if the synthesis results with high heterogeneity for the individual variation existing between the inclusion studies. A fixed-effect model was used for studies with low or moderate statistical heterogeneity, and a random-effect model was used for studies with high statistical heterogeneity. The value of P<.05 was considered statistically significant. Sources of heterogeneity were explored using sensitivity and subgroup analyses. Publication bias was quantitatively evaluated using funnel plots.
3. Results
3.1. Studies selected
The process of studies selected shown in the flow diagram, in brief, a total of 1026 studies were identified by the search strategy on line.
3.2. Study characteristics and qualities
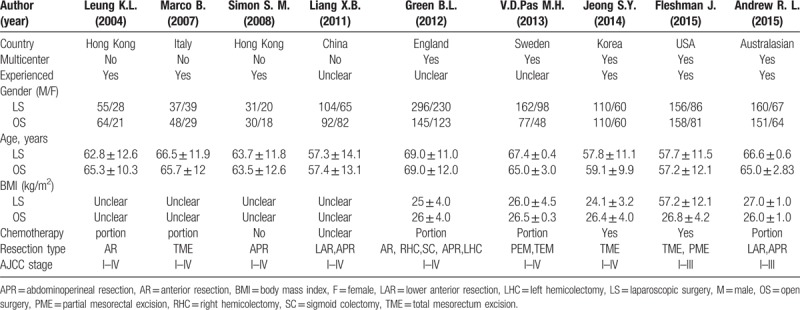
A total of 9RCTs studies based on comparing the results of laparoscopic versus open resection for rectal cancer were eligible for the meta-analysis. Four thousand twenty 6 rectal cancer patients were included, of these, 2379 patients underwent LS and 1747 patients underwent OS. As Table 1 shown, 5 of the studies were randomized multicenter clinical studies and long-term follow-up were conducted in all studies.
Table 1.
Characteristics of the studies included in meta-analysis.
Methodological quality of included trials was assessed using the Cochrane Collaboration's tool for evaluating risk of bias. In these studies, blinding techniques were hardly feasible because of the different treatment procedures and the associated adverse effects. However, the 2 reviewers judge that the outcome is not likely to be influenced by lack of blinding. All of the studies had a moderate risk of bias (Fig. 1A and B).
Figure 1.
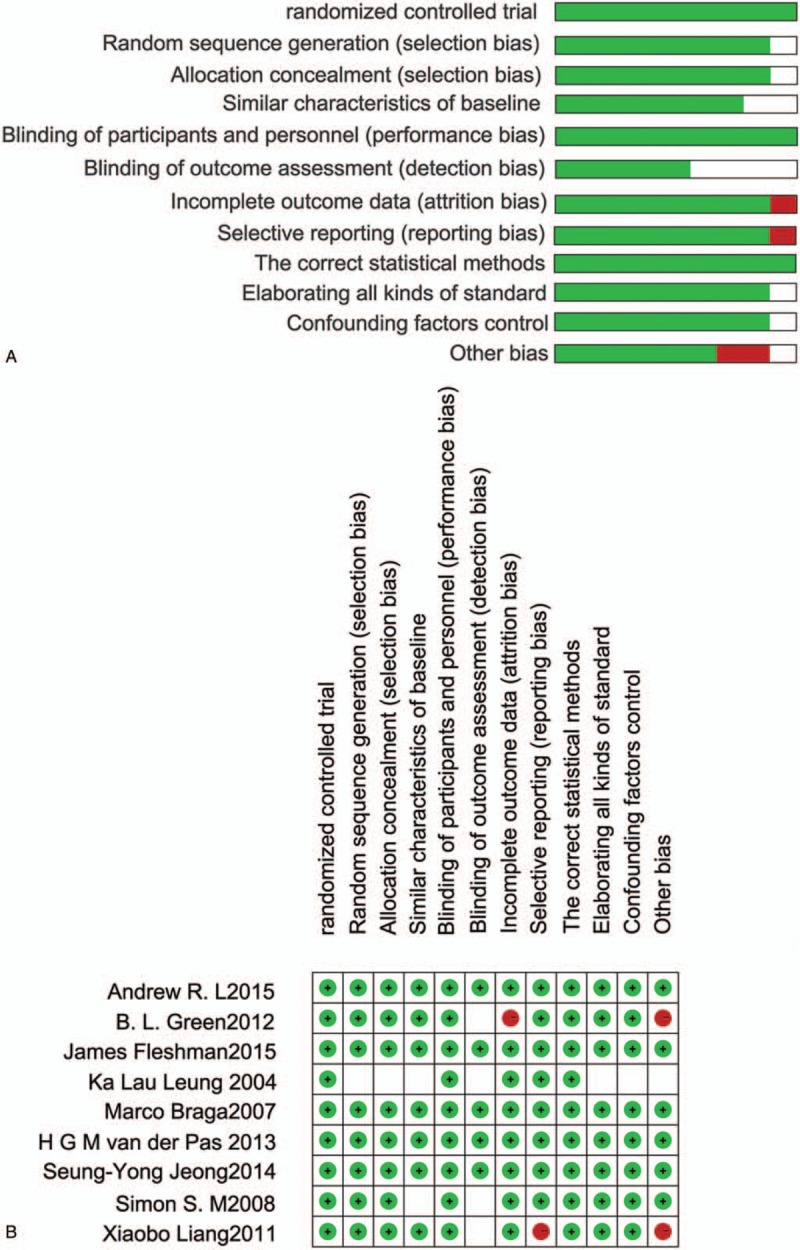
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies. (A) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study (B).
3.3. Short-term outcomes of laparoscopic surgery versus open surgery
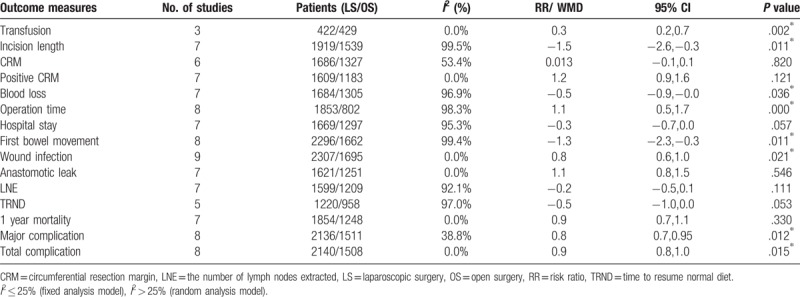
The meta-analysis results for short-term outcomes indicated no significance between laparoscopic surgery and open surgery as Table 2 shown, except for transfusion [RR = 0.344, 95%CI (0.175, 0.675), I2 = 0, P = .002], incision length [SWD = −1.487, 95%CI (−2.639, −0.334), I2 = 99.5%, P = .011], blood loss [SWD = −0.475, 95%CI (−0.918, −0.032), I2 = 96.9%, P = .036], operation time [SWD = 1.099, 95%CI (0.517, 1.682), I2 = 98.3%, P = .000], wound infection [RR = 0.762, 95%CI (0.605, 0.960), I2 = 0, P = .021], first bowel movement [SWD = −1.278, 95%CI (−2.257, −0.299), I2 = 99.4%, P = .011], major complication[effect size (ES) = 0.794, 95%CI (0.663, 0.950), I2 = 38.8%, P = .012] and total complication [ES = 0.884, 95%CI (0.800, 0.977), I2 = 0%, P = .015].
Table 2.
Results of meta-analysis of short outcomes and individual postoperative complications.
3.4. Long term outcomes of laparoscopic surgery versus open surgery
Six studies analysed 5 year survival rate between the 2 groups, which involved 1715 patients (986 laparoscopic group and 729 open group), and it revealed no significantly difference [Hazard ratio (HR) = 0.915, 95%CI (0.816, 1.025), I2 = 0, P = .124, Fig. 2A; RR = 0.998, 95%CI (0.928,1.037), I2 = 0, P = .947]. A total of 1538 patients (878 laparoscopic group and 660 open group in 5 studies) were included in the analyses of 5 year disease-free survival, and this difference was also not significant [HR = 0.909, 95%CI (0.807, 1.023), I2 = 0, P = .114, Fig. 2B; RR = 0.963, 95%CI (0.896, 1.036), I2 = 0, P = .314].
Figure 2.
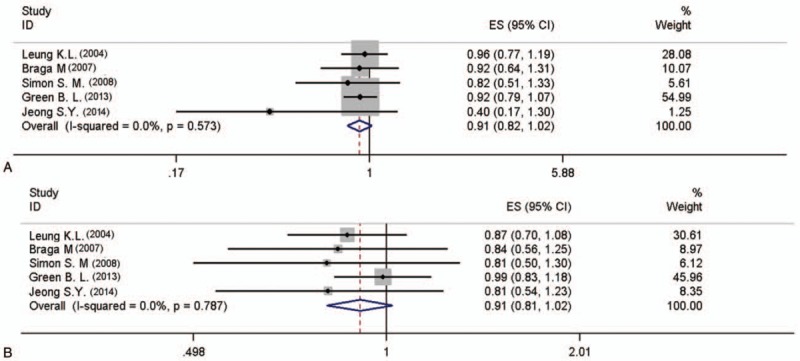
Meta-analysis of the pooled data: 5 year survival rate (A) 5 year disease-free survival (B).
3.5. Subgroup analysis
As indicated in multicenter subgroup analysis, the result of first bowel movement [3 studies in singer central, SWD = −0.324, 95%CI (−0.460, −0.189), I2 = 0, P = .000; 4 studies in multicenter, SWD = −1.858, 95%CI (−3.289, −0.427), I2 = 99.6%, P = .011, Fig. 3A] were significantly lower for LS group, and the number of lymph nodes extracted [4 studies in singer central, SWD = −0.109, 95%CI (−0.232, −0.014), I2 = 0, P = .083; 3 studies in multicenter, SWD = −0.436, 95%CI (−0.880, −0.008), I2 = 94.7%, P = 0.054, Fig. 3B] has no significance between these 2 groups.
Figure 3.
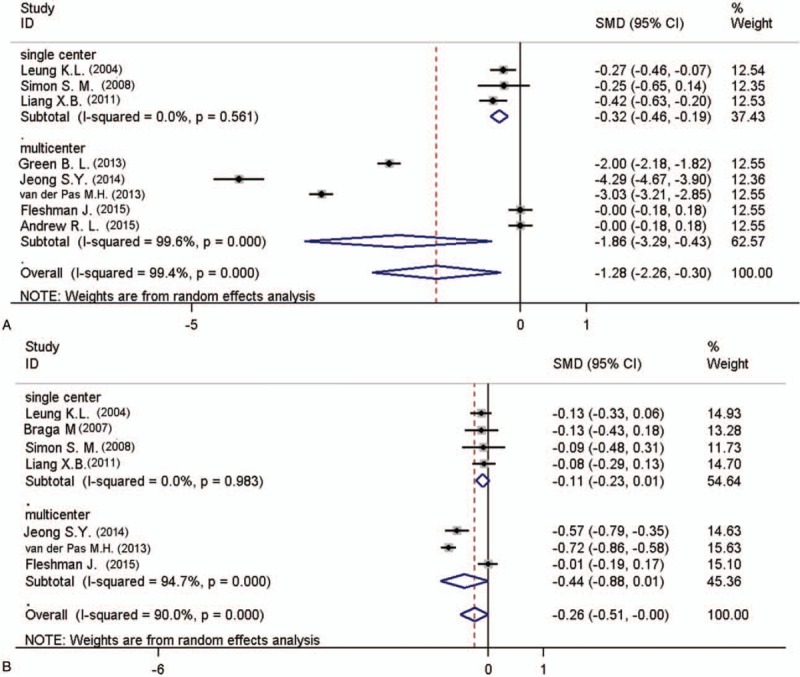
Subgroup analysis of the pooled data: first bowel movement (A) number of lymph nodes extracted (B).
In the experienced doctor surgery subgroup where blood loss [6 studies, SWD = −0.288, 95%CI (−0.433, −0.143), I2 = 58.3%, P = .000, Fig. 4A], the incision length [3 studies, SWD = 0.021, 95%CI (−0.102, 0.142), I2 = 0.0%, P = 0.324, Fig. 4B] and the rate of major complication [ES = 0.805, 95%CI (0.670, 0.967), I2 = 0%, P = .021, Fig. 4C] were significantly lower in LS group with low heterogeneity.
Figure 4.
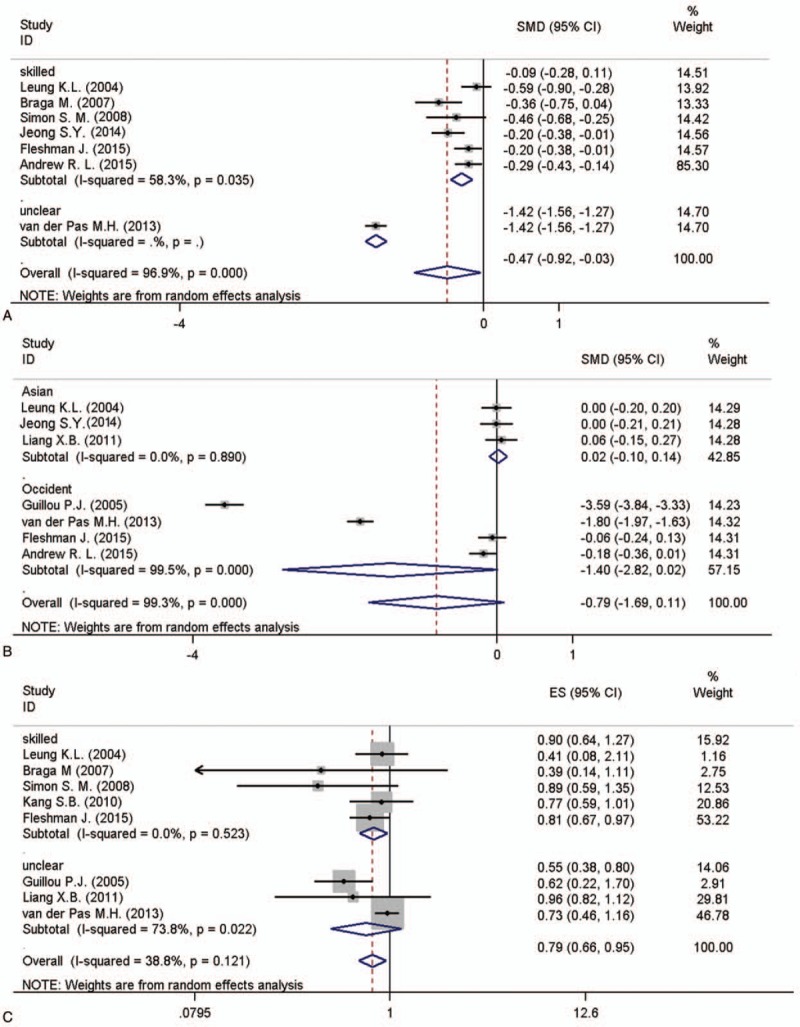
Subgroup analysis of the pooled data: blood loss (A) incision length (B) major complication (C).
3.6. Sensitivity analysis and publication bias
Sensitivity analysis was performed by trim and fill method, of which results are similar and steady for long-term outcomes and complication between pre-trim and post-trim (Fig. 5). Funnel plot analysis (Fig. 5) of the studies was performed in the meta-analysis for detecting the publication bias of the main outcomes such as 5 year mortality (Harbord test, P = .656), 5 year disease-free survival (Harbord test, P = .747), total complication (Harbord test, P = .656) and major complication (Harbord test, P = .052), None of the studies were outside of the limits of the 95%CI, and there was no evidence of publication bias or heterogeneity among the studies.
Figure 5.
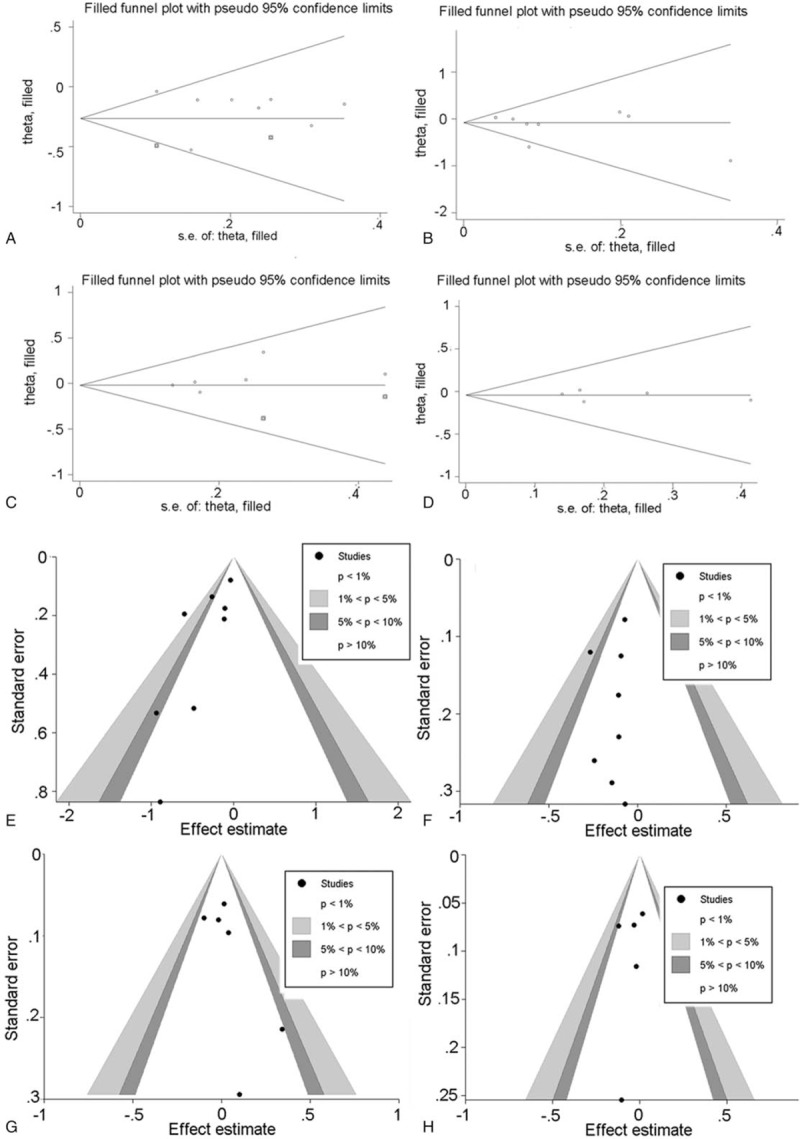
Post-trim plots of the synthetic results: major complication (A) total complication (B) 5 year survival rate (C) 5 year disease-free survival (D) funnel plots of the synthetic results: major complication (E) total complication (F) 5 year survival rate (G) 5 year-disease-free survival (H).
4. Discussion
A total of 4126 patients from 9 RCTs considered low risk of bias were analyzed in the present meta-analysis (LS 2379 vs OS 1747). In this analysis, long-term outcome shown no statistical significant differences and similar with previous reports[6,7,12,13] between the 2 groups regarding 5 year survival rate and disease-free survival,[3,14] but of which the risk trend were higher in LS group. The short term outcome of major complication and total complication were lower in LS group compared with OS group.[15] It is confident that the original data of the synthetic results come from high quality RCTs. The selection and publication biases have not been detected by bias risk analysis and sensitivity analysis shown a stable profile for these long-term outcome.
Amount studies reported optimal operation information in LS versus OS. A faster postoperation recovery would well compensate the relatively long operating time (Table 2) for the laparoscopic approach for rectal cancer as most of other studies related.[6,16,17] For considering the high heterogeneity (I2 = 98.5%), we applied a subgroup analysis of random-effects model to take into consideration the study variation, of which result shown different area may be as one important reason for the differences of the results from different studies (Asian, I2 = 7.2%; occidental, I2 = 99.2%).[18,19] The blood loss was significant lower in LS group with moderate heterogeneity taking surgical experience as grouping factors (surgical experience group, I2 = 58.3%), which indicates that ultrasonic scalpel and magnification instruments applied by highly skilled doctors would enable better identification and dissection of the vessels and significantly reduce operation bleeding.[20,21]
The positive CRM and retrieved lymph node numbers are related to local recurrence and long term survival.[22–24] In this analysis, we did not find significant differences between LS and OS at the result (Table 2) of positive CRM (RR = 1.2, P = .121; I2 = 0.0%,) and lymph node numbers (WMD = −0.2, P = .111; I2 = 92.1.0%) as James Fleshman and Andrew reported indicated that the resection outcomes of LS were comparable to that of OS.[7,25] We attributed the high heterogeneity in the synthetic results of lymph node numbers to different operation criteria from hospitals because of lower heterogeneity detected in single center subgroup (I2 = 0.0%, P = .083).[4,26]
Postoperative complication rate is a crucial index for evaluating the quality of operation. In the present analysis, no significant differences were detected for anastomotic leak[27,28] and 1 year mortality[3,29,30] (Table 2) between LS and OS groups as other studies concerns, but the rates of major postoperative complication and total postoperative complication were lower in LS group implied that LS treatment reduced postoperative complication compared to OS for rectal cancer. In the subgroup analysis, we identified skilled operation team was the main connections for the occurrence of major postoperative complication (skilled operation team, I2 = 0%; unclear team, I2 = 38.8%).[31]
In summary, our study indicated that laparoscopic surgery for rectal cancer was as safe and effective as open surgery in terms of long-term outcomes. Moreover, laparoscopic surgery reduced hospital stay, wound infection, total and major post- operative complication.
This meta-analysis has several limitations that should be considered regarding interpretation. Firstly, the small number of included RCTs may limit the statistical power. Although it is ideal for a meta-analysis to include RCTs only, the inclusion of high-quality non-RCT can improve the statistical power while maintaining an acceptable level of evidence. Secondly, high heterogeneity exists in several synthetic results (Table 2), therefore more details relating the bias between different studies, such as the race of the patients, the operation team, randomization, blinding and the oncologic results, should be recorded. Furthermore, improved standardized judgment method and reporting form of functional outcomes should be considered in the future RCTs.
Author contributions
Contribution of authors: All of the authors participated in the design, interpretation of the studies, analysis of the data and review of the manuscript; Conception and design: Zhong Lin, Qiang- Qiang Ge; Search literature: Zhong Lin, Qiang-Qiang Ge, Zheng-Li Jiang, Dan-Yang Chen; Collection and assembly of data: Zhong Lin, Qiang-Qiang Ge, Zheng-Li Jiang, Min-Fang Chen, Li-Hua Chen; Data analysis and interpretation: Peng-Zhou, Ai-Xiao Xia, Yan-Wu Zhu; Manuscript writing: Zhong Lin, Qiang-Qiang Ge, Zheng-Li Jiang; Final approval of manuscript: All authors.
Conceptualization: Zhong Lin, Zheng Li Jiang, Qiang Qiang Ge.
Data curation: Zhong Lin, Zheng Li Jiang, Dan Yang Chen, Min Fang Chen, Li Hua Chen, Peng Zhou, Ai Xiao Xia, Yan Wu Zhu, Hui Jin, Qiang Qiang Ge.
Formal analysis: Zhong Lin, Zheng Li Jiang, Dan Yang Chen, Min Fang Chen, Li Hua Chen, Peng Zhou, Ai Xiao Xia, Yan Wu Zhu, Hui Jin, Qiang Qiang Ge.
Funding acquisition: Zhong Lin, Zheng Li Jiang, Qiang Qiang Ge.
Investigation: Zhong Lin, Zheng Li Jiang, Qiang Qiang Ge.
Methodology: Zhong Lin, Zheng Li Jiang, Qiang Qiang Ge.
Project administration: Zhong Lin, Qiang Qiang Ge.
Software: Zhong Lin, Zheng Li Jiang, Dan Yang Chen, Min Fang Chen, Li Hua Chen, Peng Zhou, Ai Xiao Xia, Yan Wu Zhu, Hui Jin.
Supervision: Zhong Lin.
Validation: Zhong Lin, Qiang Qiang Ge.
Writing – original draft: Zhong Lin, Zheng Li Jiang, Qiang Qiang Ge.
Writing – review & editing: Zhong Lin, Zheng Li Jiang, Dan Yang Chen, Min Fang Chen, Li Hua Chen, Peng Zhou, Ai Xiao Xia, Yan Wu Zhu, Hui Jin, Qiang Qiang Ge.
Footnotes
Abbreviations: 95% CI = confidence interval, APR = abdominoperineal resection, AR = anterior resection, BMI = body mass index, CRM = circumferential resection margin, ES = effect size, F = female, HR = hazard ratio, LAR = lower anterior resection, LHC = left hemicolectomy, LNE = lymph nodes extracted, LS = laparoscopic surgery, M = male, OS = open surgery, PICOS = population, intervention, compare, outcomes, study, PME = partial mesorectal excision, RCTs = randomized controlled trials, RHC = right hemicolectomy, RR = risk ratio, SC = Sigmoid colectomy, SWD = standardized mean difference, TME = total mesorectum excision.
ZL and Z-LJ contribute equally to this study.
This study was supported by research grant No. 14SF03 from Taizhou, Health Department of Scientific Research Funds in Zhejiang Province (2015KYB436).
The authors have no conflicts of interest to disclose.
References
- [1].Fernandez R, Anaya DA, Li LT, et al. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg 2013;206:509–17. [DOI] [PubMed] [Google Scholar]
- [2].Feng B, Zhu QL, Xia Y, et al. Direct and indirect costs and long-term survival of laparoscopic anterior resection for rectal cancer. Med Sci Monit 2010;16:PH97–102. [PubMed] [Google Scholar]
- [3].Gong J, Shi DB, Li XX, et al. Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol 2012;18:7308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arezzo A, Passera R, Scozzari G, et al. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc 2013;27:1485–502. [DOI] [PubMed] [Google Scholar]
- [5].Van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–8. [DOI] [PubMed] [Google Scholar]
- [6].Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767–74. [DOI] [PubMed] [Google Scholar]
- [7].Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA Surg 2015;314:1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xiong B, Ma L, Zhang C, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res 2014;188:404–14. [DOI] [PubMed] [Google Scholar]
- [9].Cirocchi R, Trastulli S, Farinella E, et al. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a RCT is needed. Surg Oncol 2012;21:e111–23. [DOI] [PubMed] [Google Scholar]
- [10].Wan X, Liu WWQ, Tong JMTJ. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC 2014;14:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen H, Zhao L, An S, et al. Laparoscopic versus open surgery following neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and meta-analysis. J Gastrointest Surg 2014;18:617–26. [DOI] [PubMed] [Google Scholar]
- [12].Zhao D, Li Y, Wang S, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis of 3-year follow-up outcomes. Int J Colorectal Dis 2016;31:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ng SS, Leung KL, Lee JF, et al. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum 2009;52:558–66. [DOI] [PubMed] [Google Scholar]
- [14].Kellokumpu IH, Kairaluoma MI, Nuorva KP, et al. Short- and long-term outcome following laparoscopic versus open resection for carcinoma of the rectum in the multimodal setting. Dis Colon Rectum 2012;55:854–63. [DOI] [PubMed] [Google Scholar]
- [15].Greenblatt DY, Rajamanickam V, Pugely AJ, et al. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg 2011;212:844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Martinez-Perez A, Carra MC, Brunetti F, et al. Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: a systematic review and meta-analysis. JAMA Surg 2017;152:e165665. [DOI] [PubMed] [Google Scholar]
- [17].Braga M, Frasson M, Vignali A, et al. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum 2007;50:464–71. [DOI] [PubMed] [Google Scholar]
- [18].Huang MJ, Liang JL, Wang H, et al. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis 2011;26:415–21. [DOI] [PubMed] [Google Scholar]
- [19].Gong T, Zhou X, Dou HQ, et al. A meta-analysis of clinical outcomes after laparoscopic operation for rectal cancer. Chin J Gastrointest Surg 2010;13:831–5. [PubMed] [Google Scholar]
- [20].Zeng W-g, Zhou Z-x, Hou H-r, et al. Outcome of laparoscopic versus open resection for rectal cancer in elderly patients. J Surg Res 2015;193:613–8. [DOI] [PubMed] [Google Scholar]
- [21].Simorov A, Reynoso JF, Dolghi O, et al. Comparison of perioperative outcomes in patients undergoing laparoscopic versus open abdominoperineal resection. Am J Surg 2011;202:666–72. [DOI] [PubMed] [Google Scholar]
- [22].Ghezzi TL, Luca F, Valvo M, et al. Robotic versus open total mesorectal excision for rectal cancer: comparative study of short and long-term outcomes. Eur J Surg Oncol 2014;40:1072–9. [DOI] [PubMed] [Google Scholar]
- [23].Lezoche E, Guerrieri M, De Sanctis A, et al. Long-term results of laparoscopic versus open colorectal resections for cancer in 235 patients with a minimum follow-up of 5 years. Surg Endosc 2006;20:546–53. [DOI] [PubMed] [Google Scholar]
- [24].Kusters M, Marijnen CA, van de Velde CJ, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 2010;36:470–6. [DOI] [PubMed] [Google Scholar]
- [25].Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT Randomized CIinical Trial. JAMA Surg 2015;314:1356–63. [DOI] [PubMed] [Google Scholar]
- [26].Athanasiou C, Lockwood S, Markides GA. Systematic review and meta-analysis of laparoscopic versus open appendicectomy in adults with complicated appendicitis: an update of the literature. World J Surg 2017;41:3083–99. [DOI] [PubMed] [Google Scholar]
- [27].Ivanov P, Vasilev K, Kotashev G, et al. Laparoscopic vs open resection for rectal carcinoma--a prospective analysis. Khirurgiia 2013;1:23–9. [PubMed] [Google Scholar]
- [28].Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Brit J Surg 2013;100:75–82. [DOI] [PubMed] [Google Scholar]
- [29].Ng SS, Leung KL, Lee JF, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol 2008;15:2418–25. [DOI] [PubMed] [Google Scholar]
- [30].Liang X, Hou S, Liu H, et al. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A 2011;21:381–5. [DOI] [PubMed] [Google Scholar]
- [31].Pecorelli N, Greco M, Amodeo S, et al. Small bowel obstruction and incisional hernia after laparoscopic and open colorectal surgery: a meta-analysis of comparative trials. Surg Endosc 2017;31:85–99. [DOI] [PubMed] [Google Scholar]


