Abstract
The endometrium tissue is functionally androgen related which plays an important role in women's fertility regulation. In addition recent findings show that endometrium related pathology is closely linked to disrupted androgen biosynthesis and associated regulatory functions. These findings also suggest that androgens might play an important role in endometrium related cancer pathology with significant implications for treatment.
Based on these findings, we have assessed 50 female outpatients with endometriosis and the clinical investigations were focused on biochemical serum analysis of DHEAS, oncological markers CA-125 and CA 19-9, estradiol, thyreothropic hormone, and prolactin.
The results show significant Spearman correlations of CA-125 and CA 19-9 with dehydroepiandrosterone- DHEA-S (R = 0.52 resp. R = 0.49).
This result represents 1st reported finding documenting androgen related increase of CA-125 and CA 19-9 levels as significant markers of endometrium pathology and it is possible to assume that these potential biomarkers could have clinical importance with respect to timely diagnosis.
Keywords: CA 19-9, CA-125, dehydroepiandrosterone sulfate, endometriosis
1. Introduction
The endometrium is the inner epithelial tissue which is functionally androgen related and plays an important role in regulation of women's fertility and menstrual cycle.[1,2] The endometrial tissue creates the uterine lining and its pathology may lead to endometriosis when the tissue is present in other parts of the body mainly within the peritoneal cavity at lower abdomen or pelvis.[1,2] Recent findings suggest that androgens might play an important role in endometrium related pathology which is closely linked to disrupted androgen biosynthesis and associated regulatory functions.[2] These findings also indicate that androgens may play a role in hormone-dependent cancer pathology and these studies suggest a link between risk of endometrial cancer and androgen functions.[3,4,5,2] There are some controversial findings suggesting that dehydroepiandrosterone sulfate (DHEA-S) is associated[6] or not associated[7] with increased risk of the endometrial cancer. Recent data indicate that CA-125 and CA 19-9 molecules represent important markers of endometrial and cancer pathology.[8,9] Nevertheless according to the recent literature there is no evidence about relationships of DHEA-S with CA-125 and CA 19-9 as indicators of endometrial and cancer pathology. With respect to these findings we have tested this hypothesis and assessed 50 female outpatients with endometriosis and the clinical investigations were focused on biochemical serum analysis of DHEA-S, oncological markers CA-125 and CA 19-9, estradiol, thyreothropic hormone, and prolactin.
2. Methods
To test the above hypothesis, we have assessed 50 female outpatients mean age (32.78 ± 4.36), age range (26–44) with endometriosis who were treated at the Institute of Sexology of the Charles University Hospital in Prague. The diagnosis was confirmed by laparoscopic and histological investigations. All women included in this study had dyspareunia, pelvic pain, orgasm disorders, lubrication disorders, and irregular and painful bleeding. Most women had pains during the menstrual and non-menstrual stages; other reported symptoms were fatigue, sleep disturbances, painful sex, and partner relationship disturbances. In this study, 11% of patients had heightened Body Mass Index (BMI > 26), 5% had 1st menstruation at age 10 to 11, 33% had menstrual disorders including heavy menstrual and intermenstrual bleeding, 45% had menstrual painful symptoms. Of these 9% of patients manifested positive ultrasound changes.
Exclusion criteria were gravidity, oncological diseases, urological disorders, intestinal diseases metabolic disorders drug, and alcohol abuse including smoking. All the outpatients provided written informed consent and the study was approved by Charles University Hospital Ethical Committee and all methods were performed in accordance with the relevant guidelines and regulations.
The clinical investigations were focused on biochemical serum analysis of dehydroepiandrosterone sulfate (DHEA-S), oncological markers CA-125 and CA 19-9, estradiol, thyreothropic hormone, and prolactin. The DHEA-S is an androgen, a male sex hormone which is present in both men and women. It plays a role in the development of secondary male sexual signs in puberty and can be metabolized in the body to more potent androgens such as testosterone and androstendione or can be converted to female hormone estrogen. The DHEA-S is produced by the outer layer of adrenal cortex. To a lesser extent, it is also produced by female ovaries and male testicles. Values of hormone are high after birth and they are rapidly declining during childhood until the age of 30. The CA-125 is a glycoprotein with a high carbohydrate component and its molecular weight is at about 200 kDa. The CA-125 is produced in the fetal period by epithelial tissues and in adulthood it may occur in the normal epithelium of the fallopian tubes, cervix or bronchus. The CA-125 is particularly important as a marker of serosa membrane carcinomas and undifferentiated ovarian carcinomas and its serum concentrations may reflect tumor size.[9] The CA 19-9 is a pentasacharide with carbohydrate component containing fructose components and it belongs to a group of oncofetal antigens. In the fetal period, it is synthesized in the epithelial structures of the stomach and in adulthood its production is significantly decreased. In addition recent findings show that CA 19-9 may be produced in glandular structures of the gall bladder, pancreas, bronchus, and some gynecological tumors.[11] Some studies show that CA-19-9 may be demonstrably elevated in endometriosis and exhibit the same or decreased sensitivity as CA-125.[11]
In addition DHEA-S as an androgen hormone plays a very important role in the development of male gender but it is present in both men and women. The DHEA-S can be metabolized in the body to other androgens such as testosterone and androstendione or it can be transformed to female hormones estrogen. The DHEA-S is mainly produced by the zona reticularis of the adrenal cortex, partially produced male testicles and in pathophysiological conditions by female ovaries.[12]
3. Results
The results show significant Spearman correlations of CA-125 and CA-19-9 with DHEA-S (R = 0.52 resp. R = 0.49, Fig. 1). This result represents 1st reported finding documenting increased androgen levels as significant markers of endometrium pathology. Results of the Mann–Whitney test for the subgroups lower or higher than median DHEA-S are in agreement with these correlations (Z = -2.259, P = .024 for CA-125 and Z = -2.529, P = .011 for CA-19-9). In addition we have analyzed comparison of women who manifested ultrasound changes with other participants in the sample using Mann–Whitney test and this comparison do not show any significant differences in other assessed variables (P > .09, Z < 1.68).
Figure 1.
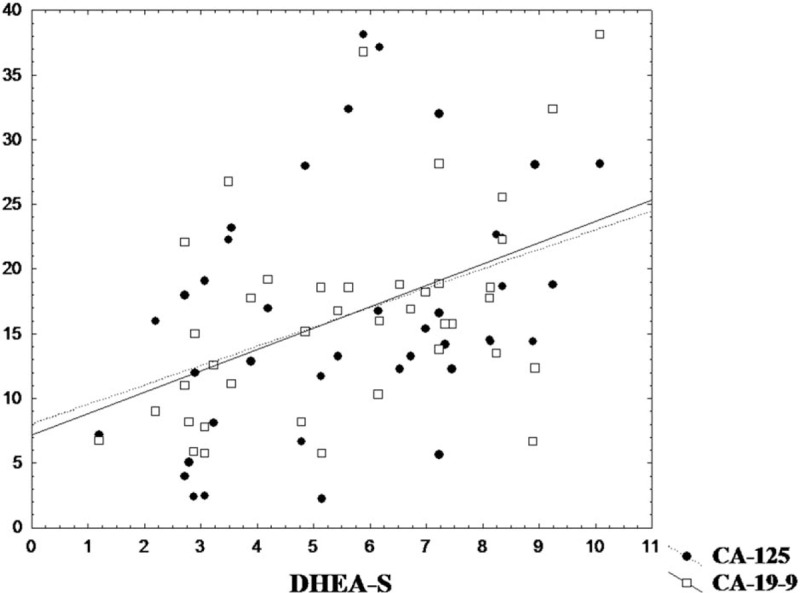
Dependence of DHEA-S with CA-125 and CA-19-9. DHEA-S = dehydroepiandrosterone sulfate.
4. Discussion
The results of this study are in agreement with the tested hypothesis focused on the relationship of DHEA-S with oncological markers CA-125 and CA 19-9. These results are in accordance with recent findings indicating that CA-125 and CA 19-9 molecules represent important markers of endometrial and cancer pathology.[8,9] According to current literature, there is no evidence of DHEA-S relationship with CA-125 and CA 19-9 as indicators of endometrial pathology and it is possible to assume that these potential biomarkers could have clinical importance with respect to timely diagnosis. The search for new biomarkers and validation of predicted biomarkers continues to be a priority of endometriosis research to shorten the time between diagnosis and treatment initiation. Mainly because diagnosis of endometriosis is generally delayed by 8 to 10 years due to misinterpretation of symptoms in juveniles and young women.[10] This research needs to be replicated in a larger group of patients which might represent a limitation of this study and interpretation of the results. Nevertheless the current results have only statistical limitations and further research including higher number of participants is warranted.
Acknowledgment
The authors thank to the closest collaborators of the Institute of Sexology and Department of Psychiatry, First Faculty of Medicine, Charles University, in Prague and Pilsen. This study was supported by Charles University project Progress and SVV.
Author contributions
Conceptualization: Ludek Fiala, Petr Bob.
Data curation: Ludek Fiala.
Formal analysis: Ludek Fiala, Petr Bob.
Funding acquisition: Jiri Raboch.
Investigation: Ludek Fiala, Petr Bob, Jiri Raboch.
Methodology: Ludek Fiala, Petr Bob.
Project administration: Ludek Fiala.
Writing – original draft: Ludek Fiala, Petr Bob.
Writing – review & editing: Jiri Raboch.
Footnotes
Abbreviations: BMI = body mass index, DHEA-S = dehydroepiandrosterone sulfate.
The authors report no conflicts of interest
References
- [1].Cloke B, Christian M. The role of androgens and the androgen receptor in cycling endometrium. Mol Cell Endocrinol 2012;358:166–75. [DOI] [PubMed] [Google Scholar]
- [2].Simitsidellis I, Saunders PT, Gibson DA. Androgens and endometrium: new insights and new targets. Mol Cell Endocrinol 2018;465:48–60. [DOI] [PubMed] [Google Scholar]
- [3].Gibson DA, et al. A role for steroid sulfatase in intracrine regulation of endometrial decidualisation. J Mol Endocrinol 2018;JME-18-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2014;20:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ito TE, Khalil EDA, Taffel M, et al. Magnetic resonance imaging correlation to intraoperative findings of deeply infiltrative endometriosis. Fertil Steril 2017;107:e11–2. [DOI] [PubMed] [Google Scholar]
- [6].Audet-Walsh E, Lepine J, Belangeret A, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocr Metab 2011;96:E330–9. [DOI] [PubMed] [Google Scholar]
- [7].Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr-Relat Cancer 2008;15:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Socolov R, Socolov D, Sindilar A, et al. An update on the biological markers ofendometriosis. Minerva Ginecol 2017;69:462–7. [DOI] [PubMed] [Google Scholar]
- [9].Hirsch M, Duffy JM, Deguara CS, et al. Diagnostic accuracy of Cancer Antigen 125 (CA125) for endometriosis in symptomatic women: a multi-center study. Eur J Obstet Gyn R B 2017;210:102–7. [DOI] [PubMed] [Google Scholar]
- [10].Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril 2017;107:523–32. [DOI] [PubMed] [Google Scholar]
- [11].Fassbender A, Burney RO, O DF, et al. Update on biomarkers for the detection of endometriosis. Biomed Res Int 2015;130854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rižner TL. The important roles of steroid sulfatase and sulfotransferases in gynecological diseases. Front Pharmacol 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]


