Abstract
Background:
In patients with hepatocellular carcinoma (HCC), the prognostic role of tumor-infiltrating lymphocytes (TILs) for survival is still controversial. A meta-analysis was performed to investigate the prognostic effect of TILs in HCC.
Methods:
We identify studies from PubMed, Embase, and the Cochrane Library to evaluate the prognostic value of TILs in patients with HCC. A meta-analysis was conducted to estimate overall survival and disease-free survival. The hazard ratio (HR) and 95% confidence interval (CI) were calculated employing fixed-effect or random-effect models depending on the heterogeneity of the included trials.
Results:
A total of 7905 patients from 46 observational studies were enrolled. For TILs subsets, the density of CD8+, FOXP3+, CD3+, and Granzyme B+ lymphocytes was significantly associated with improved survival (P < .05). The density of FOXP3+ TILs in intratumor (IT) was the most significant prognostic marker (pooled HR = 1.894; 95% CI = 1.659–2.164; P < .001). Patients with high infiltration of CD8+ TILs in IT (pooled HR = 0.676; 95% CI = 0.540–0.845; P = .001) or in margin of tumor (MT) (pooled HR = 0.577; 95% CI = 0.437–0.760; P < .001) had better OS. The pooled analysis revealed that high density of Granzyme B+ T-lymphocytes in IT was statistically significant associated with better OS (pooled HR = 0.621; 95% CI = 0.516–0.748; P < .001) and DFS (pooled HR = 0.678; 95% CI = 0.563–0.815; P < .001). It was interesting that high density of CD3+ in IT foreboded worse OS (pooled HR = 1.008; 95% CI = 1.000–1.015; P = .037), but better DFS (pooled HR = 0.596; 95% CI = 0.374–0.948; P = .029).
Conclusion:
Our findings suggested that some TIL subsets could serve as prognostic biomarkers in HCC. High-quality randomized controlled trials are needed to determine if these TILs could serve as targets for immunotherapy in HCC.
Keywords: CD8, Foxp3, hepatocellular carcinoma, prognosis, tumor-infiltrating lymphocytes
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common primary malignancies of the liver, representing the 3rd leading cause of cancer-related death worldwide. Besides early diagnosis, prompt treatment plans identified from the prediction of patients’ outcomes also contribute to successful treatment of liver cancer. Therefore, further investigation is needed to discovery precise tumor biomarkers with higher sensitivity and specificity in HCC to determine the optimal treatment programs and predict the prognosis of HCC.
Tumor-infiltrating lymphocytes (TILs), including T-cells, B cells, and natural killer (NK) cells, are one of the representative components of host antitumor immune responses.[1] There are many specific antigens such as FOXP3, CD3, CD4, CD8, CD16, CD20, CD56 and CD57, CD68, and CD169 in the cell membrane of TILs.[2] For example, FOXP3, CD3, CD4, and CD8 bind to T-cells; CD16 binds to monocytes; CD20 binds to B cells; CD56 and CD57 binds to natural killer cells; CD68 and CD169 bind to macrophages. With advances in flow cytometry and immunohistochemistry, many researches have shown that specific types of immune cells not only regulate the host defense against cancer,[3] but also accelerate tumor progression either by selecting an opportune microenvironment to survive for tumor or by creating conditions within the tumor microenvironment that simulate tumor outgrowth.[4]
The TILs might play as a prognostic biomarker in HCC. TILs have been demonstrated to predict overall survival (OS) and disease-free survival (DFS) following resection of both primary and metastatic liver tumors.[5–7] Hang et al found that high infiltration of Foxp3+ T-cells was associated with poor prognosis,[5] whereas no correlation between the infiltration of Foxp3+ T-cells and OS was found in Wang et al.[8] Chew et al revealed that patients with high density of CD8+ T-cell had poor outcome,[9] but Ramzan et al reported the opposite results.[10] So, the effect of TILs on HCC prognosis is controversial. Besides, it is not clear whether the associations between the density of TILs and prognosis vary depending on clinical factors such as the pathological stage and anatomic site. Therefore, we performed this meta-analysis to assess the prognostic effect of TILs in HCC.
2. Materials and methods
2.1. Literature search
Relevant articles were identified by 2 reviewers via an electronic search of PubMed, Embase, and Cochrane using the following keywords: (tumour or tumor or cancer or carcinoma), (hepatic or liver), (TILs or tumor-infiltrating lymphocyte or intratumoral lymphocyte or tumor-infiltrating lymphocyte or intratumoral lymphocyte), and (prognostic or prognosis or outcome). And the search time period of the electronic database was from inception to July 7, 2018. Additionally, pertinent studies were searched in reference lists of selected reports and reviews. Unpublished literature was not performed to search. Disagreement on article inclusion between the 2 reviewers was resolved via a 3rd reviewer. A 3rd researcher would determine the final results about the disagreement of the 2 reviewers.
2.2. Inclusion and exclusion criteria
Inclusion criteria for this meta-analysis were as follows: studies researching the prognostic effect of TILs or associated TIL subsets in HCC; report of TILs or associated TIL subsets in tumor surgical specimens; hazard ratio (HR) and 95% confidence interval (CI) could be extracted; when the same author or group reported results obtained from the same cohort of patients in more than one paper, the most recent report or the most informative report was selected.
Exclusion criteria for this meta-analysis were as follows: letters, reviews, case reports, animal trials, abstracts, and expert opinion were excluded; articles in which have no information on overall survival or disease-free survival; patients who only treated with immunotherapy chemotherapy, radiotherapy, interventional therapy, or liver transplantation; nonprimary tumor, such as metastatic tumor or recurrent tumor. Names of authors or journals of the articles were not considered in excluding or including the articles.
2.3. Statistical analysis
The HR and its 95% CI were used to assess the association between TILs and prognosis of HCC. If a direct report of OS and DFS were not available, then extract the survival data from Kaplan–Meier curves by Engauge Digitizer version 4.1 as described previously.[11] The extraction was performed by 2 independent researchers to decrease error. STATA version 12.0 was performed to merge the results of studies. Statistical heterogeneity between trials was assessed by Chi-squared test and was suggested significant when I2 > 50% and/or P < .05. The fixed-effect model was used when no heterogeneity was detected among studies, while the random-effect model was preferred when variance existed. Funnel plot and Egger test were performed to evaluate the publication bias.[12]
3. Results
3.1. Study selection and characteristics
A total of 1232 articles were initially gathered after duplicate removal. Among them, 58 studies reported the association between TILs and prognosis of HCC. After further screening, 12 researches were excluded (1 studying the same cohort of patients, 10 lacking enough useful data, and 1 treating with immunotherapy). Finally, a total of 46 retrospective studies including 7905 patients from America, China, France, Germany, Japan, The Netherlands, and Singaporean were included in this meta-analysis (Fig. 1).[8–10,13–55]
Figure 1.
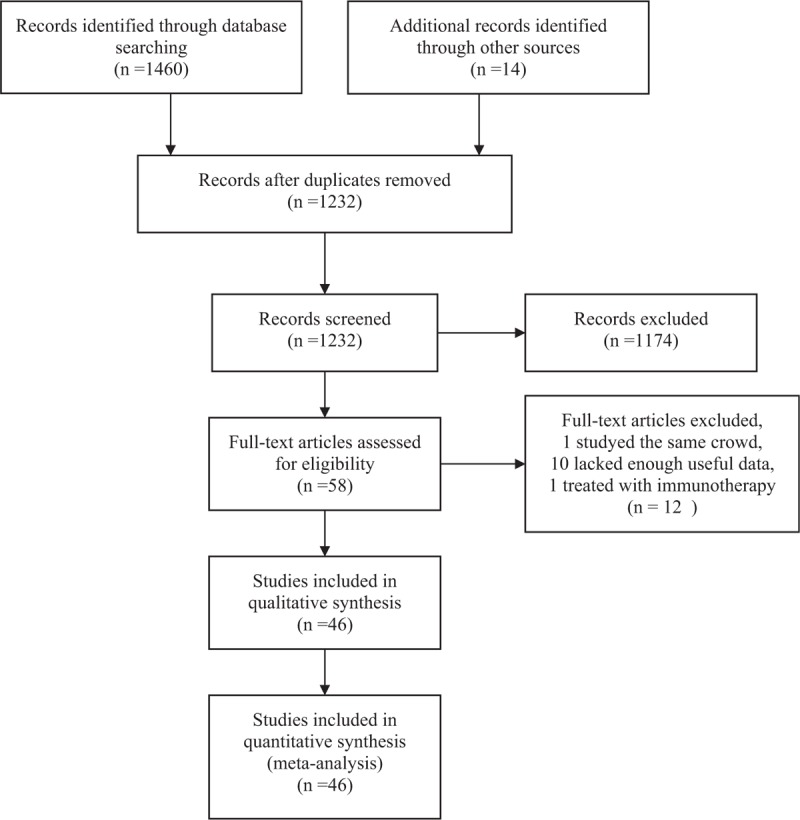
Flow diagram of study selection.
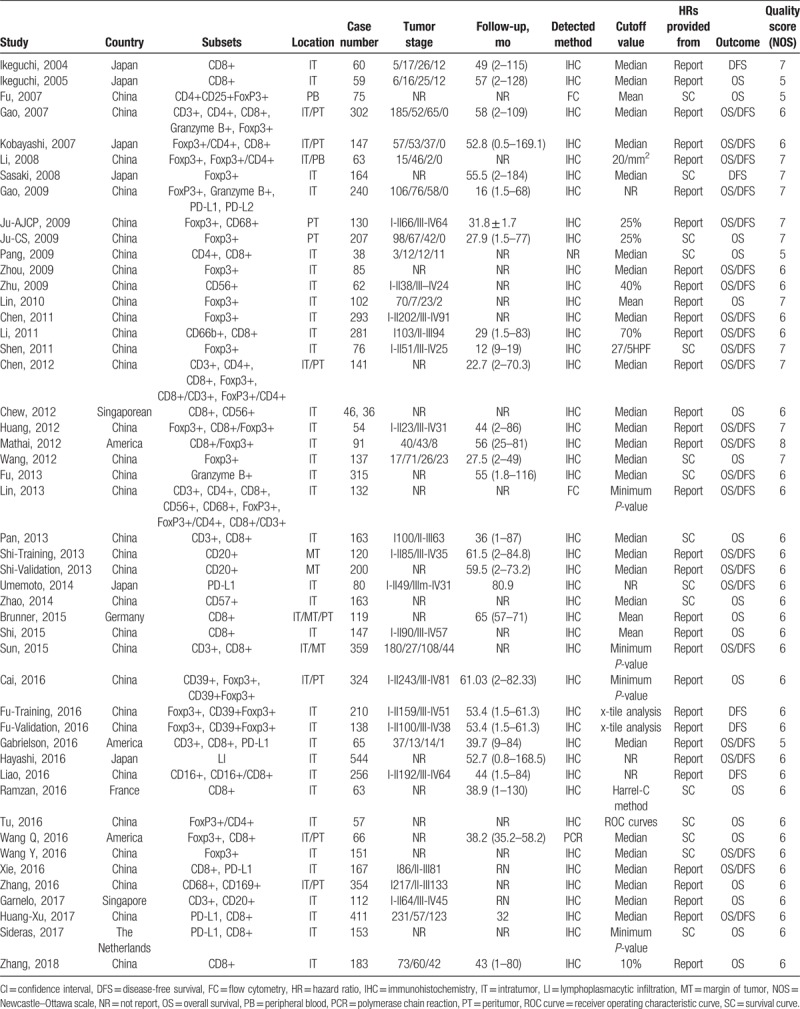
The main characteristics of included studies were displayed in Table 1. The prognostic role of TILs in intratumor (IT), margin of tumor (MT), and peritumor (PT) were evaluated in 41, 3, and 9 articles, respectively. Sample sizes of included studies ranged from 36 to 544 patients. The details of tumor stage were offered in 31 articles, and the classifications were diverse. Follow-up time was provided in 27 articles. Specific TIL subsets were detected by methods of flow cytometry (FC), immunohistochemistry (IHC), and polymerase chain reaction (PCR), respectively. All 46 studies were considered to be of adequate quality for the meta-analysis according to NOS assessment (score ≥5 points) (Table 1).
Table 1.
Main characteristics of all studies included in the meta-analysis.
3.2. Subgroup analysis
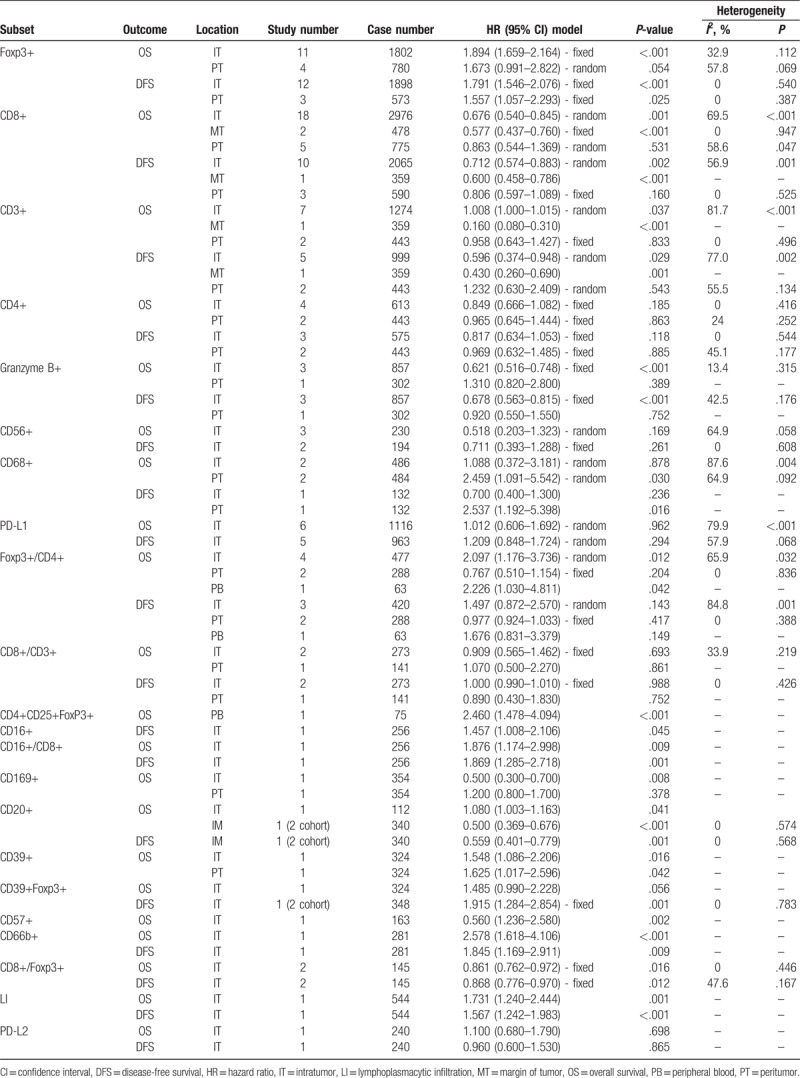
Different TIL subsets have different functions in the process of protumor or antitumor immunoreaction response. Therefore, subgroup analyses based on TIL subsets were carried out. Then, we further conducted subgroup analyses based on the distribution location of TILs. Table 2 shows the various consequences of subgroup analyses.
Table 2.
The pooled associations between TILs subsets and the prognosis of patients with hepatocellular carcinoma.
3.3. Foxp3+ T-lymphocyte subset
A total of 18 articles, including 3015 patients with HCC, researched the association between clinical outcome and the density of Foxp3+ TILs.[8,16,18–22,24,26,27,29–31,34,42,43,48,49]
3.3.1. Overall survival
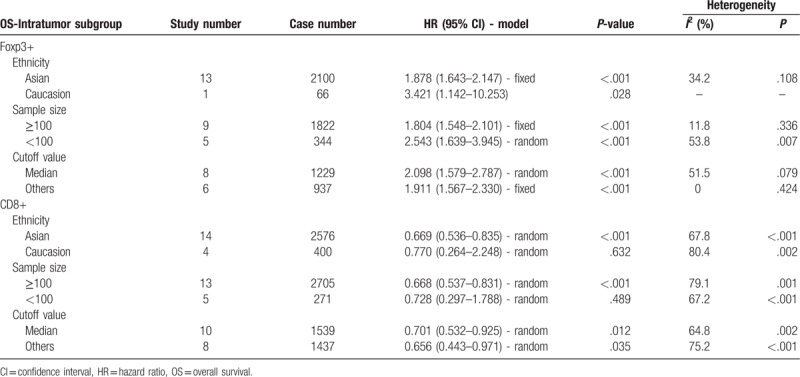
Fouteen articles focused on the relationship between OS and the density of Foxp3+ TILs in IT.[8,16,18,20,24,26,27,29–31,34,42,48,49] Four articles focused on the association between OS and the density of Foxp3+ TILs in PT.[16,21,22,30] The pooled analysis revealed that low density of infiltration of Foxp3+ TILs in IT was statistically significant associated with better OS (pooled HR = 1.894; 95% CI = 1.659–2.164; P < .001). But, there was no statistical significance between the density of Foxp3+ TILs in PT and OS (P = .054) (Fig. 2, Table 2).
Figure 2.
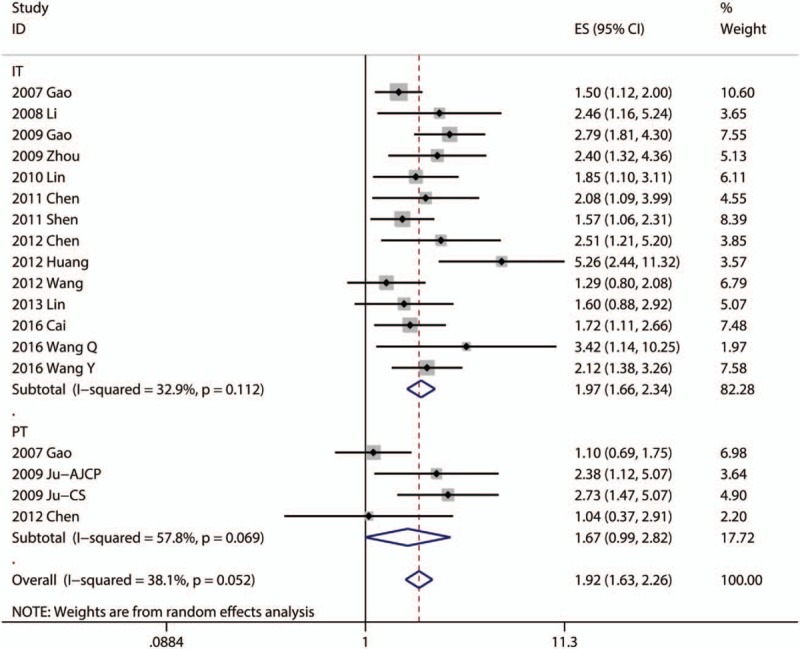
Forest plots of studies evaluating the association between Foxp3+ lymphocytes and overall survival of patients with hepatocellular carcinoma.
Subgroup analysis showed that patients with high infiltration of Foxp3+ T-lymphocytes had worse OS in the groups with Asian patients (pooled HR = 1.878, 95% CI = 1.643–2.147; P < .001), small sample size (<100; pooled HR = 2.543, 95% CI = 1.639–3.945; P < .001), large sample size (≥100; pooled HR = 1.804, 95% CI = 1.548–2.101; P < .001), median cutoff values (pooled HR = 2.098, 95% CI = 1.579–2.787; P < .001), and other cutoff values (pooled HR = 1.911, 95% CI = 1.567–2.330; P < .001). However, other subgroup showed no statistical significance (Table 3).
Table 3.
Pooled hazard ratios for OS according to subgroup analyses.
3.3.2. Disease-free survival
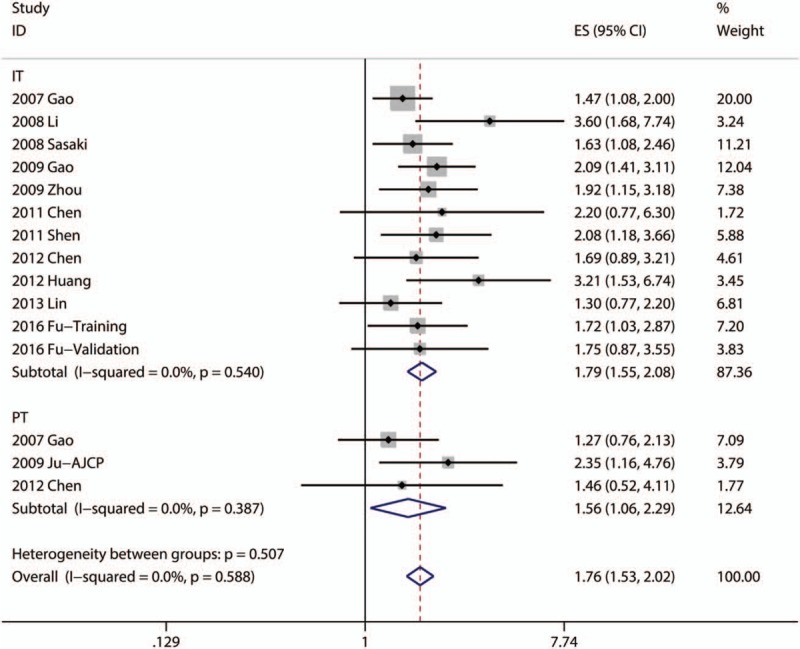
Eleven articles focused on the relationship between DFS and the density of Foxp3+ TILs in IT.[16,18–20,24,27,29–31,34,43] Four articles focused on the association between DFS and the density of Foxp3+ TILs in PT.[16,21,30] The pooled analysis revealed that low density of infiltration of Foxp3+ TILs in IT was statistically significant associated with better DFS (pooled HR = 1.791; 95% CI = 1.546–2.076; P < .001) and so as Foxp3+ TILs in PT (pooled HR = 1.557; 95% CI = 1.057–2.293; P = .025) (Fig. 3, Table 2).
Figure 3.
Forest plots of studies evaluating the association between Foxp3+ lymphocytes and disease-free survival of patients with hepatocellular carcinoma.
3.4. CD8+ T-lymphocyte subset
A total of 20 articles, including 3102 patients with HCC, researched the association between clinical outcome and the density of CD8+ TILs.[9,10,13,14,16,17,23,28,30,34,35,39–41,44,48,50,53–55]
3.4.1. Overall survival
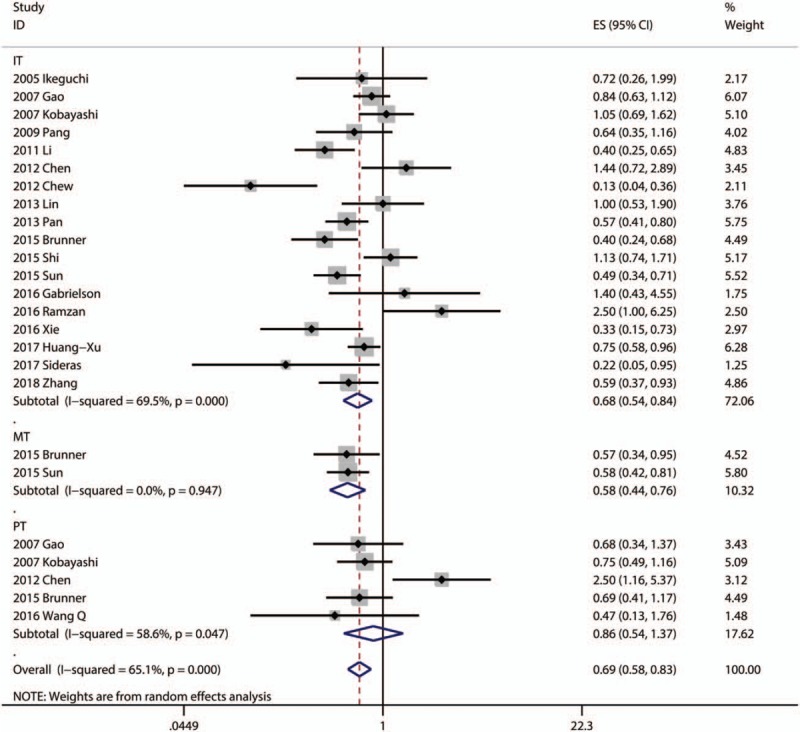
Eighteen articles focused on the relationship between OS and the density of CD8+ TILs in IT.[9,10,14,16,17,23,28,30,34,35,39–41,44,50,53–55] Two articles focused on the association between OS and the density of CD8+ TILs in MT.[39,41] Five articles focused on the association between OS and the density of CD8+ TILs in PT.[16,17,30,39,48] A random model was used because of a significant heterogeneity (P < .001, I2 = 69.5%), and the result demonstrated patients with high infiltration of CD8+ TILs in IT had better OS (pooled HR = 0.676; 95% CI = 0.540–0.845; P = .001). The pooled analysis revealed that high density of infiltration of CD8+ TILs in MT was statistically significant associated with better OS (pooled HR = 0.577; 95% CI = 0.437–0.760; P < .001). But, there was no statistical significance between the density of CD8+ TILs in PT and OS (P = .531) (Fig. 4, Table 2).
Figure 4.
Forest plots of studies evaluating the association between CD8+ lymphocytes and overall survival of patients with hepatocellular carcinoma.
Subgroup analysis showed that patients with high infiltration of CD8+ T-lymphocytes had better OS in the groups with Asian patients (pooled HR = 0.669, 95% CI = 0.536–0.835; P < .001), large sample size (≥100; pooled HR = 0.668, 95% CI = 0.537–0.831; P < .001), median cutoff values (pooled HR = 0.701, 95% CI = 0.532–0.925; P = .012), and other cutoff values (pooled HR = 0.656, 95% CI = 0.443–0.971; P = .035). However, other subgroups showed no statistical significance (Table 3).
3.4.2. Disease-free survival
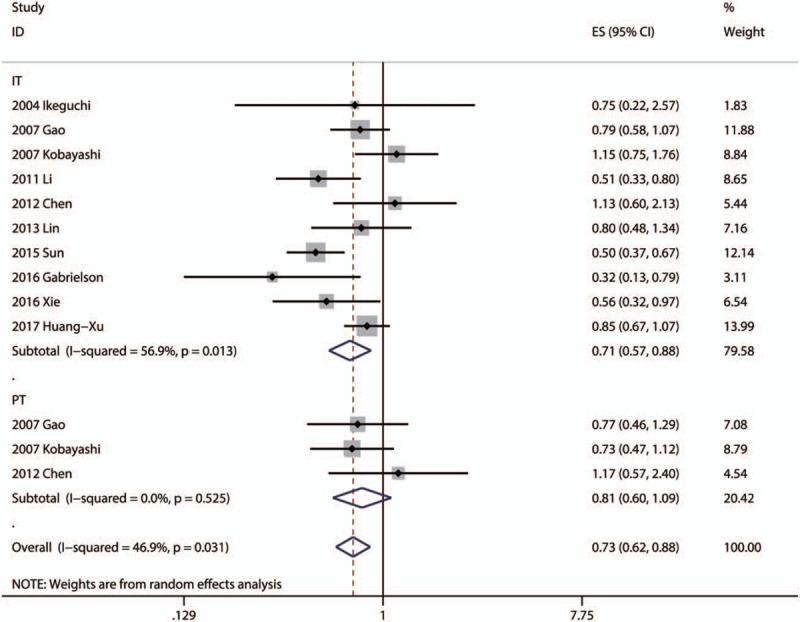
Ten articles focused on the relationship between DFS and the density of CD8+ TILs in IT.[13,16,17,28,30,34,41,44,50,53] Only 1 article focused on the association between DFS and the density of CD8+ TILs in MT.[41] Three articles focused on the association between DFS and the density of CD8+ TILs in PT.[16,17,30] The pooled analysis revealed that high density of infiltration of CD8+ TILs in IT was statistically significant associated with better OS (pooled HR = 0.712; 95% CI = 0.574–0.883; P = .002). There was no statistical significance between DFS and CD8+ TILs in PT (P = .160) (Fig. 5, Table 2).
Figure 5.
Forest plots of studies evaluating the association between CD8+ lymphocytes and disease-free survival of patients with hepatocellular carcinoma.
3.5. CD3+ T-lymphocyte subset
A total of 7 articles, including 1274 patients with HCC, researched the association between clinical outcome and the density of CD3+ TILs.[16,30,34,35,41,44,52]
3.5.1. Overall survival
Seven articles focused on the relationship between OS and the density of CD3+ TILs in IT.[16,30,34,35,41,44,52] Only 1 article focused on the association between OS and the density of CD3+ TILs in MT.[41] Two articles focused on the association between OS and the density of CD3+ TILs in PT.[16,30] The pooled analysis revealed high density of CD3+ in IT foreboded worse OS (pooled HR = 1.008; 95% CI = 1.000–1.015; P = .037). However, there was no statistical significance between OS and CD3+ TILs in PT (P = .833) (Table 2).
3.5.2. Disease-free survival
Five articles focused on the relationship between DFS and the density of CD3+ TILs in IT.[16,30,34,41,44] Only 1 article focused on the association between DFS and the density of CD3+ TILs in MT.[41] Two articles focused on the association between DFS and the density of CD3+ TILs in PT.[16,30] The pooled analysis revealed high density of CD3+ in IT foreboded better DFS (pooled HR = 0.596; 95% CI = 0.374–0.948; P = .029). However, there was no statistical significance between DFS and CD3+ TILs in PT (P = .543) (Table 2).
3.6. CD4+ T-lymphocyte subset
A total of 4 articles, including 613 patients with HCC, researched the association between clinical outcome and the density of CD4+ TILs.[16,23,30,34]
3.6.1. Overall survival
Four articles focused on the relationship between OS and the density of CD4+ TILs in IT.[16,23,30,34] Two articles focused on the association between OS and the density of CD4+ TILs in PT.[16,30] However, there was no statistical significance between OS and CD4+ TILs in IT (P = .185) or in PT (P = .863) (Table 2).
3.6.2. Disease-free survival
Three articles focused on the relationship between DFS and the density of CD4+ TILs in IT.[16,30,34] Two articles focused on the association between DFS and the density of CD4+ TILs in PT.[16,30] However, there was no statistical significance between DFS and CD4+ TILs in IT (P = .118) or in PT (P = .885) (Table 2).
3.7. Granzyme B+ T-lymphocyte subset
A total of 3 articles, including 857 patients with HCC, researched the clinical outcome of patients with Granzyme B+ T-lymphocytes infiltration.[16,20,33] The pooled analysis revealed that high density of Granzyme B+ T-lymphocytes in IT was statistically significant associated with better OS (pooled HR = 0.621; 95% CI = 0.545–0.794; P < .001) and DFS (pooled HR = 0.678; 95% CI = 0.563–0.815; P < .001) (Table 2).
3.8. PD-L1 lymphocyte subset
A total of 6 articles, including 1116 patients with HCC, researched the clinical outcome of patients with PD-L1 lymphocyte infiltration.[20,37,44,50,53,54] A random model was used because of a significant heterogeneity (P < .001, I2 = 79.9%), and the result demonstrated that there was no statistical significance between PD-L1 lymphocyte in IT and OS (P = .962). A random model was used because of a significant heterogeneity (P < .068, I2 = 57.9%), and the result demonstrated that there was no statistical significance between PD-L1 lymphocyte in IT and DFS (P = .294).
3.9. CD56+ NK cell subset
A total of 3 articles, including 230 patients with HCC, researched the clinical outcome of patients with CD56+ NK cells infiltration.[9,25,34] A random model was used because of a significant heterogeneity (P = .058, I2 = 64.9%), and the result demonstrated that there was no statistical significance between CD56+ NK cells in IT and OS (P = .169), and the same to DFS (P = .261) (Table 2).
3.10. CD68+ macrophage subset
A total of 3 articles, including 616 patients with HCC, researched the clinical outcome of patients with CD68+ macrophages infiltration.[21,34,51] A random model was used because of a significant heterogeneity (P = .004, I2 = 87.6%), and the result demonstrated that there was no statistical significance between CD68+ macrophages in IT and OS (P = .878). A random model was used because of a significant heterogeneity (P = .092, I2 = 64.9%), and the result demonstrated that high density of CD68+ macrophages in PT was statistically significant associated with better OS (pooled HR = 2.459; 95% CI = 1.091–5.542; P = .030) (Table 2).
3.11. FoxP3+/CD4+ T-lymphocytes ratio
A total of 5 articles, including 540 patients with HCC, researched the clinical outcome of patients with FoxP3+/CD4+ T-lymphocytes ratio.[17,18,30,34,47] A random model was used because of a significant heterogeneity (P = .032, I2 = 65.9%), and the result demonstrated that patients with low FoxP3+ /CD4+ T-lymphocytes ratio had better OS in IT and OS (pooled HR = 2.097; 95% CI = 1.176–3.736; P = .012) (Table 2).
3.12. Other subsets
The prognostic value of CD4+CD25+FoxP3+ TILs, CD16+ macrophages, CD39+ TILs, CD20+ B cells, CD57+ NK cells, and CD169+ macrophages in patients with HCC have been investigated in separate articles respectively.[15,36,38,42,46,51] Subgroup analysis suggested that patients with high density of CD20+ B cells in MT had better OS (pooled HR = 0.500; 95% CI = 0.369–0.676; P < .001) and DFS (pooled HR = 0.559; 95% CI = 0.401–0.779; P = .001) (Table 2).
3.13. Publication bias
We just assessed the publication bias of subgroups which contained more than 10 researches. We used Egger test to evaluate the publication bias. It indicated there was some degree of publication bias in the studies about Foxp3+ in IT with OS (P = .016) (Fig. 6A) and with DFS (P = .048) (Fig. 6B). And, there was no statistical significance of publication bias in the studies about CD8+ in IT with OS (P = .653) (Fig. 6C) or with DFS (P = .688) (Fig. 6D).
Figure 6.
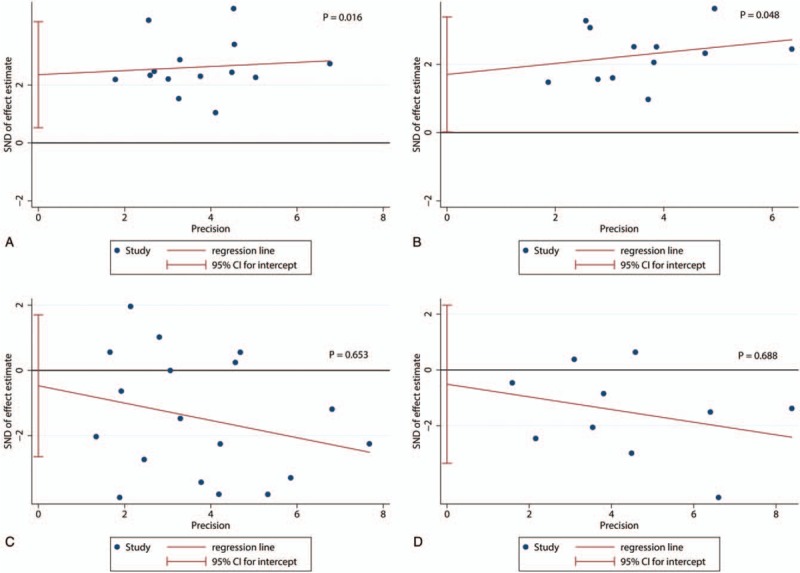
Egger tests of the association between Foxp3+ tumor-infiltrating lymphocytes (TILs) or CD8+ TILs and prognosis. (A) Egger tests of the association between Foxp3+ TILs in intratumor (IT) and overall survival (OS). (B) Egger tests of the association between Foxp3+ TILs in IT and disease-free survival (DFS). (C) Egger tests of the association between CD8+ TILs in IT and OS. (D) Egger tests of the association between CD8+ TILs in IT and DFS.
4. Discussion
The prognostic value of TILs in HCC was quantified in this meta-analysis. Because of the bidirectional role of TILs in immune microenvironment of HCC, subgroup analysis was performed to assess the protumor or antitumor role of different TIL subsets.
Different subsets of TILs have different effect in the development of HCC, which contribute to different prognosis. FOXP3, CD3, CD4, CD8, CD16, CD20, CD56 and CD57, CD68, and CD169 are mainly located in the surface of lymphocytes, including T-cells, B cells, NK cells, and macrophages. FOXP3 is surface antigen of regulatory T-lymphocytes (Tregs) that are the main effective cells in the protumor immune response.[56] Correspondingly, Granzyme B and CD8 are surface antigens of cytotoxic T-lymphocytes that play an important role of the antitumor immune response.[9,20] This is consistent with our research. CD3 is also a common surface antigen of T-lymphocytes. Pan et al and Sun et al considered that high density of CD3+ TILs in IT and MT implied a favorable prognosis, respectively.[35,41]
Furthermore, there are some other surface antigens, which located in T-lymphocytes, showing different prognostic roles. CD8+ lymphocytes include a group of heterogeneous T-lymphocytes, which can diverse programmed cell death ligand-1 (PD-L1; B7-H1; CD274), galactin-9 and herpesvirus entry mediator (HVEM). Sideras et al showed that low tumor expression of PD-L1 and Galectin-9 are associated with poor survival in patients with resected HCC.[54] On the contrary, some studies also confirmed overexpression of PD-L1 in HCC was significantly associated with tumor aggressiveness and enhanced risk for postoperative recurrence.[20,37] These conflicting results, on the association between PD-L1 and prognosis of HCC, were probably related to the lack of specificity of some anti-PD-L1 antibodies for immunohistochemical staining of PD-L1 in formalin-fixed paraffin-embedded tissues.[54] Therefore, the role of PD-L1 is very complicated.
The B cells, NK cells, and macrophages also play crucial roles in tumor-associated immune responses. The role of CD20+ (a B-lymphocyte antigen expressed on mature B cells but not on plasma cells) lymphocytes in promoting favorable outcomes in several human cancers has been reported, including breast carcinoma, cutaneous melanoma, and HCC.[36,57,58] The potential mechanisms included margin-infiltrating B lymphocytes serving as allophycocyanin, stimulating CD8+ T-lymphocytes by releasing proinflammatory cytokines, and exerting a direct killing effect.[36] CD56 and CD57 are all main markers of NK cells. Zhao et al suggested that IL-37 might mediate antitumor immune responses through recruiting CD57+ NK cells to tumor sites.[38] CD16, CD68, and CD169 are all main markers of macrophages. CD169 is mainly expressed by cells of the monocyte/macrophage lineage, and CD169+ macrophages enhanced autologous CD8+ T-cell-mediated immunity in vitro and could suppress tumor progression by enhancing CD8+ T-cell activity in human HCC.[51]
To quantify the immune microenvironment and apply it to a heterogeneous group of patients, a methodology named “Immunoscore” based on the numeration of CD3+ and CD8+ lymphocytes in 2 regions (IT and MT), was provided.[41,44] The Immunoscore was acquired by summation of the 4 binary score values, the scale being from 0 to 4. For instance, immunoscore 0 (I0) was low densities of both cell types in both regions and immunoscore 4 (I4) was high densities of both cell types in both regions.[12,59] Gabrielson et al revealed Immunoscore were significantly associated with recurrence and recurrence-free survival independently of other predictive clinicopathologic factors, and patients with a high density of CD3+ and CD8+ cells in 1 or both tumor regions (IT or MT) experienced a significant reduction in the rate of HCC recurrence.[44]
In addition, we observed that TILs are potential targets for immunotherapy, beside prognostic biomarker for gastric cancer. Recently, cancer immunotherapy has emerged as an appealing field in eliminating the micrometastatic and residual disease of cancer, which contains adoptive cell therapy, monoclonal antibody-based treatment and cancer vaccines, among which cytokine-induced killer (CIK) cells therapy is a promising option. Tumor immunotherapy is currently focusing on the signaling pathway of the programmed cell death protein 1 (PD-1; also known as CD279) binding with PD-L1. PD-1+ TIL was a powerful and specific biomarker in predicting the outcome of CIK immunotherapy in patients with HCC. The correlation between high number of PD-1+ TILs and the high number of both CD4+ and CD8+ TILs suggested that PD-1+ TILs can reflect the existence of endogenous host immune response to tumors, and additional CIK therapy, to some extent, could compensate for immunosuppressive environment and exert a synergistic antitumor effect against HCC.[60]
As we know, this is the 1st meta-analysis including more than 30 articles to evaluate the prognostic role of TILs in HCC. And there are several limitations which should be taken into consideration in interpreting the conclusions of this study. First, studies included used different cutoff values that could reduce the accuracy of TILs in the process of estimating prognosis. Second, when several HRs could not be directly acquired in the original article, we obtained them by calculating the data extracted from the survival curves. Third, some data comes from univariate analysis, which may overestimate the effect sizes of some TIL subsets compared to multivariate analysis. Fourth, there are few investigations of some subsets such as CD16+ TILs, CD39+ TILs, CD20+ B cells, CD57+ NK cells, and CD169+ macrophages. This could reduce the credibility of the data in the subgroup analysis. Fifth, there was some degree of publication bias in the studies about Foxp3+ in IT. It could be caused by a greater percentage of Asian articles. Finally, no prospective randomized trials are reported; therefore, future research should be directed at performing prospective randomized trials.
5. Conclusion
In conclusion, our meta-analysis provides strong evidence that TILs would serve as an effective marker for evaluating the prognosis of patients with HCC. Especially, high density of Foxp3+ TILs in the IT was a poor prognostic factor, and high density of CD8+ TILs in IT was a good prognostic factor. More randomized controlled trials were needed to further verify the results of this meta-analysis.
Acknowledgments
The authors thank all their colleagues at the Department of General Surgery, Wujin Hospital, affiliated with Jiangsu University.
Author contributions
Ding W and Xu XZ contributed equally to the work as co-first authors; Ding W and Tan YL designed the study and wrote the manuscript; Ding W and Xu XZ designed the research; Qan Y, Xue WB, and Wang YB performed the research and analyzed the data; Du JG and Jin L contributed analytical tools; and Tan YL designed the study and edited the manuscript as the corresponding author.
Conceptualization: Yulin Tan.
Data curation: Wei Ding, Jianguo Du, Lei Jin.
Methodology: Yan Qian, Wenbo Xue, Yibo Wang.
Software: Wei Ding.
Writing – original draft: Wei Ding, Xuezhong Xu.
Writing – review & editing: Wei Ding, Yulin Tan.
Yulin Tan orcid: 0000-0002-3278-7624.
Footnotes
Abbreviations: CI = confidence interval, CIK = cytokine-induced killer, DFS = disease-free survival, FC = flow cytometry, HCC = hepatocellular carcinoma, HR = hazard ratio, HVEM = herpesvirus entry mediator, IHC = immunohistochemistry, IT = intratumor, LI = lymphoplasmacytic infiltration, MT = margin of tumor, NK = natural killer, NOS = Newcastle–Ottawa scale, NR = not report, OS = overall survival, PB = peripheral blood, PCR = polymerase chain reaction, PT = peritumor, ROC curve = receiver operating characteristic curve, SC = survival curve, TILs = tumor-infiltrating lymphocytes, Treg = regulatory T-lymphocytes.
WD and XX contributed equally to this study.
This study was supported by the Science and Technology Project of Bureau of Changzhou Municipal Wujin District (WS201515).
The authors have no conflicts of interest to disclose.
References
- [1].Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol 2010;15:544–51. [DOI] [PubMed] [Google Scholar]
- [2].Sprinzl MF, Galle PR. Immune control in hepatocellular carcinoma development and progression: role of stromal cells. Semin Liver Dis 2014;34:376–88. [DOI] [PubMed] [Google Scholar]
- [3].Shirabe K, Motomura T, Muto J, et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. Int J Clin Oncol 2010;15:552–8. [DOI] [PubMed] [Google Scholar]
- [4].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [5].Katz SC, Bamboat ZM, Maker AV, et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann Surg Oncol 2013;20:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Katz SC, Donkor C, Glasgow K, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB 2010;12:674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Khan H, Pillarisetty VG, Katz SC. The prognostic value of liver tumor T cell infiltrates. J Surg Res 2014;191:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang F, Jing X, Li G, et al. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver Int 2012;32:644–55. [DOI] [PubMed] [Google Scholar]
- [9].Chew V, Chen J, Lee D, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012;61:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ramzan M, Sturm N, Decaens T, et al. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int 2016;36:434–44. [DOI] [PubMed] [Google Scholar]
- [11].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Trans Med 2012;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ikeguchi M, Oi K, Hirooka Y, et al. CD8+ lymphocyte infiltration and apoptosis in hepatocellular carcinoma. Eur J Surg Oncol 2004;30:53–7. [DOI] [PubMed] [Google Scholar]
- [14].Ikeguchi M, Hirooka Y. Interleukin-2 gene expression is a new biological prognostic marker in hepatocellular carcinomas. Onkologie 2005;28:255–9. [DOI] [PubMed] [Google Scholar]
- [15].Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132:2328–39. [DOI] [PubMed] [Google Scholar]
- [16].Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586–93. [DOI] [PubMed] [Google Scholar]
- [17].Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 2007;13:902–11. [DOI] [PubMed] [Google Scholar]
- [18].Li SP, Peng QQ, Ding T, et al. Clinical significance of regulatory T cells proportion in the peripheral blood and tumor tissue in primary hepatocellular carcinoma [in Chinese]. Zhonghua Zhong Liu Za Zhi 2008;30:523–7. [PubMed] [Google Scholar]
- [19].Sasaki A, Tanaka F, Mimori K, et al. Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol 2008;34:173–9. [DOI] [PubMed] [Google Scholar]
- [20].Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971–9. [DOI] [PubMed] [Google Scholar]
- [21].Ju MJ, Qiu SJ, Fan J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol 2009;131:498–510. [DOI] [PubMed] [Google Scholar]
- [22].Ju MJ, Qiu SJ, Gao Q, et al. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci 2009;100:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pang YL, Zhang HG, Peng JR, et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immunother 2009;58:877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou J, Ding T, Pan W, et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer 2009;125:1640–8. [DOI] [PubMed] [Google Scholar]
- [25].Zhu LY, Zhou J, Liu YZ, et al. Prognostic significance of natural killer cell infiltration in hepatocellular carcinoma. Chin J Cancer 2009;28:1198–202. [DOI] [PubMed] [Google Scholar]
- [26].Lin GH, Wang J, Li SH, et al. Relationship and clinical significance of TGF-beta1 expression with Treg cell infiltration in hepatocellular carcinoma. Chin J Cancer 2010;29:403–7. [DOI] [PubMed] [Google Scholar]
- [27].Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS One 2011;6:e24671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497–505. [DOI] [PubMed] [Google Scholar]
- [29].Shen SL, Liang LJ, Peng BG, et al. Foxp3+ regulatory T cells and the formation of portal vein tumour thrombus in patients with hepatocellular carcinoma. Canad J Surg 2011;54:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen KJ, Zhou L, Xie HY, et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol 2012;29:1817–26. [DOI] [PubMed] [Google Scholar]
- [31].Huang Y, Wang FM, Wang T, et al. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 2012;86:329–37. [DOI] [PubMed] [Google Scholar]
- [32].Mathai AM, Kapadia MJ, Alexander J, et al. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol 2012;36:980–6. [DOI] [PubMed] [Google Scholar]
- [33].Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013;58:139–49. [DOI] [PubMed] [Google Scholar]
- [34].Lin SZ, Chen KJ, Xu ZY, et al. Prediction of recurrence and survival in hepatocellular carcinoma based on two Cox models mainly determined by FoxP3+ regulatory T cells. Cancer Prev Res (Phila) 2013;6:594–602. [DOI] [PubMed] [Google Scholar]
- [35].Pan QZ, Pan K, Zhao JJ, et al. Decreased expression of interleukin-36alpha correlates with poor prognosis in hepatocellular carcinoma. Cancer Immunol Immunother 2013;62:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shi JY, Gao Q, Wang ZC, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res 2013;19:5994–6005. [DOI] [PubMed] [Google Scholar]
- [37].Umemoto Y, Okano S, Matsumoto Y, et al. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 2014;50:65–75. [DOI] [PubMed] [Google Scholar]
- [38].Zhao JJ, Pan QZ, Pan K, et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci Rep 2014;4:5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brunner SM, Rubner C, Kesselring R, et al. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 2015;61:1957–67. [DOI] [PubMed] [Google Scholar]
- [40].Shi J, Dong Q, Qin L, et al. Phenotype and distribution of infiltrating lymphocytes in liver cancer tissues. Chin J Clin Oncol 2015;42:559–63. [Google Scholar]
- [41].Sun C, Xu J, Song J, et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 2015;6:35602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cai XY, Ni XC, Yi Y, et al. Overexpression of CD39 in hepatocellular carcinoma is an independent indicator of poor outcome after radical resection. Medicine 2016;95:e4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fu YP, Yi Y, Cai XY, et al. Overexpression of interleukin-35 associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Br J Cancer 2016;114:767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gabrielson A, Wu Y, Wang H, et al. Intratumoral CD3 and CD8 T-cell densities associated with relapse-free survival in HCC. Cancer Immunol Res 2016;4:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hayashi A, Shibahara J, Misumi K, et al. Histologic assessment of intratumoral lymphoplasmacytic infiltration is useful in predicting prognosis of patients with hepatocellular carcinoma. PLoS One 2016;11:e0155744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liao R, Jiang N, Tang ZW, et al. Systemic and intratumoral balances between monocytes/macrophages and lymphocytes predict prognosis in hepatocellular carcinoma patients after surgery. Oncotarget 2016;7:30951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tu JF, Ding YH, Ying XH, et al. Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep 2016;6:35056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang Q, Luan W, Warren L, et al. Prognostic role of immune cells in hepatitis B-associated hepatocellular carcinoma following surgical resection depends on their localization and tumor size. J Immunother 2016;39:36–44. [DOI] [PubMed] [Google Scholar]
- [49].Wang Y, Liu T, Tang W, et al. Hepatocellular carcinoma cells induce regulatory T cells and lead to poor prognosis via production of transforming growth factor-beta1. Cell Physiol Biochem 2016;38:306–18. [DOI] [PubMed] [Google Scholar]
- [50].Xie QK, Zhao YJ, Pan T, et al. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology 2016;5:e1181252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang Y, Li JQ, Jiang ZZ, et al. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J Pathol 2016;239:231–41. [DOI] [PubMed] [Google Scholar]
- [52].Garnelo M, Tan A, Her Z, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut 2017;66:342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang CY, Wang Y, Luo GY, et al. Relationship between PD-L1 expression and CD8+ T-cell immune responses in hepatocellular carcinoma. J Immunother 2017;40:323–33. [DOI] [PubMed] [Google Scholar]
- [54].Sideras K, Biermann K, Verheij J, et al. PD-L1, Galectin-9 and CD8(+) tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017;6:e1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang M, Pang HJ, Zhao W, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC cancer 2018;18:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang Y, Liao H, Zhang Y, et al. Prognostic value of tumor-infiltrating FoxP3+ T cells in gastrointestinal cancers: a meta analysis. PLoS One 2014;9:e94376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ladanyi A, Kiss J, Mohos A, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 2011;60:1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mahmoud SM, Lee AH, Paish EC, et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2012;132:545–53. [DOI] [PubMed] [Google Scholar]
- [59].Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007;67:1883–6. [DOI] [PubMed] [Google Scholar]
- [60].Lianyuan T, Dianrong X, Chunhui Y, et al. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Liver Int 2018;19:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]


