Abstract
Rationale:
Inflammatory myofibroblastic tumor (IMT) is a rare soft tissue lesion, originally reported in the lungs. Occurrence of the IMT was also documented in the digestive system, but rare in the urinary system, especially in the urachus, and little is presently known about IMT.
Patient concerns:
This study reported a very rare case of urachal IMT in an elderly female patient at the age of 77 who was diagnosed with a lower abdominal mass 2 months ago.
Diagnosis:
The preoperative diagnosis was urachal carcinoma, which was confirmed to be anaplastic lymphoma kinase (ALK) negative urachal IMT by postoperative histopathology and immunohistochemistry tests.
Interventions:
Laparoscopic radical urachal carcinoma resection and partial bladder resection was performed under general anesthesia, and the tumor was completely removed.
Outcomes:
There was no recurrence and metastasis over 22 months of follow-up.
Lessons:
The urachal IMT occurs mainly in males and nonelderly people with ALK positive while in females with ALK negative. The most common clinical manifestations of urachal IMT are lower abdominal masses; it is very important to distinguish whether the tumor originates from the bladder or the urachus, because the surgical treatment options are completely different. Currently the complete surgical removal of the tumor is the best treatment option for urachal IMT. No other adjuvant therapy is required after operation. All urachal IMT after follow-up showed no recurrence and metastasis, suggesting a good prognosis. However, IMT has malignant potential and it requires a long-term close follow-up check.
Keywords: genitourinary, histopathology, immunohistochemistry, inflammatory mycofibroblastic tumor (IMT), negative for ALK, urachus
1. Introduction
Inflammatory myofibroblast tumor (IMT) refers to a relatively rare soft tissue lesion consisting of fusiform myofibroblasts, fibroblasts, and some inflammatory cells (lymphocytes, plasma cells); IMT was also known as inflammatory pseudotumor (IPT), fibrin granulation granuloma, plasma cell granuloma, myxoid hamartoma, myofibroblastic tumor, pseudosarcoma, and inflammatory fibrosarcoma and other names in the past.[1] However, IMT was grouped into the tumor category since it was later found to have metastases and recurrences. Although, the clinical incidence of this disease is low, most cases are benign, and the prognosis is good; there is still a certain degree of malignant, invasive, recurrent, and even distant metastasis. Due to the complex structure of IMT, the current understanding of the disease is still controversial.
Present studies have shown that IMT can occur at any age, anybody site, more common in children and adolescents, no significant gender differences.[2] IMT was first detected in the lungs and was later reported in the many other sites. However, the occurrence of IMT in the urogenital system is rare, it occurs mainly in the bladder of the urinary system,[1] followed by the kidney, retroperitoneal, renal pelvis, ureter, prostate, testis, etc. The occurrence of IMT is extremely rare in the urachus. The urachal tube is a tubular structure extending from the dome of the bladder to the umbilicus. It disappears before birth and degenerates into a fibrous cord connecting the dome of the bladder to the umbilicus (i.e., the umbilical mid-limus ligament), located within the loose connective tissue between the abdominal fascia and the peritoneum (Retzius gap).[3] Urachal tubes are about 5 to 6 cm in length, which can be divided into upper urinary bladder, inner bladder muscle, and inner bladder mucosa. When insufficient degeneration of the urachal duct occurs, it may cause sinus tract, cyst, diverticulum, tumor, and other lesions. Malignant urachal adenocarcinomas are the most common tumors occurring in the urachal region as compared to benign urachal inflammatory myofibroblastic tumors which are very rare. The pathogenesis of urachal inflammatory myofibroblastic tumor remains unclear since there have been few reports in the past, and most of the literature is case report. Therefore, there is still a lack of complete and systematic information about clinical diagnosis and treatment. This study reported a case of urachal IMT and summarized some conclusions about the disease basing on this case-related information and a review of previous literature. Thus, this report is designed to provide the information and experience and serve as a reference for current understanding of the disease, and to promote further research on urachal IMT.
2. Case presentation
A female patient, 77 years old, was admitted to hospital on June 25, 2016 because she “discovered her lower abdominal mass 2 months ago.” She had a history of diabetes for more than 10 years and a history of hypertension for 4 years. Except for renal cysts, there were no other urinary-related diseases and surgical history. Physical examination found an ellipsoidal mass of approximately 9.0 × 9.0 cm in size located in the suprapubic region of the suprapubic bladder. The mass has an unclear boundary, a hard texture, a fixed position, no tenderness, and pulsating blood vessels. Abdominal CT examination (plain scan + enhancement) showed irregular soft tissue masses between the bladder and pelvic cavity with blurred edges. The surrounding structure is not clear, the size of the mass on the transverse section is about 4.7 × 4.0 cm, and is obviously unevenly strengthened. The bladder wall, thickening of the pelvic wall, and enlarged lymph nodes in the pelvic fat space were initially considered as urachal carcinoma (Fig. 1A and B). Cystoscopy examination showed irregular topography of the bladder wall, smooth surface. The finding from a biopsy from the bulge exhibited the remaining bladder and urethra mucosa were smooth, bilateral ureteral orifice were clear, peristalsis was normal, there was no any newly formed objects and stones in the bladder (Fig. 1C). A grayish white tissue of about 0.2 cm in diameter was examined presurgery and pathological results showed surface coating of the urothelium, intrinsic stroma edema, a few chronic inflammatory cell infiltration changes, and no invasion of tumor cells in the bladder mucosa. Because of the small size, small number, and limited depth of biopsy, the pathological findings have some limitations. According to the clinical manifestations of patients and the described examination results above, preoperative diagnosis was urachal carcinoma. After the stabilization of blood glucose and blood pressure with drug administration, laparoscopic radical urachal carcinoma resection and partial bladder resection was performed under general anesthesia, and the tumor was completely removed. Intraoperative findings: The size of the tumor was approximately 9.0 × 10.0 × 5.0 cm in the abdomen. The cavity contained necrosis and liquefaction, the anterior wall adhered to the rectus abdominis, the posterior wall adhered to the anterior wall of the bladder, and the bladder had normal bladder capacity. Anterior wall thickening, stiffness, pelvic lymph nodes were observed.
Figure 1.
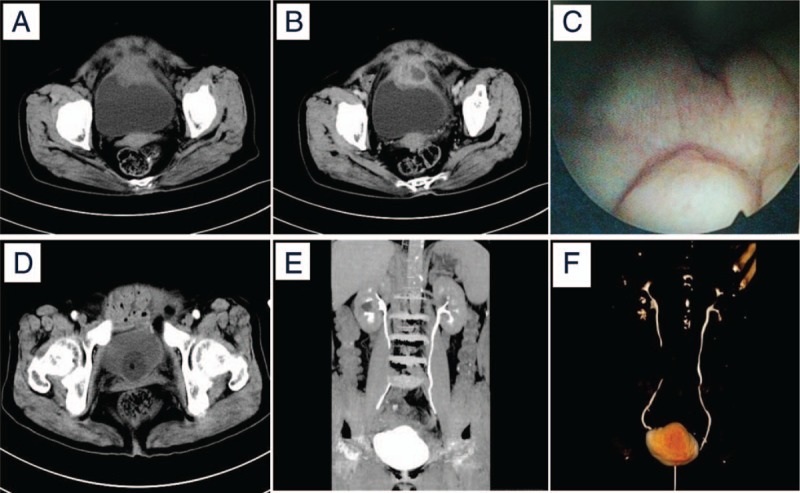
Images of computed tomography (CT) and cystoscopy. (A and B) Abdominal CT (plain scan + enhancement) images showing an irregular soft tissue mass between the bladder and pelvic cavity with obvious uneven enhancement, blurred edges and unclear surrounding structures. (C) Image of cystoscopy examination showing irregular topography of the bladder wall and bladder mucosa was smooth. (D) Abdominal CT (plain scan) showed no local tumor recurrence and lymphadenopathy. (E and F) The coronal reconstruction and three-dimensional reconstruction of the urinary system by time-lapse imaging showed that the kidneys, ureters and bladders were well-filled, and no obvious contrast agent extravasation was observed. The images (D–F) were taken after 6 months of the surgery and indwelling catheterization. CT = computed tomography.
Postoperative pathology report showed: findings from gross specimen examination include that urachal lumps after incision was gray and white. The size of the nonshaped tissue was approximately 9.5 × 10.0 × 5.0 cm, and the size of a cyst was approximately 4.5 × 3.5 × 5.5 cm. Some necrosis was detected on the inner wall of the capsule, and the rest of the tissue was gray-red, gray-yellow, and there was also a little lymphoid tissue in the texture as shown in Figure 2 (Fig. 2C); light microscopic examination revealed that the spindle cells were diffusely growing, arranged in a bundle, swirling, The cell size and shape are more uniform, the cytoplasm is red-stained, interspersed with muscle tissue, and invasive growth. There are a large number of inflammatory cell proliferation, interstitial edema, mucinous changes, and diffuse infiltration of inflammatory cells in some regions. Small abscess formation (Fig. 2A and B); Immunohistochemistry test showed positive results included CD34 (blood vessel+) (Fig. 3B), SMA(+) (Fig. 3C), Vim(3+) (Fig. 3 D), Ki-67(1%+), and negative results included ALK(−) (Fig. 3A), Des(−),CK(−), EMA(−), and S-100(−); special staining: VG(red) (Fig. 3E), masson (blue) (Fig. 3F), suggesting muscle fiber sources. Pathological diagnosis confirmed the urinary inflammatory myofibroblastic tumors and tumor residues in the lymphoid tissue.
Figure 2.
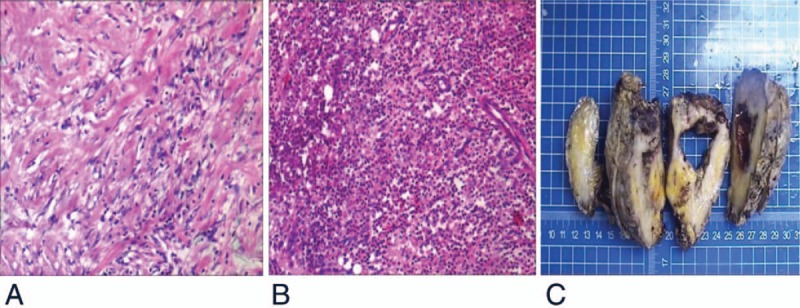
Histopathology images of urachal inflammatory myofibroblastic tumor. (A) Spindle cells with infiltration of inflammatory cell; (B) tumor tissue with small focal abscess; (C) the section of urachal lumps showing grayish white tissue and a cavity with a little necrosis on the inner wall. (A and B) Tissue sections were stained with hematoxylin and eosin (H&E). Magnification ×100.
Figure 3.
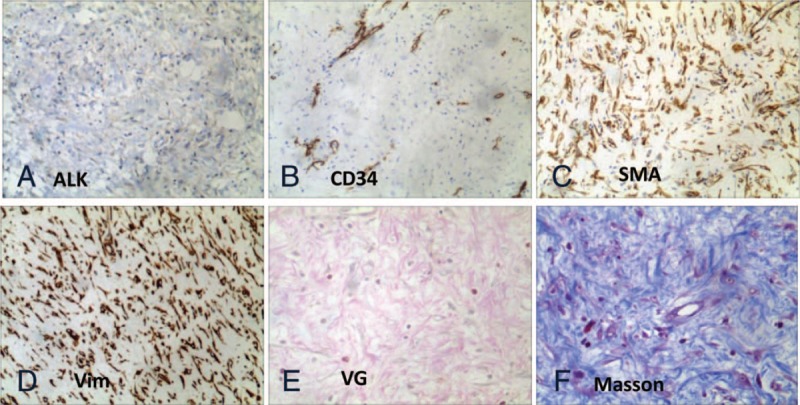
Immunohistochemistry images of urachal inflammatory myofibroblastic tumor showing (A) ALK: negative; (B) vascular-CD34: positive; (C) smooth muscle actin of the tumor cells: positive; (d) vimentin of the tumor cells: positive. Special staining showed that (E) the tumor cells appeared red color after VG-stained and (F) the tumor cells appeared blue color after Masson-stained, indicating that the tumor cells originated from muscle fiber. Magnification: A–D = 100×, and E and F = 200×.
The patient came back to our hospital again after 6 months of the surgery because of her abdominal discomfort. It was suspected due to urinary extravasation. After treatment of indwelling catheterization, the review of the middle and lower abdomen CT (plain scan + enhancement + three-dimensional reconstruction) presented no retroperitoneal and pelvic lymph nodes, while double both kidneys, ureters and bladders were well filled, with no obvious change in contrast agent leakage and no local recurrence (Fig. 1D–F). No other adjuvant therapy was performed after the operation. The patient was followed-up for more than 22 months with good condition showing no occurrence of recurrence or metastasis of the tumor.
3. Discussion
Inflammatory myofibroblastic tumor is a differentiated myofibroblast spindle cell with a large number of plasma cells and/or lymphocyte infiltrating mesenchymal tissue-derived borderline tumors. It belongs to low-grade malignant tumors and has potential metastasis. The most common metastases occur in the mesentery and retroperitoneum. The vast majority occurs in single lesions with local growth, and can infiltrate the adjacent tissues and organs. The early literature reported that this type of spindle cell proliferation was a reactive change after inflammation, so inflammatory pseudotumor became the most commonly used synonym. Later, in-depth study revealed that the spindle cells in the lesion are the main component, and some related cases reported that it had tumor characteristics such as local invasion, recurrence, distant metastasis, etc. Thus, it was classified as a tumor category and gradually realized as a true tumor. In 2002, the WHO International Group of Tissue Classification for Soft Tissue Tumors defined it as “a tumor composed of differentiated myofibroblast spindle cells, often accompanied by plasma cells and/or lymphocyte infiltration,” and thereafter it is classified as fibroblasts or myofibroblast tumors with intermediate and a low number of metastatic.[4] Since then most scholars called the disease inflammatory myofibroblastic tumors. IMT was first discovered clinically in the lungs by Brunn[5] in 1939. At that time, people knew very little about this tumor, and it was only then gradually recognized and found in various organs of the human body. There was no report of IMT in the urinary system until 1980 when Roth[6] first reported the discovery of such tumors in the bladder. Since then, other scholars have found this tumor in other parts of the urinary system, but rarely found in the urinary tract, and particularly urachal IMT is the rarest. Literature search showed there has been only 7 cases of urachal IMT, of which 3 were adult cases, and the remaining 4 cases were children.[7–13] The previously reported cases were all nonelderly patients, so this is the first case of urachal IMT reported in an elderly patient.
The cause of the disease is still unclear, and may be associated with the following related factors, including: surgery, trauma, inflammation, abnormal repair, overexpression of interleukin-6 or CyclinD1, human herpes virus, papilloma virus, EB virus, or special bacterial Infections.[14,15] The pathogenesis of IMT is also being studied and discussed and most people believe that it is due to a genetic mutation. Some scholars have reported that the lesion is associated with chromosomal aberrations and identified the breakage of the p22–24 band of chromosome 2 in the ALK gene region, specifically involving clonal chromosome aberrations of 2p23.[16] There is also evidence of abnormalities in ALK and p80 and chromosomal rearrangements of 2p23 found in IMT.[17] A recent study has shown that about 50% of IMT have a clonal rearrangement of the ALK gene.[18] The immunohistochemistry of ALK leading to activation of ALK protein expression is also helpful for the identification of similar tumors.[19] Chromosome analysis and the ploidy number of DNA can contribute to benign and malignant diagnosis.[20] TSuzuki et al[21] also confirmed that ALK-1 immunostaining is useful in differential identification of IMT and malignant spindle cell tumors. In addition, ALK reactivity may be a useful prognostic indicator of IMT, distal metastasis occurs mainly in ALK-negative IMT, but local recurrence or not is not associated with ALK expression.[22] ALK positive can be used as a specific marker to identify with other tumors, but ALK negative is not an indicator to exclude IMT. Histopathologically, the pathological morphology of inflammatory myofibroblastic tumors can be divided into 3 histological subtypes: mucinous, spindle-shaped, and fibro-carcinogenic. Pathological type can be mainly one of them, it can also be 2 or 3 kinds coexist. IMT is generally expressed as a mixture of spindle cells, composed of myofibroblasts, fibroblasts, and tissue cells, and is often mistakenly considered as smooth muscle or rhabdomyosarcoma.
Table 1 summarizes the details of the demographic characteristics of 8 urachal IMT patients, including age, gender, clinical manifestations (symptoms, location, and size of tumors), and diagnostic features (tumor manifestations in CT, pathological immunohistochemistry), treatment status (surgical resection, adjuvant therapy), and prognosis (follow-up time, recurrence). These 8 urachal IMT cases includes present reported case the other 7 cases retrieved in literature.
Table 1.
Summary of 8 cases of the urachal inflammatory myofibroblastic tumors.
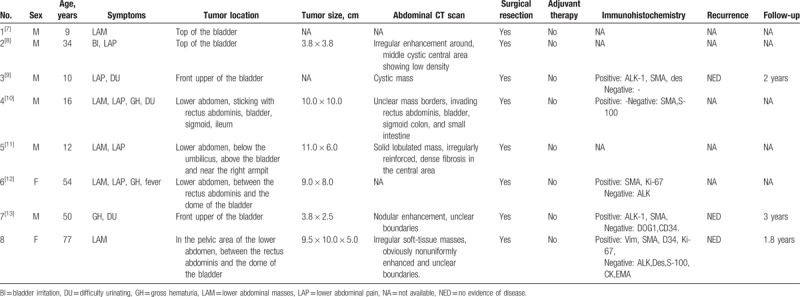
As shown in Table 1, immunohistochemistry tests show that among the 4 urachal IMT patients, 2 males are ALK positive (ages 10 and 50) while 2 females are ALK negative (ages 54 and 77). It can be concluded that ALK-positive urachal IMT patients are male children and adults, and ALK-negative cases occur in adult females. In addition, it was observed that urachal IMT mainly occurs in men and nonelderly people (6/8), and less frequently in women and the elderly (1/8). However, because there is no statistical analysis of a large number of cases to provide direct evidence, the accuracy and reliability of these conclusions need to be confirmed by more in-depth studies with more cases in the future.
Clinical manifestations of urachal IMT can be different due to the location, size, whether it violates the bladder and other factors. From Table 1 it shows that the tumors were small for the cases 2 and 7, but due to tumor invasion and oppression of the bladder, hematuria and bladder irritation occurred in both patients. While for the case 4, the patient had a large tumor and a wide range of invasiveness, which resulted in associated abdominal pain, hematuria, difficulty urinating, and abdominal masses. The present case (the case 8 in Table 1) had a large tumor size and tight adhesion to the bladder. The cystoscopy and tissue biopsy analysis revealed that the tumor did not invade the mucosal layer. Therefore, the patient only had a painless mass but no gross hematuria and bladder irritation were observed. In addition, the most common clinical manifestation of urachal IMT are found in these 8 cases as lower abdominal masses. Therefore, if a patient has a lower abdominal mass which is excluded from the bladder, female uterus attachment, and lower gastrointestinal tract, doctors are recommended to consider whether or not the patient has umbilical IMT. Some scholars believe that common clinical manifestations of urachal malignancies include hematuria, pain, urinary tract irritation, abdominal masses, and bacteriuria, umbilical drainage, and other symptoms.[23] However, the latter 2 have not been detected in the existing urachal IMT cases. Bacterial urine may be caused by a violation of the bladder infection caused by the distal urachal tumor. The umbilical drainage may be related to the proximal urachal tumor invading the abdominal wall and necrosis bursts out of the urinary sinus that has never closed.
According to the diagnostic information of the patients in Table 1, the CT findings revealed that the IMT masses were located near the bladder in the lower abdomen and all showed irregular enhancement, while the borders were unclear and the center of the tumor could be cystic low density or high density of dense fibrosis. The findings of the urachal IMT are very similar to the CT findings of urachal malignancies, and this means that urachal IMT does not have specific imaging characteristics it is difficult to distinguish with the naked eye during surgery. However, the use of B-ultrasonography, CT, MRI, and other imaging instruments can still provide valuable information for the diagnosis of this disease, such as the anatomical site of the tumor, the adjacent relationship, the range of involvement, and the internal structure. In addition, the preoperative pathological biopsy of urachal IMT has certain limitations due to its small size, small number, and limited depth, and the urinary catheter IMT is difficult to distinguish with the naked eye during surgery, so the urachal IMT definitive diagnosis still needs to be determined by postoperative histopathology and immunohistochemical examination.
Because the surgical approaches for urachal IMT and bladder cancer are radically different, it is especially important to identify the tissue origin of these tumors. Urachal tumors can be easily identified when they occur above the bladder without extending to the bladder wall. However, when the tumor occurs in the bladder mucosa and muscle layers, it is difficult to determine whether the tumor originates from the urachus or the bladder. The 6 diagnostic criteria of Wheeler and Mustofi [24] are useful criteria for distinguishing malignant tumors of bladder tumor and urachal origin, and can also be used for the diagnosis of urachal IMT. The case can be considered urachal primary tumors if it meets these 6 diagnostic criteria: the tumor is located in the dome or anterior wall of the bladder, the tumor is located in the bladder muscle but not in the mucosa, the tumor invades the bladder wall and continuously spreads to the lower abdominal wall, visible urachal tube residuals, clear boundaries between the tumor and the overlying bladder mucosa, and the absence of glandular or polypoid proliferations of the bladder mucosa. In addition, histologically, inflammatory myofibroblastic tumors should be differentiated from the following tumors including lesions such as desmoid, leiomyoma, neurofibromas, leiomyosarcoma, adult sarcomatoid carcinoma, embryonic nevus-like tumor, rhabdomyosarcoma of children, Nodular fasciitis, spindle cell nodules, etc.[13] Clinically, urachal IMT should be differentiated from urachal carcinoma, urachal cyst, other urachal inflammatory lesions, and bladder cancer.
For treatment, complete surgical removal of the tumor is the preferred treatment option for the urachal IMT. Due to the relatively mature minimally invasive technique in our hospital, a complete laparoscopic resection of the urachal neoplasm and removal of some of its invaded lymph nodes with enlarged pelvic and pelvic cavities were the decision for this case. This procedure has minimal trauma to the patient with a good prognosis, and the patient was discharged one week after surgery. Since IMT is considered to be a benign or low-grade tumor, the prognosis is generally good. Most scholars believe that there is no need for further treatment after appropriate surgical resection, such as adjuvant radiotherapy and chemotherapy.[25] Although the effects of radiotherapy and chemotherapy are not clear, some scholars suggested that adjuvant chemotherapy can be given to invasive IMT.[26] There have also been some studies with the attempt of some other conservative treatments. Such as antibiotic treatment after the disappearance of lesions.[27] There was also a report of the successful treatment of IMT cases using prednisone and COX-2 inhibition anti-inflammatory.[28] Afterward, there are still some reports in the literature on the use of corticosteroids for the treatment of IMT with good results.[29] At present, the latest research progress has revealed that the ALK pathway plays a very important role in IMT. ALK-targeted inhibitors can be used for the treatment of ALK-dependent or positive IMT cases that have been transferred or unresectable.[30] All the 8 patients (Table 1) underwent surgical treatment of complete resection of the tumors without any adjuvant therapy. Three patients had no evidence of tumor recurrence after long-term follow-up and the remaining 5 cases showed no follow-up information. Therefore, there is no follow-up treatment experience in cases of recurrence or metastasis of urachal IMT. If later urachal IMT or other IMT cases have recurrence or metastasis, adjuvant chemoradiotherapy and/or ALK targeting inhibitors may also be considered for treatment. Therefore, continuous in-depth study and analysis for the treatment methods of urachal IMT is needed in the future.
4. Conclusion
Urachal IMT is extremely rare and difficult to diagnose. Currently, it is mainly based on the patient's clinical manifestations and information provided by the auxiliary examination to initially determine the source of the tumor, the degree of malignancy, the extent of the violation, and then use this information to provide guidance for surgical treatment. ALK positive has certain specificity that can be used to differentiate the IMT from other tumors and provide some help for diagnosis. The effectiveness of conservative treatments such as antibiotics, nonsteroidal anti-inflammatory drugs, and corticosteroids also requires to be confirmed by more direct and definitive proof from further research in future. Invasive tumors may be considered to give adjuvant chemotherapy, but the effect is not clear. Among ALK-dependent IMT patients whose IMT are metastatic or surgically unresectable can be treated with ALK-targeted inhibitors. This report has made the following conclusions basing on analyzing the existing urachal IMT cases in literature: urachal IMT mainly occurs in nonelderly males; ALK-positive urachal IMT occurs in male children and adults, while ALK-negative IMT occurs in adult females; the most common clinical manifestations of urachal IMT is the formation of lower abdominal mass, Therefore, if the patient has a lower abdominal mass which is excluded from the bladder, uterine attachments, and the lower digestive tract, it is recommended to consider for umbilical IMT; it is very important to distinguish whether tumors originate from the bladder or the urachus by imaging examination, because the surgical treatment plans of the 2 are very different; preoperative pathological biopsy results have some limitations, and accurate diagnosis requires postoperative histopathology and immunohistochemistry tests; the current surgical resection of the tumor is the best treatment option for urachal IMT. No other adjuvant therapy is required after the operation. All the follow-up urachal IMT shows no case of recurrence and metastasis, suggesting a good prognosis. Since the IMT has malignant potential, long-term close follow-up is still recommended.
Acknowledgments
We would like to express our great appreciation to the First Affiliated Hospital of Nanchang University. We also thank all the teachers and students who took part in the research design and the field investigations. GW led the study and authorship of this paper. KW drafted the paper and conducted the analysis. HZ and YL provided writing assistance. SX critically revised the manuscript for intellectual content. QL, CZ, and XZ were involved in the data collection and analysis.
Author contributions
Conceptualization: Gongxian Wang.
Data analysis: Shuyan Xia.
Data curation: Kai Wang, Qi Lu, Cheng Zhang, Xiaochen Zhou.
Formal analysis: Kai Wang, Qi Lu, Gongxian Wang.
Investigation: Kai Wang.
Manuscript preparation: Shuyan Xia.
Supervision: Gongxian Wang.
Writing – original draft: Kai Wang, Hui Zhou.
Writing – review & editing: Yuanan Lu.
Footnotes
Abbreviations: ALK = anaplastic lymphoma kinase, CK = cytokeratin, CT = computed tomography, Des = desmin, DNA = deoxyribonucleic acid, EMA = epitheial membrane antigen, HE = hematoxylin-eosin stain, IMT = inflammatory myofibroblastic tumor, IPT = inflammatory pseudotumor, Ki-67 = antigen KI67, S-100 = soluble protein-100, SMA = smooth muscle actin, VG = Van Gieson, Vim = vimentin.
Ethical Statement: The procedures employed were in accordance with the Declaration of Helsinki. The patient provided informed consent prior to clinical materials collection and her personal information was maintained anonymously. This study was approved by the National Natural Science Foundation Review board (81760457, 2018).
The authors have no conflicts of interest to disclose.
References
- [1].Gwynn ES, Clark PE. Inflammatory myofibroblastic tumor associated with renal cell carcinoma. Urology 2005;66:880. [DOI] [PubMed] [Google Scholar]
- [2].Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859–72. [DOI] [PubMed] [Google Scholar]
- [3].Mazeau P, Curinier S, Kandem-Simo A, et al. Prenatal diagnosis and evolution of patent urachus. J Gynecol Obstet Biol Reprod 2013;43:393–6. [DOI] [PubMed] [Google Scholar]
- [4].Fletcher CDM, Unni K, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Cancer 2002;177:1365–76. [Google Scholar]
- [5].Brunn H. Two interesting benign lung tumor of contradictory histopathology. J Thorac Surg 1939;9:119–31. [Google Scholar]
- [6].Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology 1980;16:635–7. [DOI] [PubMed] [Google Scholar]
- [7].Kaskas M, Helfrich P, Dabrowski A, et al. Inflammatory pseudotumor of the urachus. A case. Presse Med 1992;21:1374–6. [PubMed] [Google Scholar]
- [8].Drissi M, Amil T, Lebbar K, et al. Inflammatory pseudo-tumor of the urachus: a case report. Ann Durol 2002;36:138–41. [DOI] [PubMed] [Google Scholar]
- [9].Nascimento AF, Dal CP, Cilento BG, et al. Urachal inflammatory myofibroblastic tumor with ALK gene rearrangement: a study of urachal remnants. Urology 2004;64:140. [DOI] [PubMed] [Google Scholar]
- [10].Tunca F, Sanli O, Demirkol K, et al. Inflammatory pseudotumor of urachus mimicking invasive carcinoma of bladder. Urology 2006;67:623. [DOI] [PubMed] [Google Scholar]
- [11].Sharma RK, Jain VK, Mukherjee S, et al. Inflammatory pseudotumor of the urachus. Urotoday Int J 2012;5:94. [Google Scholar]
- [12].Wu HL, Gong LG, Xiao XL, et al. One case:urachal primary inflammatory myofibroblastic tumor (in Chinese). J Pract Radiol 2015;31:170. [Google Scholar]
- [13].Venkatesh K, Madhusudhan HR. Anaplastic lymphoma kinase positive inflammatory myofibroblastic tumor of the urachus: a rare neoplasm in an unusual location. Indian J Pathol Microbiol 2016;59:93–5. [DOI] [PubMed] [Google Scholar]
- [14].Lee SH, Fang YC, Luo JP, et al. Inflammatory pseudotumour associated with chronic persistent Eikenella corrodens infection: a case report and brief review. J Clin Pathol 2003;56:868–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alaani A, Hogg R, Warfield AT, et al. Air bag injury as a cause of inflammatory myofibroblastic pseudotumour of the subglottic larynx progressing to myositis ossificans. Acta Otolaryngol 2005;125:674–7. [DOI] [PubMed] [Google Scholar]
- [16].Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res 1999;59:2776–80. [PubMed] [Google Scholar]
- [17].Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol 2001;14:569–76. [DOI] [PubMed] [Google Scholar]
- [18].Mariño-Enríquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol 2011;35:135–44. [DOI] [PubMed] [Google Scholar]
- [19].Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 2001;25:1364–71. [DOI] [PubMed] [Google Scholar]
- [20].Jones EC, Clement PB, Young RH. Inflammatory pseudotumor of the urinary bladder. A clinicopathological, immunohistochemical, ultrastructural, and flow cytometric study of 13 cases. Am J Surg Pathol 1993;17:264–74. [DOI] [PubMed] [Google Scholar]
- [21].Tsuzuki T, Magigalluzzi C, Epstein JI. ALK-1 expression in inflammatory myofibroblastic tumor of the urinary bladder. Am J Surg Pathol 2004;28:1609–14. [DOI] [PubMed] [Google Scholar]
- [22].Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509–20. [DOI] [PubMed] [Google Scholar]
- [23].Behrendt M, De JJ, Van RB. Urachal Cancer: contemporary review of the pathological, surgical, and prognostic aspects of this rare disease. Minerva Urol Nefrol 2016;68:172–84. [PubMed] [Google Scholar]
- [24].Lippincott Williams and Wilkins, Wolters Kluwer Health, Petersen RO, Sesterhenn IA, Davis CJ. Urinary bladder, Urologic Pathology. 3rd edn. 2009. [Google Scholar]
- [25].Jochum W, H?Nggi D, Bruder E, et al. Inflammatory myofibroblastic tumor of the small intestine. J Am Coll Surg 2002;194:502–6. [DOI] [PubMed] [Google Scholar]
- [26].Kovach SJ, Fischer AC, Katzman PJ, et al. Inflammatory myofibroblastic tumors. J Surg Oncol 2004;11:385–91. [DOI] [PubMed] [Google Scholar]
- [27].Alezra E, Delforge X, Buisson P, et al. Complete resolution of inflammatory myofibroblastic tumor of the bladder after antibiotic therapy. Arch Pediatr 2016;23:612–5. [DOI] [PubMed] [Google Scholar]
- [28].Berger A, Kim C, Hagstrom N, et al. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumor with anti-inflammatory therapy. Urology 2007;70:372. [DOI] [PubMed] [Google Scholar]
- [29].Li JY, Yong TY, Coleman M, et al. Bilateral renal inflammatory pseudotumour effectively treated with corticosteroid. Clin Exp Nephrol 2010;14:190–8. [DOI] [PubMed] [Google Scholar]
- [30].Mossé YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children's oncology group study. J Clin Oncol 2017;35:3215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]


