Abstract
Autoimmune hepatitis (AIH) is an autoimmune liver disease that is characterized by a progressive destruction of the liver parenchyma and the development of liver fibrosis. We aimed to examine the relationship between circulating cytokines/chemokines and the Mac-2 binding protein glycosylation isomer (M2BPGi) levels in Japanese patients with autoimmune hepatitis (AIH).
We investigated the relationship between circulating cytokines/chemokines and M2BPGi levels in Japanese patients with AIH. Seventy-seven patients with well-documented AIH were enrolled in the National Hospital Organization (NHO)-AIH-liver-network database. We measured the serum levels of 20 cytokines in 31 selected AIH patients before and after steroid treatment using multisuspension cytokine array.
Eleven cytokines and soluble adhesion molecules were increased in untreated AIH patients compared with treated AIH patients. Among these cytokines and soluble adhesion molecules, soluble intercellular adhesion molecule-1 (sICAM-1) and interferon-γ-inducible protein 10 (IP-10) were most downregulated by steroid therapy in AIH patients. We measured serum sICAM-1 and IP-10 by ELISA and found the levels were significantly higher in AIH patients (n = 77) compared with chronic viral hepatitis C patients (n = 32). Furthermore, there was a positive correlation between sICAM-1 or IP-10 and alanine aminotransferase, total bilirubin, and circulating M2BPGi levels. M2BPGi levels were increased in AIH patients with high stages of liver fibrosis. Additionally, M2BPGi levels were correlated with the histological grade of inflammation in AIH. Circulating M2BPGi levels were significantly reduced by steroid treatment in AIH patients.
sICAM-1 and IP-10 are useful markers to assess immune-mediated hepatitis activity in AIH and they correlate with circulating M2BPGi. Serum M2BPGi levels increased in untreated AIH patients with active hepatitis and were decreased by steroid therapy. M2BPGi reflects autoimmune-mediated hepatic inflammation as well as liver fibrosis.
Keywords: autoimmune hepatitis, cytokine, interferon-γ-inducible protein 10, soluble intercellular adhesion molecule-1, Wisteria floribunda agglutinin positive Mac-2-binding protein
1. Introduction
Autoimmune hepatitis (AIH) is an immune-mediated liver disease characterized by elevated serum-IgG and circulating autoantibodies.[1] Corticosteroids alone or in combination with immunosuppressants are the mainstay therapies for patients with AIH.[2] In most cases, these therapies result in biochemical remission, a treatment end-point in AIH.[3] However, some AIH patients develop liver cirrhosis and liver failure at initial presentation.[4,5] Hepatic inflammation is regulated by the activities of immune cells including lymphocytes, Kupffer cells, hepatocytes, and hepatic stellate cells.[6] Serum cytokines/chemokines are thought to be linked to the pathogenesis of AIH by activating these hepatic immune cells.[7] Previous studies reported that levels of circulating inflammatory cytokines/chemokines reflect liver damage in patients with AIH.[8] However, the relationship between cytokines/chemokines and hepatic inflammation in AIH patients remains unresolved.
Mac-2 binding protein glycosylation isomer (M2BPGi) is a serum biomarker for liver fibrosis caused by chronic viral hepatitis and nonalcoholic fatty liver disease.[9,10] Although M2BPGi levels are correlated with liver fibrosis, serum M2BPGi values are increased in patients with acute liver injury suggesting M2BPGi may reflect liver inflammation.[11] More recently, Umemura et al reported that M2BPGi levels represented a simple and reliable surrogate marker of liver fibrosis in patients with primary biliary cholangitis (PBC).[12] Nishikawa et al also reported that M2BPGi was a useful marker for assessing liver histological findings in AIH patients.[13] However, there is no available data regarding the effects of immunosuppressive treatments on M2BPGi levels in patients with AIH.
In the present study, we analyzed the serum levels of cytokines/chemokines and examined the relationship between these cytokines and clinical parameters to elucidate putative biomarkers for immune-mediated liver injury in patients with AIH. Another aim of the present study was to examine the relationship between M2BPGi levels and inflammatory cytokines and liver histological findings in patients with AIH.
2. Patients and methods
2.1. Study population
Patients with well-documented and untreated AIH were enrolled from the National Hospital Organization (NHO)-AIH-liver-network database, a multicenter registry for Japanese patients with AIH.[5] The diagnosis of AIH was made according to the diagnostic criteria defined by International Autoimmune Hepatitis Group[14], including a raised IgG level and presence of organ-specific and non-organ-specific autoantibodies, presence of interface hepatitis and portal plasma cell infiltration on liver histology and the absence of other liver diseases of known etiology. Patients were excluded from the study if there was histological evidence of cholangitis or non-alcoholic steatohepatitis. Patients positive for hepatitis B virus surface antigen or HCV RNA were also excluded. Patients with other causes of liver disease, such as excess alcohol or drug use, were excluded based on reviews of their appropriate history and investigations. As controls, patients with chronic hepatitis C (untreated CHC, n = 32; female/male = 16/16; mean age, 56.2 ± 7.8 years; aspartate aminotransferase (AST), 43.4 ± 23.6 IU/L; alanine transaminase (ALT), 54.1 ± 39.0 IU/L), total bilirubin (TB), 0.8 ± 0.4 mg/d were included. As controls for autoimmune disease, SLE patients (n = 27; female/male = 22/5; untreated SLE = 12) satisfying American College of Rheumatology classification criteria were included. Mean age was 35.2 ± 13.4 years, mean age at diagnosis was 30.0 ± 12.5 years, mean disease duration 4.5 ± 5.6 years. Aspartate aminotransferase (AST), 38.1 ± 35.5 IU/L; alanine transaminase (ALT), 38.4 ± 72.9 IU/L); total bilirubin (TB), 0.8 ± 0.8 mg/d. Fifteen patients had been treated with steroid (mean prednisolone doses; 21.3 ± 21.9 mg/day), and 9 patients had been treated with steroid plus immunosuppressive agents. Nine patients were complicated with lupus nephritis and 6 patients were complicated with neuropsychiatric SLE (NPSLE). The study was approved by the Ethics Committee of the NHO Central Internal Review Board and participating NHO liver-network hospitals. Written informed consent was obtained from each individual.
2.2. Multiplex cytokine assay
We performed a multiplex cytokine bead assay blindly and in parallel using the Bio-plex Pro Human Cytokine assay (Bio-Rad, Hercules, CA) and Milliplex MAP Human Cytokine/Chemokine panel 1 (Millipore, Billerica, MA) according to the manufacturers’ instructions as described previously.[15] The subjects’ baseline demographic characteristics were compared using Fisher's exact test for discrete variables and the Wilcoxon test for continuous variables. The Kruskal–Wallis test followed by Dunn's multiple comparisons test was used to compare cytokine levels between groups. To rank the cytokine levels, we performed a multivariate classification algorithm termed random forest analysis (RFA),[16] using the R package RandomForest (http://cran.r-project.org/web/packages/randomForest/) ver. 4.6–12 software, as previously described.[17]
2.3. Enzyme-linked immunosorbent assay for sICAM-1and IP-10
Serum concentrations of sICAM-1and IP-10 were measured using human sICAM-1 or IP-10 immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instruction.
2.4. Measurement of M2BPGi
Serum M2BPGi level was directly measured with the HISCL M2BPGi reagent kit (Sysmex, Kobe, Japan) using an automatic immunoanalyzer HISCL-5000 (Sysmex, Hyogo, Japan). M2BPGi levels were indexed using the following equation: Cut-off Index (C.O.I.) = ([M2BPGi]sample-[M2BPGi]NC)/([M2BPGi]PC)-[M2BPGi]NC), where [M2BPGi]sample represents the M2BPGi count of the serum sample, PC is positive control, and NC is negative control. The positive control was supplied as a calibration solution standardized to yield a C.O.I. of 1.0. The positive control was supplied as a calibration solution. The range of measurement is 0.1 to 20 COI[18].
2.5. Staging of hepatic fibrosis
Liver biopsies were taken by fine-needle aspiration (16G or 18G sonopsy) guided by US. Liver tissue specimens were fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin. They were evaluated for hepatic fibrosis stage by a pathologist according to the criteria of Desmet et al.[19]
2.6. Statistical analysis
The Chi-square test and Mann–Whitney U tests were applied to detect significant associations. Correlations between continuous variables were analyzed by the Pearson correlation test. Results for non-normally distributed continuous variables were summarized as mean or medians (interquartile ranges) and were compared by the Mann–Whitney U test. Paired data were analyzed by non-parametric tests using the Wilcoxon signed-rank test for the comparison of paired data.
3. Results
3.1. Baseline characteristics of patients
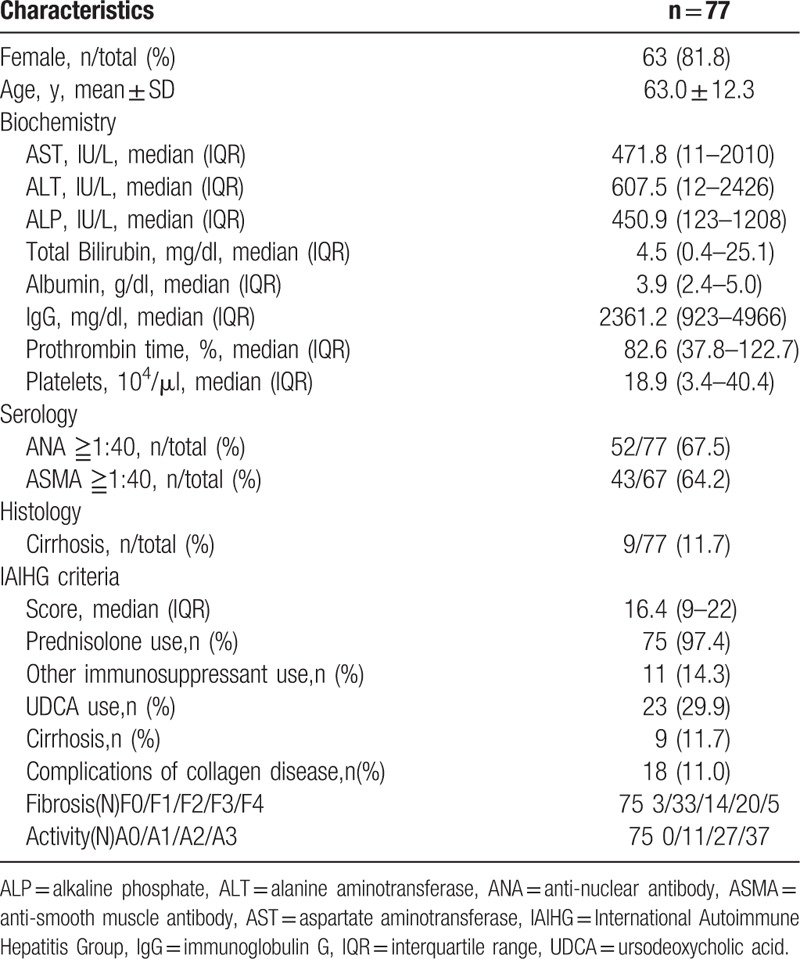
The demographic data of the enrolled AIH patients are shown in Table 1. There were 14 males and 63 females with a median (range) age of 64 (21–83) years. Using the AIH scoring system,[14] 49 patients had more than 15 points (definite AIH) and the remaining 28 patients had 10 to 15 points (probable AIH). Regarding histological findings, in terms of the degree of inflammation activity, A3 was observed in 37 patients, A2 in 27 and A1 in 11. In terms of liver fibrosis stage, F4 was observed in 1 patient, F3 in 24, F2 in 14, F1 in 33, and F0 in 3. The M2BPGi values (pretreatment) in all AIH patients ranged between 0.33 and 16.9 (mean value 4.87). In 2 patients, liver biopsy was not performed due to medical reasons.
Table 1.
Baseline characteristics of 77 Japanese autoimmune hepatitis (AIH) Type 1 patients.
3.2. Identification of combinational biomarkers specific for AIH in attack by Random Forest Analysis and Logistic Regression Analysis
We selected 31 untreated AIH patients with paired serum samples before and after treatment and measured their serum levels of multiple cytokine/chemokines using the multiplex cytokine bead assay. The serum levels of 11 cytokines (IL-4, IL-8, IL-10, IL-18, TNF-α, sICAM-1, sVCAM-1, CCL-3, CCL-4 CCL-22, and CXCL10) were significantly elevated in the untreated AIH patients compared with those receiving 4 weeks of steroid therapy (Table 2). To determine the most important predictor to discriminate untreated active AIH from treated inactive AIH, we ranked the cytokines by importance using the random forest analysis (RFA). Hierarchical clustering with heatmaps based on the Spearman correlation coefficients between active AIH and inactive AIH (Fig. 1). Although the exact identity of T cells that contribute to pathogenesis of AIH remains unknown, possible alterations in theTh1 (cellular immunity) and Th2 (humoral immunity) balance may play a role in liver cell injury.[20] It appears that an increased Th2 cytokines, such IL-10, IL-4 and proinflammatory cytokines/chemokines are increased in untreated AIH patients,[7] whereas we focused on the cytokines most downregulated by steroid treatment, sICAM-1 and CXCL-10 (interferon-γ-inducible protein 10;IP-10). We subsequently measured the serum concentrations of sICAM-1 and IP-10 in all AIH patients by specific ELISAs (Fig. 2). Serum levels of sICAM-1 and IP-10 were elevated in AIH patients compared with chronic hepatitis C patients (sICAM-1; AIH: 1732.8 ± 1076.8 ng/ml, CHC: 605.7 ± 85.7 ng/ml, IP-10; AIH: 914.5 ± 732.6 pg/ml, CHC: 487.9 ± 356.8 pg/ml) or SLE patients (sICAM-1; 461.4 ± 157.1 ng/ml, IP-10;735.6 ± 870.6 pg/ml).
Table 2.
Cytokine profile of autoimmune hepatitis (AIH) patients pre or post treatment pg/ml (∗ng/ml).
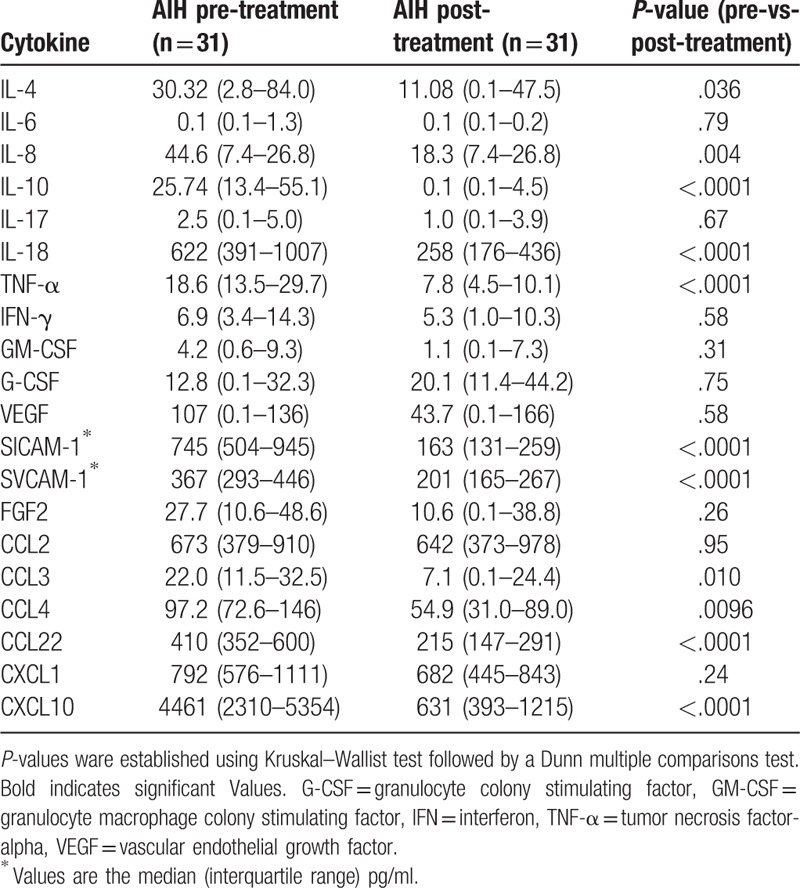
Figure 1.
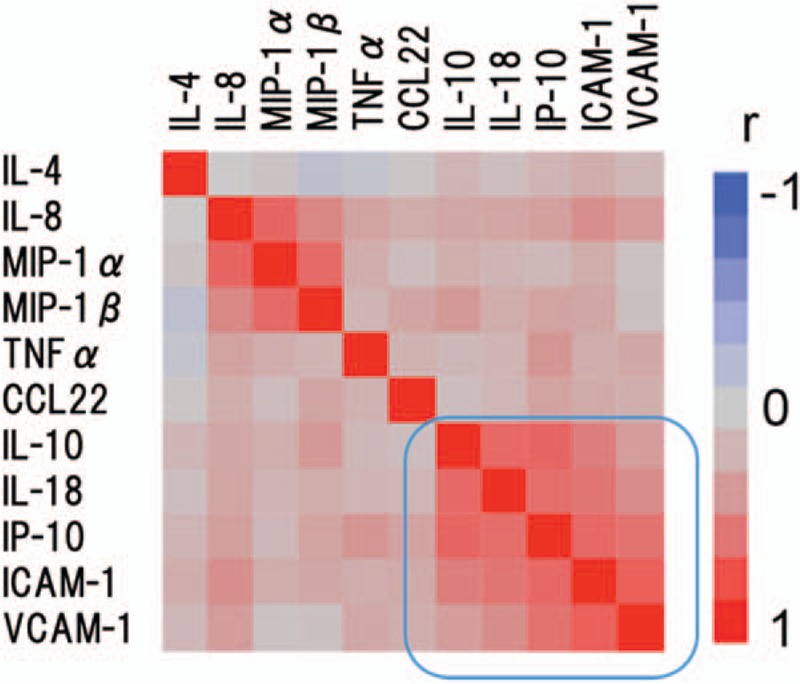
Hierarchical clustering with a Spearman correlation heat map of serum cytokine levels between active AIH before treatment and inactive autoimmune hepatitis (AIH) after treatment. AIH = autoimmune hepatitis.
Figure 2.
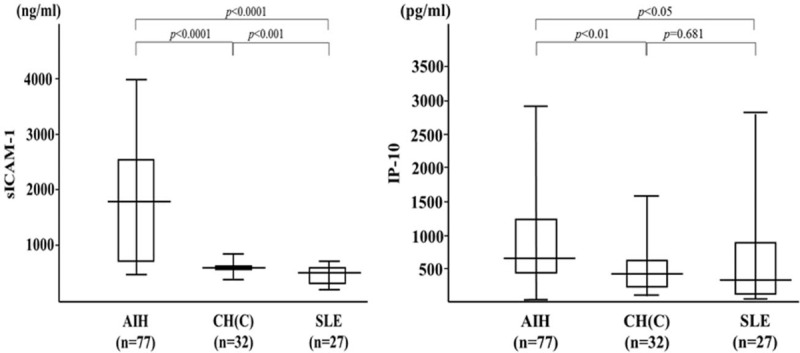
Serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and IP-10 in autoimmune hepatitis (AIH) patients (n = 77), patients with chronic hepatitis C (HCV, n = 32) and patients with SLE (n = 27). The vertical lines indicate the range and the horizontal boundaries of the boxes represent the first and third quartiles. Results were compared by non-parametric Mann–Whitney U test. IP-10 = interferon-γ-inducible protein 10, sICAM-1 = soluble intercellular adhesion molecule-1. AIH = autoimmune hepatitis.
3.3. Relationship between circulating sICAM-1 and IP-10 and liver function tests
To investigate the relationship between sICAM-1 or IP-10 and standard liver function parameters, we examined correlations between circulating sICAM-1 and serum levels of ALT or TB. A positive correlation was observed between serum levels of sICAM-1 and ALT or TB (Fig. 3). Similarly, a positive correlation was observed between serum levels of IP-10 and ALT or TB (Fig. 4).
Figure 3.
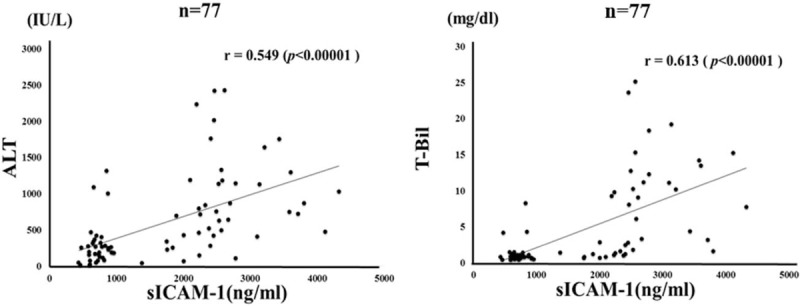
Correlations between serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and ALT/TB levels in patients with autoimmune hepatitis (AIH). Serum sICAM-1 significantly correlated with serum ALT and TB level. Statistics and regression line are represented by the solid line. AIH = autoimmune hepatitis, ALT = alanine aminotransferase, sICAM-1 = soluble intercellular adhesion molecule-1, TB = total bilirubin.
Figure 4.
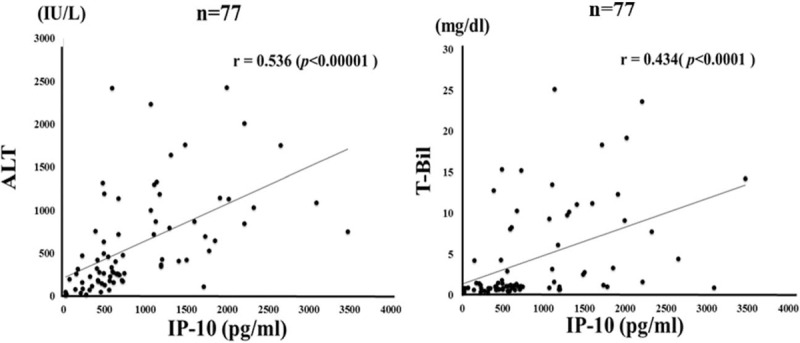
Correlations between serum levels of IP-10 and ALT/TB levels in patients with autoimmune hepatitis (AIH). Serum IP-10 significantly correlated with serum ALT level. Statistics and regression line are represented by the solid line. AIH = autoimmune hepatitis, ALT = alanine aminotransferase, IP-10 = interferon-γ-inducible protein 10, TB = total bilirubin.
3.4. Relationship between M2BPGi and liver fibrosis or necroinflammation
We demonstrated that serum levels of M2BPGi were elevated in AIH patients compared those with chronic hepatitis C as well as those with another autoimmune disease, SLE (Fig. 5). To evaluate whether serum M2BPGi correlated with liver histology, we evaluated serum M2BPGi levels in AIH patients sub-grouped according to the staging of liver fibrosis (Fig. 6). The mean M2BPGi values in each fibrosis stage (F0–F4) were 4.0 ± 3.0 COI in F0–F1, 4.7 ± 2.4 COI in F2, and 6.1 ± 4.3 COI in F3+F4. The values of M2BPGi were increased according to the stage of liver fibrosis (Fig. 6). The M2BPGi values at each liver inflammation grading (A1–A3) were also evaluated by necroinflammatory grade (Fig. 6). The mean M2BPGi values for each necroinflammatory grade (A1–A3) were 3.3 ± 3.4 COI in A1, 4.6 ± 3.0 COI in A2, and 5.4 ± 3.8 COI in A3. The values of M2BPGi were increased according to the grade of liver inflammation (Fig. 6). Whereas there was no significant correlation between serum levels of sICAM-1/IP-10 and liver fibrosis or inflammation scores (Fig. 7). In addition, there was a correlation between sICAM-1 or IP-10 levels and those of M2BPGi in AIH patients (Fig. 8). We stratified AIH patients by excluding patients with advance liver fibrosis stage (> F3) to minimize the effect of liver fibrosis against the values of circulating M2BPGi. We found a strong correlation between serum sICAM-1 or IP-10 levels and M2BPGi in AIH patients without advance liver fibrosis stage (Fig. 9).
Figure 5.
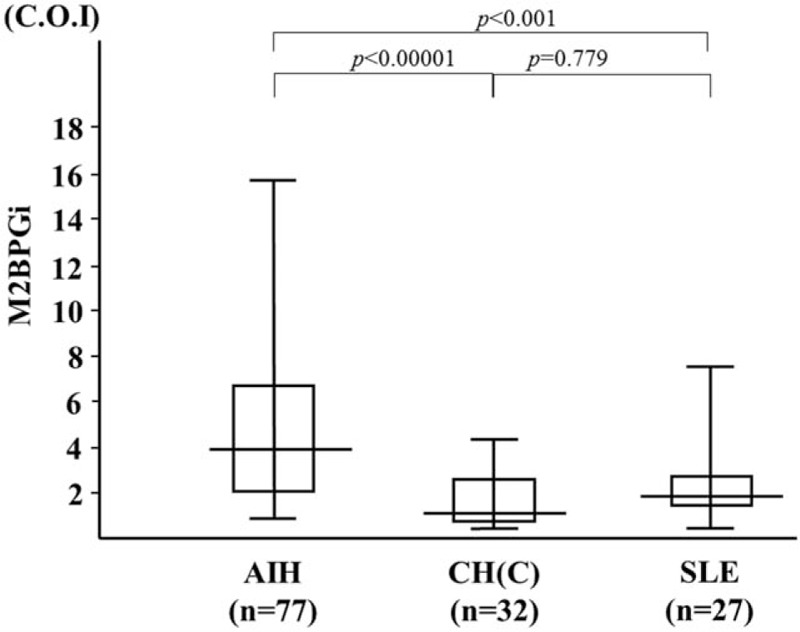
Serum levels of M2BPGi in autoimmune hepatitis (AIH) patients (n = 77), patients with chronic hepatitis C (HCV, n = 32) and patients with systemic lupus erythematosus (SLE, n = 27). The vertical lines indicate the range and the horizontal boundaries of the boxes represent the 1st and 3rd quartiles. Results were compared by non-parametric Mann–Whitney U test. AIH = autoimmune hepatitis, M2BPGi = Mac-2 binding protein glycosylation isomer.
Figure 6.
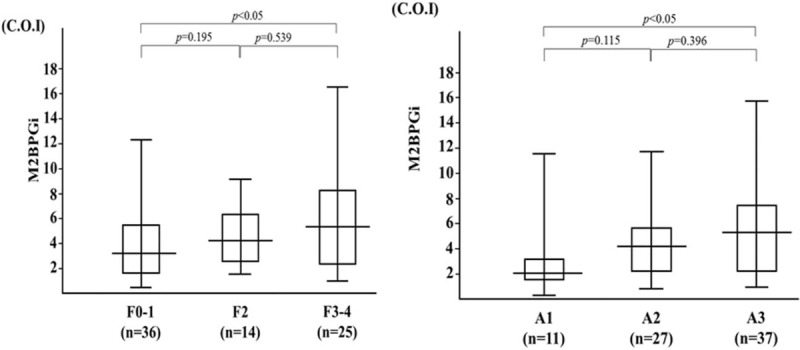
Serum levels of M2BPGi increased according to liver fibrosis stage and liver inflammation grading. The vertical lines indicate the range and the horizontal boundaries of the boxes represent the first and third quartile. Results were compared by non-parametric Mann–Whitney U test. M2BPGi = Mac-2 binding protein glycosylation isomer.
Figure 7.
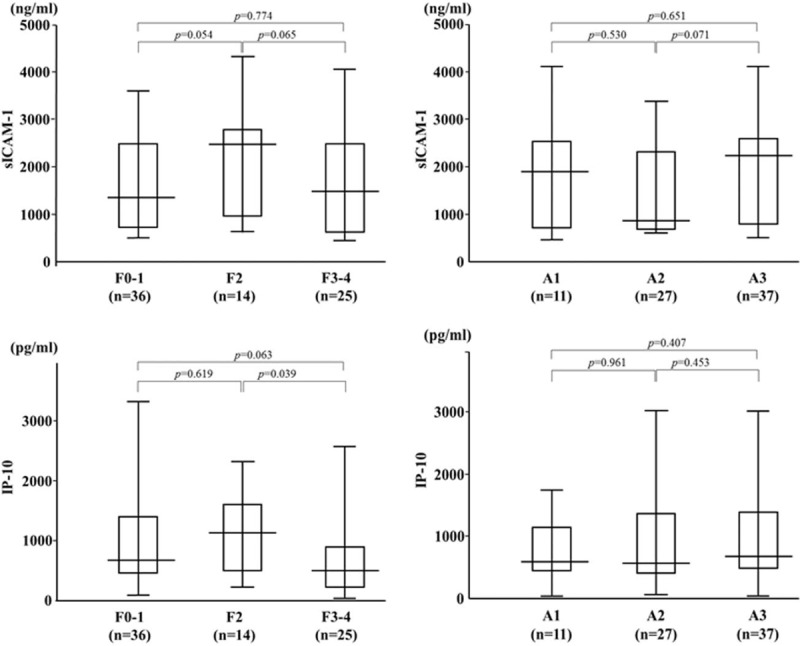
Serum levels of sICAM-1/IP-10 and according to liver fibrosis stage or inflammatory grading. The vertical lines indicate the range and horizontal boundaries of the boxes represent the 1st and 3rd quartile. Results were compared by non-parametric Mann–Whitney U test. IP-10 = interferon-γ-inducible protein 10, sICAM-1 = soluble intercellular adhesion molecule-1.
Figure 8.
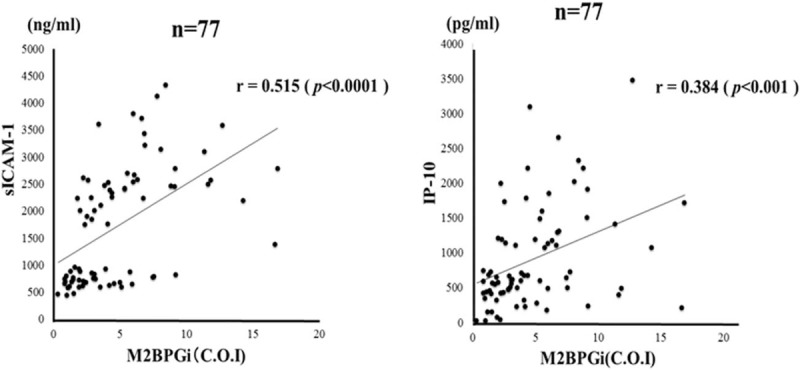
Correlations between serum levels of sICAM-1/IP-10 and serum M2BPGi levels in patients with autoimmune hepatitis (AIH). Serum sICAM-1 and IP-10 significantly correlated with serum M2BPGi levels. Statistics and regression line are represented by the solid line. AIH = autoimmune hepatitis, COI: cutoff index, IP-10 = interferon-γ-inducible protein 10, sICAM-1 = soluble intercellular adhesion molecule-1, M2BPGi = Mac-2 binding protein glycosylation isomer.
Figure 9.
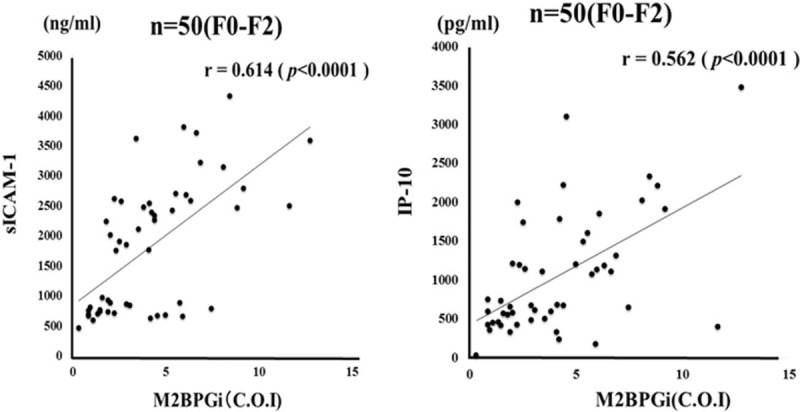
Correlations between serum levels of sICAM-1/IP-10 and serum levels of M2BPGi in autoimmune hepatitis (AIH) patients stratified by excluding patients with advance liver fibrosis stage (> F3). Serum sICAM-1 level significantly correlated with serum M2BPGi levels. Statistics and regression line are represented by the solid line. AIH = autoimmune hepatitis, COI: cutoff index, IP-10 = interferon-γ-inducible protein 10, sICAM-1 = soluble intercellular adhesion molecule-1, M2BPGi = Mac-2 binding protein glycosylation isomer.
3.5. Changes in M2BPGi by corticosteroid therapy
Circulating levels of M2BPGi were measured before and after corticosteroid therapy in paired serum samples from 57 AIH patients. Serum levels of sICMA-1 and IP-10 were downregulated by corticosteroid therapy in AIH patients (Fig. 10A, B). Similarly, M2BPGi was upregulated in untreated AIH patients and downregulated following glucocorticoid treatment of the same patients (Fig. 10C). There was a significant difference in M2BPGi values before and after corticosteroid therapy in AIH patients.
Figure 10.
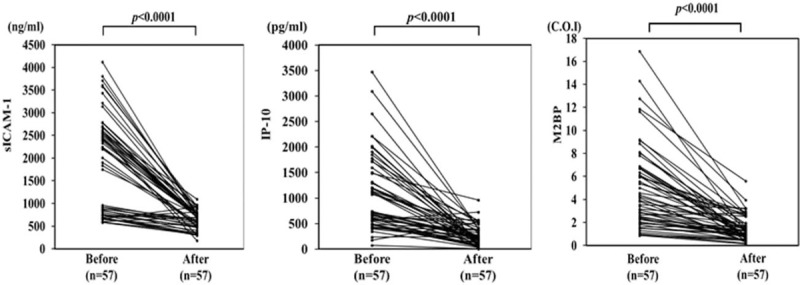
Changes in individual sICAM-1, IP-10 and M2BPGi values before and after (4weeks) steroid treatment in 57 autoimmune hepatitis (AIH) patients. Paired samples from the same subjects were compared by Wilcoxon signed-rank test. COI: cutoff index. IP-10 = interferon-γ-inducible protein 10, M2BPGi = Mac-2 binding protein glycosylation isomer.
4. Discussion
A comparative study of serum levels of various cytokines and chemokines in AIH patients before and after steroid therapy revealed the increased expression of a series of proinflammatory cytokines and chemokines. Among these cytokines/chemokines, we focused on combinational biomarkers for the acute phase of AIH consisting of IP-10 and sICAM-1. In our study, IP-10 levels were correlated with AIH disease severity, which is in line with previous studies of other liver diseases. Intracellular adhesion molecule-1 (ICAM-I), a member of the immunoglobulin (Ig) supergene family,[21] plays an important role in the recruitment of various inflammatory cells, antigen presentation and lymphocyte cytotoxicity.[22] Inflammatory cytokines, such as TNF-α and IL-1β enhance the expression of ICAM-I on hepatocytes.[23] Levels of sICAM-1 are increased in inflammatory conditions of the liver indicating they might be related to the severity of liver disease or response to treatment.[24] Our demonstration of elevated sICAM-1 levels may be explained by the cytokine-induced upregulation of ICAM-1 expression by lymphocyte infiltration and inflamed or damaged hepatocytes. These findings suggest that elevated levels of circulating sICAM-1 could be linked with cytokine-mediated liver inflammatory process in AIH.
Of note, serum sICAM-1 or IP-10 levels correlated with circulating l M2BPGi levels in the present study. Previous studies demonstrated that M2BPGi was a prognostic factor for liver fibrosis in various chronic liver diseases.[9] However, the interactions of serum M2BPGi and levels of serum cytokines in autoimmune liver diseases remain unclear. To the best of our knowledge, this is the 1st study to investigate the relationship between serum M2BPGi and proinflammatory cytokines in patients with liver diseases. Our data indicate that serum M2BPGi values are elevated in patients with AIH, and that they correlate with serum IP-10 and sICAM-1 levels, which are involved in promoting active AIH from inactive AIH. Furthermore, elevated serum M2BPGi levels were rapidly reduced following immunosuppressive treatment in AIH patients. These findings suggest that M2BPGi may not be just a liver fibrosis marker but also a useful biomarker for immune-mediated hepatic injury. Umemura et al reported that M2BPGi levels were significantly correlated with histological activity grade in PBC patients.[12] Mishikawa et al demonstrated that M2BPGi was a useful predictor for liver histological findings in patients with AIH.[13] Our results demonstrating that M2BPGi values correlated with liver inflammation in AIH are consistent with the finding of Nishikawa et al.[12] In our study, elevated levels of the liver-specific biomarker M2BPGi were not increased in patients with SLE, another autoimmune disorder. However, M2BPGi values correlated with the circulating levels of IP-10 and sICAM-1 in SLE patients. These results may increase our understanding of the role of M2BPGi in autoimmune disease and its implications in the cytokine network. M2BPGi is a useful biomarker for liver fibrosis in chronic liver disease.[25] It is unclear whether circulating M2BPGi values are influenced by immune-mediated hepatic inflammation in AIH. Our data confirmed that circulating M2BPGi values correlated with autoimmune-mediated liver damage in AIH and that its levels were rapidly reduced by steroid treatment. These findings suggest that M2BPGi is likely to reflect other factors not limited to liver fibrosis such as hepatic inflammation or injury. Hepatic stellate cells (HSCs) secrete M2BPGi, which may serve as a messenger between HSCs and Kupffer cells via Mac2, which is expressed in Kupffer cells.[26] Therefore, M2BPGi is a surrogate marker for assessing HSC activation. Our findings suggest that cytokines/chemokines are implicated in HSC activation, M2BPGi induction and the interaction between HSCs and Kupffer cells via M2BPGi. These findings also suggest that M2BPGi levels are influenced by hepatic inflammation and decreased following immunosuppressive treatment reflecting immune-mediated liver inflammation and damage in AIH.
There were limitations in our study. First, this was a retrospective study. Second, liver biopsy has a limitation of sampling error for assessing the degree of fibrosis and liver inflammation. Third, our sample size was relatively small for analysis.
5. Conclusion
Serum levels of M2BPGi were increased in patients with untreated AIH and were decreased following steroid treatment resulting in a clinical improvement. This marker might reflect liver fibrosis as well as autoimmune-mediated hepatic inflammation in parallel to the induction of hepatitis-related inflammatory cytokines.
Author contributions
Conceptualization: Kiyoshi Migita.
Data curation: Yoshiro Horai, Hideko Kozuru, Seigo Abiru, Kazumi Yamasaki, Atsumasa Komori, Yuya Fujita, Tomoyuki Asano, Shuzo Sato, Eiji Suzuki, Naoki Matsuoka, Hiroko Kobayashi, Hiroshi Watanabe, Atsushi Naganuma, Noriaki Naeshiro, Kaname Yoshizawa, Hajime Ohta, Hironori Sakai, Masaaki Shimada, Hideo Nishimura, Minoru Tomizawa, Keisuke Ario, Haruhiko Yamashita, Hiroshi Kamitsukasa, Hiroshi Kohno.
Formal analysis: Tomohiro Koga.
Investigation: Minoru Nakamura, Hiroshi Furukawa.
Supervision: Atsushi Takahashi, Atsushi Kawaami, Hiromasa Ohira, Hiroshi Yatsuhashi.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, IP-10 = interferon-γ-inducible protein 10, M2BPGi = Mac-2 binding protein glycosylation isomer, NHO = National Hospital Organization, sICAM-1 = soluble intercellular adhesion molecule-1.
Ethical Approval and Consent to participate: The study was approved by the Ethics Committee of the NHO Central Internal Review Board and participating NHO liver-network hospitals (H30-0313001). Written informed consent was obtained from each individual.
Consent for publication: Not applicable.
Funding: This work was supported by a grant from the National Hospital Organization.
Conflict of Interest: KM has received research grants from Chugai, Pfizer, and AbbVie. Rest of the authors declares that they have no competing interests.
References
- [1].Krawitt EL. Autoimmune hepatitis. N Engl J Med 2006;354:54–66. [DOI] [PubMed] [Google Scholar]
- [2].Ohira H, Abe K, Takahashi A, et al. Autoimmune hepatitis: recent advances in the pathogenesis and new diagnostic guidelines in Japan. Intern Med 2015;54:1323–8. [DOI] [PubMed] [Google Scholar]
- [3].Miyake Y, Iwasaki Y, Terada R, et al. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. J Hepatol 2005;43:951–7. [DOI] [PubMed] [Google Scholar]
- [4].Migita K, Watanabe Y, Jiuchi Y, et al. Evaluation of risk factors for the development of cirrhosis in autoimmune hepatitis: Japanese NHO-AIH prospective study. J Gastroenterol 2011;46Suppl 1:56–62. [DOI] [PubMed] [Google Scholar]
- [5].Migita K, Watanabe Y, Jiuchi Y, et al. Japanese NHO-Liver-network study group Hepatocellular carcinoma and survival in patients with autoimmune hepatitis (Japanese National Hospital Organization-autoimmune hepatitis prospective study). Liver Int 2012;32:837–44. [DOI] [PubMed] [Google Scholar]
- [6].Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–63. [DOI] [PubMed] [Google Scholar]
- [7].Longhi MS, Ma Y, Mieli-Vergani G, et al. Aetiopathogenesis of autoimmune hepatitis. J Autoimmun 2010;34:7–14. [DOI] [PubMed] [Google Scholar]
- [8].Kamijo A, Yoshizawa K, Joshita S, et al. Cytokine profiles affecting the pathogenesis of autoimmune hepatitis in Japanese patients. Hepatol Res 2011;41:350–7. [DOI] [PubMed] [Google Scholar]
- [9].Toshima T, Shirabe K, Ikegami T, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol 2015;50:76–84. [DOI] [PubMed] [Google Scholar]
- [10].Abe M, Miyake T, Kuno A, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol 2015;50:776–84. [DOI] [PubMed] [Google Scholar]
- [11].Morio K, Imamura M, Daijo K, et al. Wisteria floribunda agglutinin positive Mac-2-binding protein level increases in patients with acute liver injury. J Gastroenterol 2017;52:1252–7. [DOI] [PubMed] [Google Scholar]
- [12].Umemura T, Joshita S, Sekiguchi T, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol 2015;110:857–64. [DOI] [PubMed] [Google Scholar]
- [13].Nishikawa H, Enomoto H, Iwata Y, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res 2016;46:613–21. [DOI] [PubMed] [Google Scholar]
- [14].Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929–38. [DOI] [PubMed] [Google Scholar]
- [15].Koga T, Migita K, Sato S, et al. Multiple serum cytokine profiling to identify combinational diagnostic biomarkers in attacks of familial Mediterranean fever. Medicine 2016;95:e3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Breiman L. Random forests. Mach Learn 2001;45:5–32. [Google Scholar]
- [17].Kokkonen H, Soderstrom I, Rocklov J, et al. Up-regulation of cytokines and hemokines predates the onset of rheumatoid arthritis. Arthritis Rheum 2010;62:383–91. [DOI] [PubMed] [Google Scholar]
- [18].Yamasaki K, Tateyama M, Abiru S, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology 2014;60:1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513–20. [PubMed] [Google Scholar]
- [20].Liberal R, Longhi MS, Mieli-Vergani G, et al. Pathogenesis of autoimmune hepatitis. Best Pract Res Clin Gastroenterol 2011;25:653–64. [DOI] [PubMed] [Google Scholar]
- [21].Staunton DE, Dustin ML, Erickson HP, et al. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 1990;61:243–54. [DOI] [PubMed] [Google Scholar]
- [22].Meyer DM, Dustin ML, Carron CP. Characterization of intercellular adhesion molecule-1 ectodomain (sICAM-1) as an inhibitor of lymphocyte function-associated molecule-1 interaction with ICAM-1. J Immunol 1995;155:3578–84. [PubMed] [Google Scholar]
- [23].Kaplanski G, Farnarier C, Payan MJ, et al. Increased levels of soluble adhesion molecules in the serum of patients with hepatitis C. Correlation with cytokine concentrations and liver inflammation and fibrosis. Dig Dis Sci 1997;42:2277–84. [DOI] [PubMed] [Google Scholar]
- [24].Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol 2001;35:582–9. [DOI] [PubMed] [Google Scholar]
- [25].Ito K, Murotani K, Nakade Y, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels and liver fibrosis: a meta-analysis. J Gastroenterol Hepatol 2017;32:1922–30. [DOI] [PubMed] [Google Scholar]
- [26].Bekki Y, Yoshizumi T, Shimoda S, et al. Hepatic stellate cells secreting WFA+ -M2BP: Its role in biological interactions with Kupffer cells. J Gastroenterol Hepatol 2017;32:1387–93. [DOI] [PubMed] [Google Scholar]


