Abstract
To investigate the feasibility of intravoxel incoherent motion (IVIM) diffusion-weighted imaging (DWI) and diffusion kurtosis imaging (DKI) in discriminating soft tissue sarcoma from vascular anomalies.
Twenty-two patients with lower extremity soft tissue sarcoma and 15 patients with lower extremity vascular anomalies underwent IVIM-DWI and DKI. IVIM model generated true diffusion (D), perfusion fraction (f), and pseudo-diffusion coefficient (D∗). DKI model generated mean kurtosis (MK) and mean diffusion (MD). These parameters were measured by 2 radiologists separately through drawing region of interest. Intraclass correlation coefficient (ICC) was calculated to evaluate the inter-reader viability in measurement. The Mann–Whitney test was used to compare the parameters between vascular anomalies and soft tissue sarcoma. Receiver operating characteristic curves were constructed for assessing diagnostic accuracies.
ICC was more than 0.8 for apparent diffusion coefficient (ADC), D, D∗, f, MK, and MD. Mean ADC, D, and MD were significantly lower in soft tissue sarcoma versus vascular anomalies (P < .05). Mean D∗ and f were not significantly different (P > .05). Soft tissue sarcoma had significantly higher MK than vascular anomalies (P < .05). Areas under curve for ADC, D, MK, and MD were 0.876, 0.885, 0.894, and 0.812, respectively.
IVIM and DKI are feasible in discriminating soft tissue sarcoma from vascular anomalies.
Keywords: diffusion kurtosis imaging, intravoxel incoherent motion, lower extremity, soft tissue sarcoma, vascular anomalies
1. Introduction
Discrimination of benign and malignant lesions is important for formulating treatment plans. Cell density, vascularization, and structure complexity differ between benign and malignant lesions,[1–4] so could be used for discrimination. Besides pathology, noninvasive imaging techniques were able to provide correlated information.[5–8] For example, apparent diffusion coefficient (ADC) that partly reflects tumor cell density could be measured with conventional diffusion-weighted imaging (DWI).
Since ADC is affected by both water molecules diffusion and microcirculation perfusion, intravoxel incoherent motion (IVIM) model can be used to separate diffusion from perfusion.[9] Water molecular true diffusion (D), pseudo-diffusion coefficient (D∗), and perfusion fraction (f) are generated with IVIM model. D∗ and f can reflect vascularization of tumor. As diffusion deviates from Gauss distribution at high b-values,[10] diffusion kurtosis imaging (DKI) model was raised to deal with non-Gauss distribution. Kurtosis is generated with DKI model. Mean kurtosis (MK) can reflect complexity of tissue structure.[11]
The feasibility of IVIM and DKI in differentiating tumors has been proved by many studies.[2,8,12,13] However, there is few studies evaluating lower extremity soft tissue sarcomas with IVIM and DKI. The purpose of the current study is to use IVIM and DKI to discriminate soft tissue sarcoma from vascular anomalies that are the most common soft tissue lesions in the lower extremities.
2. Materials and methods
2.1. Patients
This study was approved by the university Institutional Review Broad. Informed consent was obtained from each patient before study. Inclusion criteria were that lower extremity vascular anomalies confirmed by pathology, lower extremity soft tissue sarcoma confirmed by pathology, and IVIM and DKI performed before operation/biopsy. Exclusion criteria were chemotherapy/radiotherapy (for tumors) performed before MRI, sclerotherapy (for vascular anomalies) performed before MRI. Twenty-two patients with lower extremity soft tissue sarcomas and 15 patients with lower extremity vascular anomalies during January 2016 and December 2017 were analyzed.
A whole body 3T scanner (Skyra, Siemens, Erlangen, Germany) was used for scanning. IVIM-DWI and DKI were performed on the transverse plane. The main parameters of IVIM were as follows: Repetition time (TR), 3000 milliseconds; echo time (TE), 61 milliseconds; field of view (FOV), 20 × 20 cm or greater for fitting tumor size; thickness, 4 mm; number of slice, 14 or more; matrix, 120 × 120; and Grappa = 2; b = 0, 10, 20, 30, 40, 50, 75, 100, 150, 200, 400, 700, 1000, and 1500 s/mm2. The main parameters of DKI were as follows: TR, 3370 milliseconds; TE, 68 milliseconds; FOV, 22 × 22 cm or greater for fitting tumor size; thickness, 4 mm; number of slices, 14 or more; matrix, 160 × 160; Grappa = 2; directions, 3; and b = 0, 100, 700, 1400, and 2100 s/mm2.
2.2. Data analysis
A noncommercial software (DKI_tool_3_4) was used for data postprocessing. ADC map, D map, D∗ map, and f map were generated with IVIM. MK map and mean diffusion (MD) map were generated with DKI. Region of interest (ROI) with different areas (4.78–112.63 mm2) were separately drawn by 2 radiologists with 7 and 9 years’ experience. ROI was first drawn on b = 0 map, and then copied to other parameter maps. ROI should be placed on the solid part of tumor, avoiding liquefied necrosis, bleeding area or vessels. ROI were drawn on the slice that had the largest tumor size, as well as 4 slices above and below.
2.3. Statistics analysis
SPSS (version 22.0, IBM, New York) was used to perform all statistical analysis. Intraclass correlation coefficient (ICC) was calculated to determine inter-reader viability in measurement of ADC, D, f, D∗, MK, and MD; 0.8 to 0.99 was considered excellent inter-reader reproducibility; 0.4 to 0.79 was considered acceptable reproducibility. ADC, D, f, D∗, MK, and MD were compared between soft tissue sarcoma and vascular anomalies using a Mann–Whitney test. Receiver operating characteristic (ROC) curves were constructed for assessing diagnostic accuracies. P values < .05 were considered as significant differences.
3. Results
Thirty-seven patients (mean age = 37.4 ± 12.5 years, male:female = 20:17) with soft tissue sarcoma (n = 22) or vascular anomalies (n = 15) in the lower extremities during January 2016 and December 2017 were included. Soft tissue sarcomas were as follows: fibrosarcoma (n = 7), synovial sarcoma (n = 2), myofibrosarcoma (n = 4), leiomyosarcoma (n = 2), epitheliosarcoma (n = 1), rhabdomyosarcoma (n = 2), and liposarcoma (n = 4).
ICC for ADC, D, f, D∗, MK, and MD were 0.84, 0.85, 0.82, 0.83, 0.88, and 0.86, respectively. As all ICC above 0.8, only the results from Reader 1 were reported in the following text for the sake of simplicity.
Mean ADC, D, f, D∗, MK, and MD of soft tissue sarcoma and vascular anomalies are shown in Table 1. ADC, D, and MD were significantly lower in soft tissue sarcoma versus vascular anomalies (P < .05, see Table 1). MK of soft tissue sarcoma was significantly higher than that of vascular anomalies (0.82 ± 0.56 vs 0.43 ± 0.32, P < .05). D∗ or f was not significantly different (P > .05, see Table 1).
Table 1.
Intravoxel incoherent motion/diffusion kurtosis imaging parameters were compared between vascular anomalies and soft tissue sarcoma using a Mann–Whitney test.

Area under curve (AUC), cutoff, sensitivity, and specificity for ADC, D, MK, and MD are shown in Table 2. MK had the highest AUC. D had the highest sensitivity.
Table 2.
Diagnostic accuracies for ADC, D, MK, and MD.
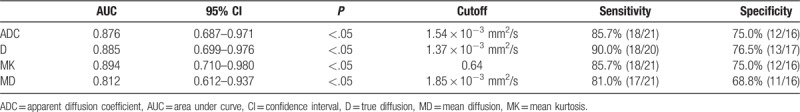
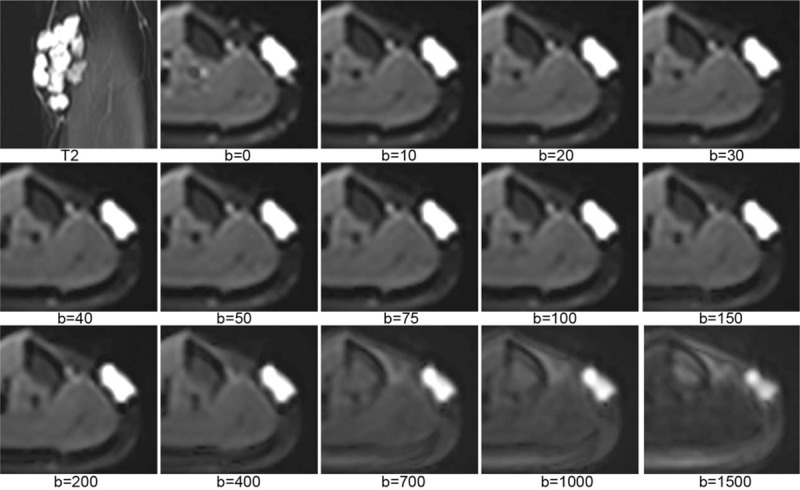
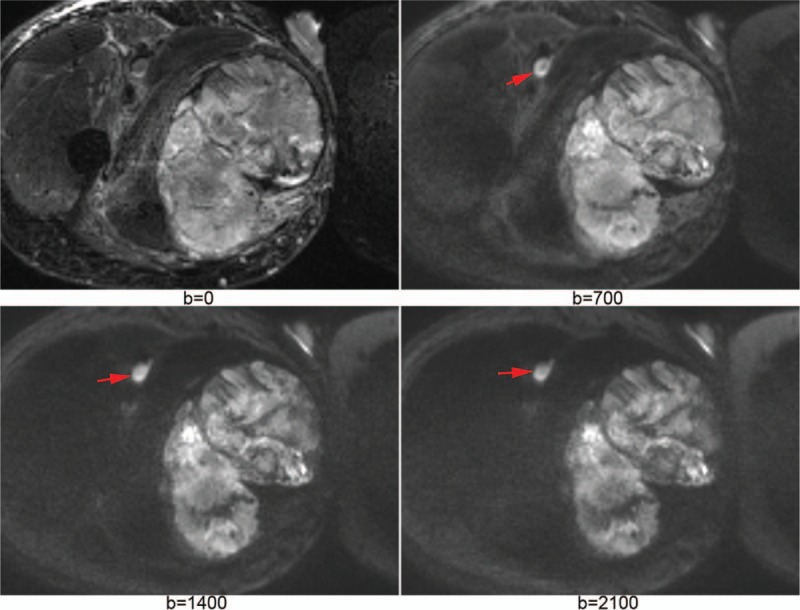
Figure 1 shows IVIM images of a vascular anomaly. Figure 2 shows DKI images of a rhabdomyosarcoma. Figure 3 shows source images and calculated parameter maps of a fibrosarcoma. Figures 4 and 5 show ROC curves for IVIM and DKI, respectively.
Figure 1.
The first image (saggital FSE T2) displayed a vascular anomaly at left calf. The others were intravoxel incoherent motion images with b values from 0 to 1500 s/mm2. As b value increased, signal intensity of the lesion decreased.
Figure 2.
A rhabdomyosarcoma of right thigh was well displayed on diffusion kurtosis imaging. On b = 2100 s/mm2 map, the tumor was still hyperintensity, as well as metastatic lymph node (arrows), while the normal muscles were very low signal.
Figure 3.
A fibrosarcoma at right hip was well displayed on b = 0 map (A) and b = 700 s/mm2 map (B). Apparent diffusion coefficient map (C) and D map (D) were generated with intravoxel incoherent motion model. Mean kurtosis map (E) and mean diffusion map (F) were generated with diffusion kurtosis imaging model.
Figure 4.
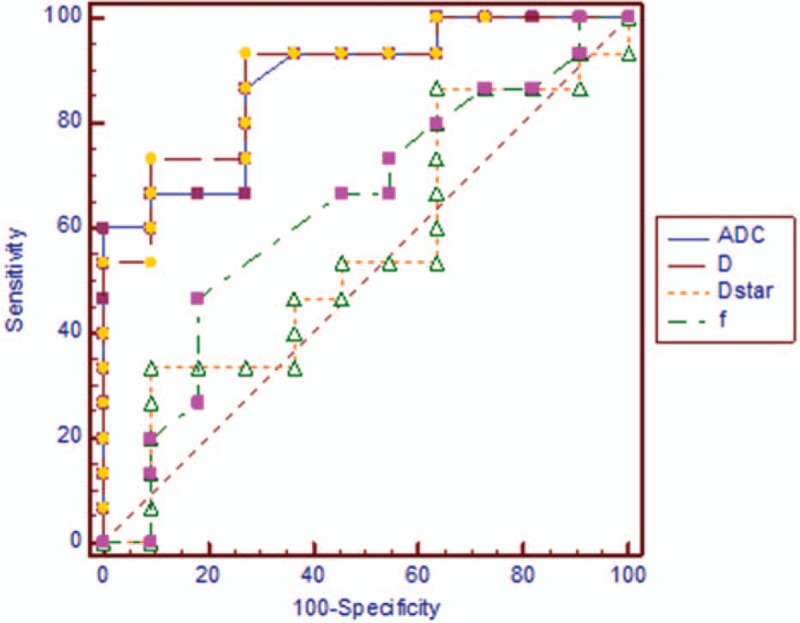
Receiver operating characteristic curves for intravoxel incoherent motion. Apparent diffusion coefficient and D were better than f and D∗ in discriminating soft tissue sarcoma from vascular anomalies.
Figure 5.
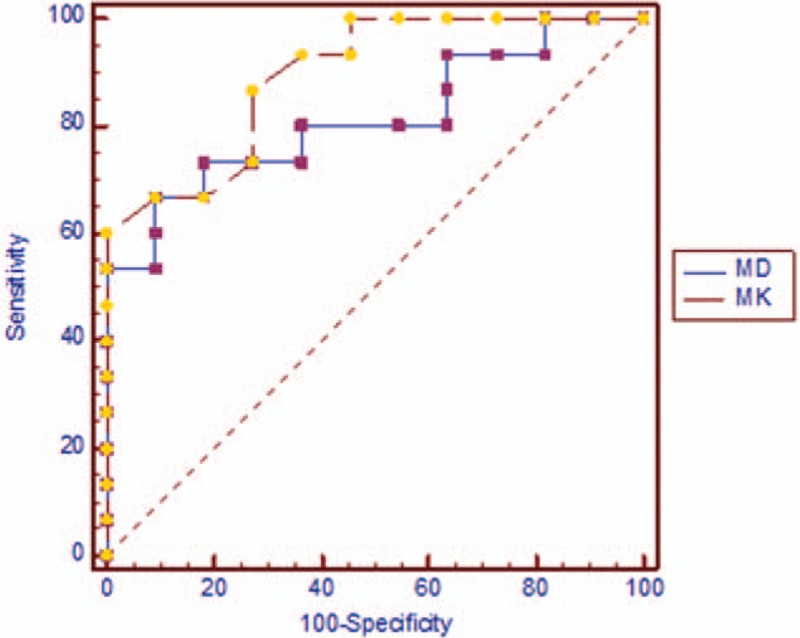
Receiver operating characteristic curves for diffusion kurtosis imaging. Mean kurtosis was better than mean diffusion in the discrimination of soft tissue sarcoma and vascular anomalies.
4. Discussion
The current study investigated the feasibility of IVIM and DKI in discriminating soft tissue sarcoma from vascular anomalies. The most important findings were that ADC, D, MD, and MK were significantly different between soft tissue sarcoma and vascular anomalies; D and MK were accurate in discriminating soft tissue sarcoma from vascular anomalies.
IVIM model describes true water molecular diffusion with D. Pseudo-diffusion caused by blood movement in capillaries[14] is described with D∗. Perfusion fraction is described with f, which reflects abundance of capillaries. Both D∗ and f are related to vascularization.[15] DKI model has solved the problem of non-Gauss distribution at high b-values, and can reflect structure complexity of tissue.[16] Diffusion heterogeneity can be assessed with MK. The greater the MK, the more complex the structure.[17]
We found that ADC, MD, and D were lower in soft tissue sarcoma versus vascular anomalies. A possible explanation is that soft tissue sarcomas have higher cell density and narrower extracellular space,[18] which result in more restricted diffusion. We observed that D was lower than ADC in every case. D has eliminated the influence of perfusion, while ADC still includes perfusion contribution. True diffusion within tumor can be described with D only. Our study found that AUC of ADC was higher than that of MD. A possible explanation is that IVIM used more b values than DKI. The accuracy in ADC calculation could be improved with more b values.[19]
Most publications considered IVIM model necessary for highly vascularized lesions, as IVIM could separate true diffusion from pseudo-diffusion.[20] Our study results supported this view. We observed that D∗ was higher than D by an order of magnitude in most cases. Soft tissue sarcomas with rich blood supply are generally highly vascularized. Vascular anomalies are also highly vascularized due to numerous dysplastic capillaries. Thus perfusion-related pseudo-diffusion effect could not be neglected for them, highlighting the importance of IVIM. We found that D had higher AUC than ADC. Thus it can be concluded that IVIM-DWI is more suitable than conventional DWI in the discrimination of soft tissue sarcoma and vascular anomalies.
There was no significant difference in D∗ or f between soft tissue sarcoma and vascular anomalies. One possible explanation is that they are both rich in blood supply and highly vascularized. D∗ and f may be inaccurate in discrimination due to overlap.
We found that MK was higher in soft tissue sarcoma versus vascular anomalies. It could be indicated that diffusion of soft tissue sarcoma deviated from Gauss distribution more. Soft tissue sarcoma may have more complex structure compared with vascular anomalies. We found that MK had the highest AUC, while D had the highest sensitivity and specificity. MK and D seemed most accurate in discriminating soft tissue sarcoma from vascular anomalies.
Our study has some limitations. First, the sample size is small. As lower extremity soft tissue sarcomas are uncommon diseases, we only collected 22 cases during nearly 2 years. We need to collect more cases in future to strength the statistical power. Second, other soft tissue tumors were not analyzed in this study, because we focused on the evaluation of soft tissue sarcoma. Third, we did not perform a comparison between malignant and benign soft tissue tumors. Such a study may include more patients. However, multiple categories in soft tissue tumor family make such studies heterogeneous.
In conclusion, IVIM and DKI are suitable for discriminating soft tissue sarcoma from vascular anomalies. MK and D are accurate for the discrimination.
Author contributions
Conceptualization: Gang Wu, Xiaoming Li.
Data curation: Gang Wu, Xuanlin Liu, Yan Xiong, Jun Ran, Xiaoming Li.
Formal analysis: Gang Wu, Xuanlin Liu, Xiaoming Li.
Funding acquisition: Xiaoming Li.
Investigation: Gang Wu, Xuanlin Liu, Yan Xiong, Jun Ran, Xiaoming Li.
Methodology: Gang Wu, Yan Xiong, Jun Ran.
Project administration: Xuanlin Liu, Jun Ran, Xiaoming Li.
Resources: Yan Xiong.
Software: Gang Wu, Xuanlin Liu, Yan Xiong, Jun Ran.
Supervision: Yan Xiong, Xiaoming Li.
Validation: Gang Wu, Xuanlin Liu, Xiaoming Li.
Visualization: Jun Ran.
Writing – original draft: Gang Wu, Xiaoming Li.
Writing – review & editing: Xuanlin Liu, Xiaoming Li.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, AUC = area under curve, DKI = diffusion kurtosis imaging, DWI = diffusion-weighted imaging, ICC = intraclass correlation coefficient, IVIM = intravoxel incoherent motion, MD = mean diffusion, MK = mean kurtosis, ROC = receiver operating characteristic, ROI = region of interest.
Funding: National Natural Science Foundation of China (NSFC, No. 31630025, 81571643 and 81320108013).
The authors have no conflicts of interest to disclose.
References
- [1].Balliu E, Vilanova JC, Pelaez I, et al. Diagnostic value of apparent diffusion coefficients to differentiate benign from malignant vertebral bone marrow lesions. Eur J Radiol 2009;69:560–6. [DOI] [PubMed] [Google Scholar]
- [2].Park S, Kwack KS, Chung NS, et al. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging of focal vertebral bone marrow lesions: initial experience of the differentiation of nodular hyperplastic hematopoietic bone marrow from malignant lesions. Skeletal Radiol 2017;46:675–83. [DOI] [PubMed] [Google Scholar]
- [3].Muller U, Kubik-Huch RA, Ares C, et al. Is there a role for conventional MRI and MR diffusion-weighted imaging for distinction of skull base chordoma and chondrosarcoma? Acta Radiol 2016;57:225–32. [DOI] [PubMed] [Google Scholar]
- [4].Oh E, Yoon YC, Kim JH, et al. Multiparametric approach with diffusion-weighted imaging and dynamic contrast-enhanced MRI: a comparison study for differentiating between benign and malignant bone lesions in adults. Clin Radiol 2017;72:552–9. [DOI] [PubMed] [Google Scholar]
- [5].Douis H, Davies MA, Sian P. The role of diffusion-weighted MRI (DWI) in the differentiation of benign from malignant skeletal lesions of the pelvis. Eur J Radiol 2016;85:2262–8. [DOI] [PubMed] [Google Scholar]
- [6].Ahlawat S, Khandheria P, Subhawong TK, et al. Differentiation of benign and malignant skeletal lesions with quantitative diffusion weighted MRI at 3T. Eur J Radiol 2015;84:1091–7. [DOI] [PubMed] [Google Scholar]
- [7].Cao J, Xiao L, He B, et al. Diagnostic value of combined diffusion-weighted imaging with dynamic contrast enhancement MRI in differentiating malignant from benign bone lesions. Clin Radiol 2017;72:793.e1–9. [DOI] [PubMed] [Google Scholar]
- [8].Lim HK, Jee WH, Jung JY, et al. Intravoxel incoherent motion diffusion-weighted MR imaging for differentiation of benign and malignant musculoskeletal tumours at 3 T. Br J Radiol 2018;91:20170636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505. [DOI] [PubMed] [Google Scholar]
- [10].Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005;53:1432–40. [DOI] [PubMed] [Google Scholar]
- [11].Zhang YD, Wu CJ, Bao ML, et al. New RESOLVE-based diffusional kurtosis imaging in MRI-visible prostate cancer: effect of reduced b value on image quality and diagnostic effectiveness. Am J Roentgenol 2016;207:330–8. [DOI] [PubMed] [Google Scholar]
- [12].Sun K, Chen X, Chai W, et al. Breast cancer: diffusion kurtosis MR imaging-diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 2015;277:46–55. [DOI] [PubMed] [Google Scholar]
- [13].Nogueira L, Brandao S, Matos E, et al. Application of the diffusion kurtosis model for the study of breast lesions. Eur Radiol 2014;24:1197–203. [DOI] [PubMed] [Google Scholar]
- [14].Mao W, Zhou J, Zeng M, et al. Chronic kidney disease: pathological and functional evaluation with intravoxel incoherent motion diffusion-weighted imaging. J Magn Reson Imaging 2018;47:1251–9. [DOI] [PubMed] [Google Scholar]
- [15].Gao Q, Lu S, Xu X, et al. Quantitative assessment of hyperacute cerebral infarction with intravoxel incoherent motion MR imaging: initial experience in a canine stroke model. J Magn Reson Imaging 2017;46:550–6. [DOI] [PubMed] [Google Scholar]
- [16].Sheng RF, Wang HQ, Jin KP, et al. Histogram analyses of diffusion kurtosis indices and apparent diffusion coefficient in assessing liver regeneration after ALPPS and a comparative study with portal vein ligation. J Magn Reson Imaging 2018;47:729–36. [DOI] [PubMed] [Google Scholar]
- [17].Wang F, Jin D, Hua XL, et al. Investigation of diffusion kurtosis imaging for discriminating tumors from inflammatory lesions after treatment for bladder cancer. J Magn Reson Imaging 2018;48:259–65. [DOI] [PubMed] [Google Scholar]
- [18].Age B. CT and MRI findings of soft tissue adult fibrosarcoma in extremities. Biomed Res Int 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Koyama H, Ohno Y, Seki S, et al. Value of diffusion-weighted MR imaging using various parameters for assessment and characterization of solitary pulmonary nodules. Eur J Radiol 2015;84:509–15. [DOI] [PubMed] [Google Scholar]
- [20].Marzi S, Stefanetti L, Sperati F, et al. Relationship between diffusion parameters derived from intravoxel incoherent motion MRI and perfusion measured by dynamic contrast-enhanced MRI of soft tissue tumors. NMR Biomed 2016;29:6–14. [DOI] [PubMed] [Google Scholar]


