Abstract
Background:
Shexiang Baoxin Pill (SBP) is one of the most commonly used traditional Chinese patent medicines for cardiovascular diseases. This systematic review was designed to provide rigorous therapeutic efficacy and safety evidence on the use of SBP combined with trimetazidine in elderly patients with heart failure (HF) secondary to ischaemic cardiomyopathy (ICM).
Methods:
Relevant randomized controlled trials (RCTs) investigating the clinical efficacy of SBP combined with trimetazidine in treating ICM-associated HF were widely searched in electronic databases, including PubMed, Cochrane library, EMBASE, CBM, CNKI, VMIS, and Wanfang up to January 1, 2018. The methodological quality of each trial was assessed according to the Cochrane Reviewers’ Handbook 5.0. Meta-analysis was performed by using Review Manager 5.3.
Results:
Eighteen RCTs (N = 1532) that met the criteria were included in the review for the assessment of methodological quality. Meta-analysis showed that, when compared with conventional therapy, SBP combined with trimetazidine significantly improved the clinical efficacy and indices of cardiac function (including increasing left ventricular ejection fraction [LVEF] and 6-minute walk distance [6-MWD], decreasing left ventricular end-diastolic diameter [LVEDD] and left ventricular end-systolic diameter [LVESD]) without serious adverse reactions.
Conclusion:
This work provides evidence of the benefit of SBP combined with trimetazidine for the treatment of HF secondary to ICM. More high quality and well-designed RCTs are needed to confirm these findings.
Keywords: heart failure, ischaemic cardiomyopathy, meta-analysis, Shexiang Baoxin Pill, trimetazidine
1. Introduction
Ischaemic cardiomyopathy (ICM) is one of the cardiovascular diseases defined as diffuse akinesis of the left ventricle with systolic dysfunction caused by chronic myocardial ischaemia.[1] To date, ICM is still the most common cause of heart failure (HF), accounting for approximately 60% of cases worldwide.[2] Due to the terminal stage of various heart diseases, it is believed that HF is associated with a significantly higher rate of mortality and morbidity in human beings.[3] Despite the multiple remarkable advances in interventional cardiology and modern optimal medical therapy for the treatment of ICM-related HF, patients with ICM have a poor prognosis even after comprehensive revascularization and especially with LV systolic dysfunction.[1] One of the most important challenges for drug advancement is the improvement of a more effective and rational drug for ICM due to HF.
Over the last few decades, traditional Chinese medicine (TCM) has provided opportunities for the treatment of various diseases. Simultaneously, integrative medicine has emerged as an increasingly useful and complementary approach to allopathic medicine. As a result, TCM as a whole medical system has become an accepted and integral component of integrative medicine.[4] Shexiang Baoxin Pill (SBP), a treasured TCM formula for cardiovascular diseases, is derived from a classical TCM named Suhexiang Pill prescription recorded in Prescriptions of the Bureau of Taiping People's Welfare Pharmacy. There are 7 substances present in SBP, including Moschus, Radix Ginseng, Cortex Cinnamomi, Borneolum Syntheticum, Styrax, Calculus Bovis, and Venenum Bufonis.[5] Moschus is the main component with notable activity and lower adverse effects in the treatment of angina pectoris and chest tightness.[6] It has been widely used for the treatment of coronary heart disease and myocardial ischaemia in clinics.[7] SBP has been widely used in the treatment of coronary heart disease and myocardial ischaemia.[8] Recent years, a number of studies have shown SBP's characteristics on attenuating mitochondrial injury of cardiomyocytes and the mechanisms are related to anti-inflammatory, anti-oxidative stress, improving lipid metabolism and protecting mitochondrial function.[9,10] Thus, SBP exhibited prominent therapeutic effects on cardiovascular disease and metabolic syndrome.
Trimetazidine is one of the anti-angina pectoris cardiovascular drugs. A large number of preliminary studies have shown that trimetazidine maintains normal energy metabolism in ischaemic or hypoxic cells and increases reduced levels of intracellular ATP. Moreover, trimetazidine reduces left ventricular work load and improves both the clinical condition and quality of life of elderly patients with ischaemic heart disease.[11] A previous study preliminarily demonstrated that SBP has a cardio-protective function by simultaneously reducing the area of myocardial infarction area and promoting angiogenesis.[12] In recent years, an increasing number of references have reported the clinical efficacy of combined SBP with trimetazidine in elderly patients with ICM and HF without serious adverse events or reactions.[13] Nevertheless, until recently, there has been a lack of comprehensive evidence to support the efficacy and safety of SBP. Therefore, to promote the rational application of Chinese patent medicine in clinical practice, this research aimed to evaluate the benefits efficacy and side effects of SBP therapy for ICM based on results from randomized controlled trials (RCTs).
This study was performed to assess whether SBP combined with trimetazidine is associated with improved therapeutic efficacy and cardiac function indexes, including left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD). Simultaneously, 6-minute walk distance (6-MWD), plasma brain natriuretic peptide (BNP) level, N-terminal pro-brain natriuretic peptide (NT-ProBNP), serum hypersensitive C-reactive protein (hs-CRP), and side effects are discussed to identify the appropriate scientific evidence regarding SBP in the treatment of ICM with HF. This study may also independently provide more useful evidence that will stimulate further research on the therapeutic value of SBP. Thus, an extended meta-analysis with detailed outcomes regarding clinical efficacy and adverse reactions is reported (Fig. 1).
Figure 1.
Review process of the whole study.
2. Materials and methods
2.1. Protocol and registration
This manuscript had been registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/#joinuppage) and the registration number is: CRD42018087688.
2.2. Ethics approval and consent to participate
Due to this study does not involve animal and patient experiments, the ethics approval and consent to participate are not applicable.
2.3. Search strategy
A comprehensive systematic search concerning the clinical efficacy and safety of SBP combined with trimetazidine in treating elderly patients with HF secondary to ICM was performed to identify all published RCTs from inception to January 1, 2018. The databases included PubMed, Cochrane library, EMBASE, Chinese Biomedical Database (CBM), China National Knowledge Infrastructure (CNKI), VIP medicine information system (VMIS), and Wanfang. Cochrane Library and Wiley Library were also used to retrieve related papers. All unclear questions were addressed by contacting the study authors by e-mail. The following search terms were used: “Shexiang Baoxin Pill” [Mesh terms] OR “Heart pill of musk” [Mesh terms] AND “trimetazidine” [Mesh terms] AND “heart failure” [Mesh terms] AND “ischaemic cardiomyopathy” [Mesh terms]. The searched results were downloaded for further evaluation.
2.4. Inclusion criteria
Two investigators (JXW and JW) independently examined the titles and abstracts of all searched databases to access the trials for inclusion. Based on the search strategy outlined above, the full texts of articles were retrieved if there was any doubt about including an article. A table with the English translation of all the titles and the English abstracts was reviewed by 2 other reviewers (XHL and YXY). The inclusion criteria were: randomized controlled trials (RCTs) with any length of follow-up; the diagnostic criteria of included trials were based on the internationally accepted criteria for the diagnosis of HF secondary to ICM in elderly patients; one or more outcome measures were included, such as clinical efficacy, LVEF, LVEDD, LVESD, BNP, NT-ProBNP, and 6-MWD, and adverse reactions during the scheduled treatment and follow-up. Any discrepancy in opinion regarding inclusion was resolved by consensus.
2.5. Exclusion criteria
Trials meeting the following conditions were excluded: non-RCTs; failed account of diagnostic criteria; absence of any quantitative outcome measures included in trials; others: including duplicate publications reporting the same trials, non-clinical experiments, reviews, mechanism research, or animal experiment.
2.6. Data collection process
The basic information about patients or participants such as intervention, comparisons, outcomes (total efficacy rate, safety), and type of RCTs was extracted and summarized by 2 investigators (JXW and LZ) independently. Any processes for obtaining and confirming data were carried out by 2 investigators (XHL, YXY). Each RCT was validated independently by 2 reviewers (JW, YLZ) who were blinded to the authors or results. All reviewers independently performed the screening of studies, selection, validation, and data extraction. The predesigned data extraction template was used to retrieve information from the included studies. Disagreements on the assessment of data were resolved by discussion and consensus was reached in all cases. Any unclear information would contact with authors. A number of responders and the total number of participants in the experimental and control groups for each study were extracted for dichotomous outcomes. The mean change and standard deviation for the mean in each trial were extracted along with the total number for continuous outcomes (e.g., LVEF, LVEDD, LVESD, 6-MWD).
2.7. Observation index
In this systematic review and meta-analysis, the observation indexes were clinical efficacy and safety of SBP, which were clinically relevant when evaluating the pharmacology of SBP in relation to the probable mechanisms. According to the New York Heart Association (NYHA) classification, clinical efficacy is defined on 3 levels: markedly effective rate: patients achieve complete remission or cardiac function improves above level II; effective rate: patients achieve partial remission or cardiac function improves to level I. Signs and symptoms are relieved to a certain degree; ineffective rate: patient with cardiac insufficiency improves to level I, or signs and symptoms are not significantly improved. In severe cases, death ensues. The total effective rate is equal to the markedly effective rate plus the effective rate. As for safety of SBP, all the adverse reactions of SBP reported in literature were fully recorded in this study, which may help the clinician make a right decision when using SBP for the treatment of HF secondary to ICM. That is, if the existing adverse reactions of SBP affect the patient's condition, it will be used as an appropriate regimen.
2.8. Risk of bias in individual studies
The methodological quality assessment was carried out using the Cochrane Handbook for Systematic Reviews of Interventions.[14,15] Six domains including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) were used for the methodological quality of each included trials. For all the relevant outcomes in the relevant domains, the quality of each item was classified using a nominal scale: “Yes” (low risk of bias), “No” (high risk of bias), or “Unclear” (unclear risk of bias).
2.9. Statistical analysis
Statistical analysis was performed by Review Manager 5.3 software (The Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre). For measurement data, dichotomous variables were presented as risk ratio (RR), while continuous outcomes were presented as the mean difference (MD) with 95% confidence intervals (CIs). As a quantitative measure of inconsistency, the I-square (I2) statistic was used to assess heterogeneity. A fixed effect model was performed for minor heterogeneity when I2 was <50%. Random effect model was applied when I2 was >50%. Subgroup analysis was used to evaluate the 2 combination therapy schedules of the control group. Moreover, sensitivity analysis was performed to evaluate the reliability of the meta-analysis results. Potential publication bias was evaluated using funnel plot analyses.
3. Results
3.1. Identification of eligible studies
A total of 396 articles were identified as potentially eligible records. As shown in Fig. 1, the removal of 275 duplicate publications left 121 articles for further screening. For the preserved records, 79 were excluded based on title and abstract, and 42 full articles were used for further assessment. Among the latter, 3 articles were non-RCTs, 5 papers were reviews of animal models, 2 articles were non-relevant RCTs, 6 trials were low quality, and 8 papers for other reasons were excluded. Finally, 18 studies[16–33] with 1532 patients with HF secondary to ICM who met the criteria were included in the meta-analysis. The flow diagram of the study screening is shown in Fig. 2.
Figure 2.
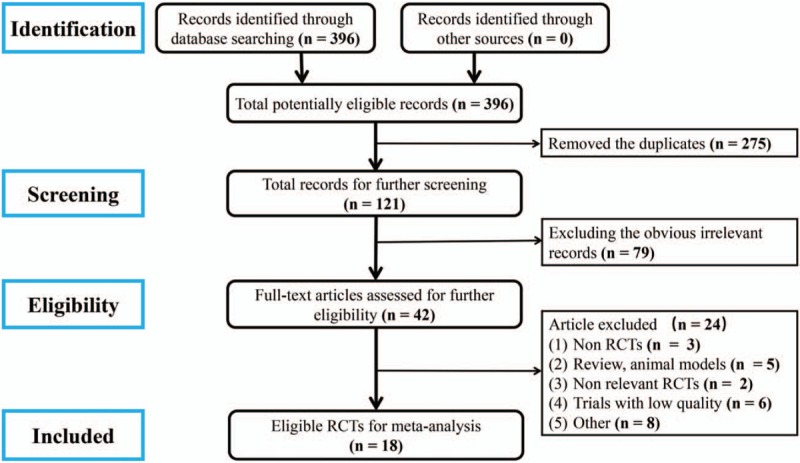
Flow diagram of study selection.
3.2. Characteristics of the included trials
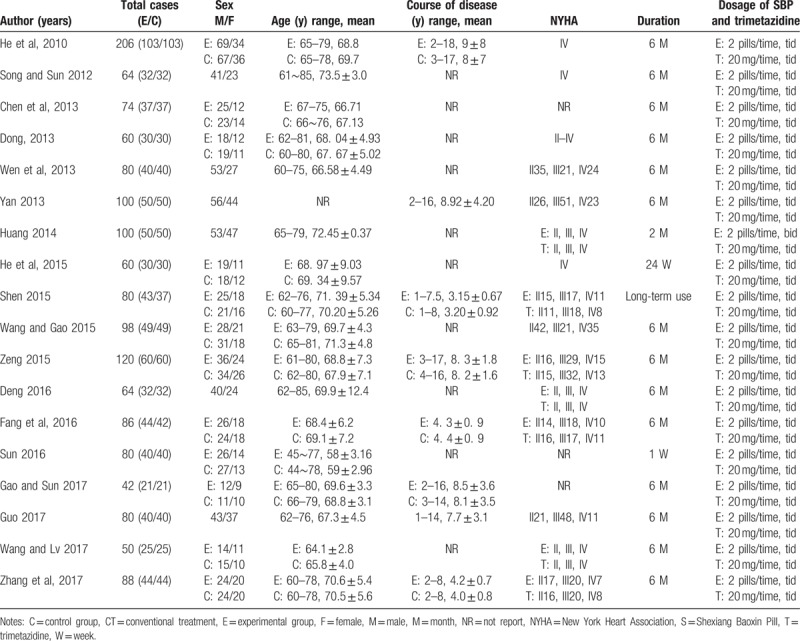
The characteristics of the 18 included studies with 1532 patients (770 patients in the experimental group and 762 patients in the control group) were investigated. As shown in Table 1, 1922 male patients accounting for 60.2% and 610 female patients accounting for 39.8% were included in this systematic review. The age of the participants ranged from 44 to 85 years. Among them, 8 studies[16,21,24,26,28,30,31,33] reported the course of the disease from 1 to 18 years. Fifteen research studies[16–17,19–28,31–33] reported the NYHA classification between II and IV according to the “Criteria Committee of the New York Heart Association.”[34] Additionally, the duration of 77.8% of the trials was 6 months. For the dosage of SBP and trimetazidine; SBP was 2 pills, 3 times a day and trimetazidine was 20 mg, 3 times a day. Finally, the intervening measures between the experimental group and the control group were clearly outlined in Table 1. Conventional treatment (CT) included nitrates, statins, angiotensin converting enzyme inhibitors (ACEI), β-receptor blockers, diuretics, and calcium channel blockers. Since trimetazidine is conventionally used for cardiovascular disease, the control group was defined as conventional treatment with or without trimetazidine. The characteristics of included studies are shown in Table 1.
Table 1.
Characteristics of included studies.
3.3. Methodological quality of included trials
The methodological quality for each included study was evaluated according to the Cochrane risk of bias estimation. All the included trials were RCTs, among which 3 trials used random number tables[30–31,33] and 2 trials that employed lottery randomization[21,25] were designated as low risk. However, 4 trials that used therapeutic randomization method[24,26,28–29] were designated as high risk in random sequence generation and allocation concealment. None of the studies reported blinding of participants and personnel or blinding of outcome assessment. Incomplete outcome data and selective reporting were performed in 1 record[16] (Fig. 3).
Figure 3.
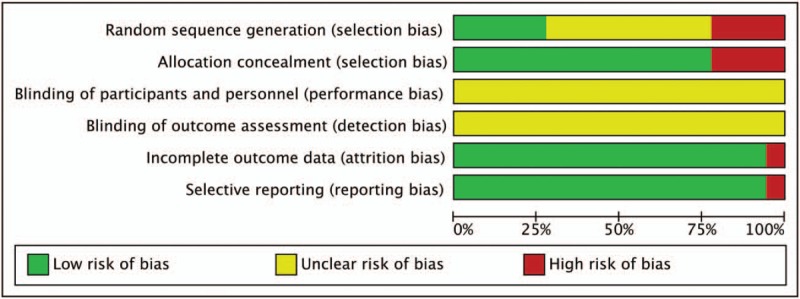
Methodological quality assessment of the risk of bias for each included study.
3.4. Clinical efficacy
The clinical efficacy was the most commonly used measure to evaluate the therapeutic efficacy of SBP combined with trimetazidine in patients with ICM. Sixteen trials[16–17,19–23,25–33] provided data comparing the clinical efficacy between the experimental and control groups (Fig. 4). A fixed-effect model was used for the meta-analysis of these studies. The results showed that the combination of SBP and trimetazidine was associated with a relatively greater improvement in the total efficacy rate in the treatment of ICM secondary to HF (RR = 1.25, 95%CI [1.19, 1.325], P < .00001).
Figure 4.
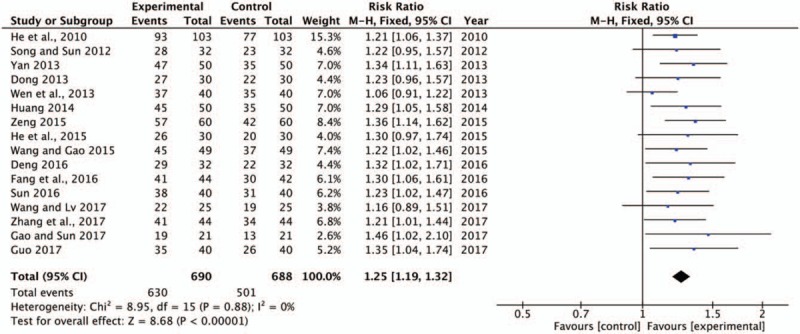
Forest plot of clinical efficacy rate comparing the experimental group and control groups. P and I2 are the criteria for the heterogeneity test, ♦ pooled risk ratio, –▪– risk ratio, and 95% CI. CI = confidence intervals.
3.5. LVEF improvement
LVEF can be stable and reliable in reflecting left ventricular function. It is an important indicator for the diagnosis of myocardial pump function and has been widely used for the assessment of HF and for the evaluation of drug efficacy in clinical diagnosis and drug research. Among the included literatures, 11[16–17,20–21,23–26,28,30–31] studies showed that the increase in LVEF was significantly better in the SBP combined with trimetazidine and conventional treatment groups than in the control group. As shown in Fig. 5, there was substantial heterogeneity in the LVEF improvement (P < .00001, I2 = 84%). A random effects model was used to pool this meta-analysis. Meta-analysis showed that the LVEF of patients in the experimental group was much higher than that in the control group (MD = 6.68, 95% CI [5.92, 7.43], P < .00001). Sensitivity analysis found that Lin et al[13] demonstrated significant heterogeneity compared with the other study.
Figure 5.
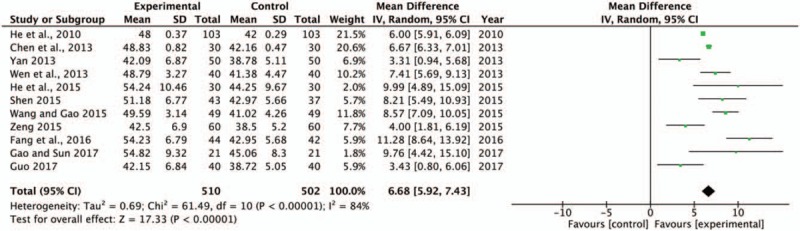
Forest plot of LVEF comparing the experimental group and control group. P and I2 are the criterion for the heterogeneity test, ♦ pooled mean difference, –▪– mean difference and 95% CI. LVEF = left ventricular ejection fraction.
3.6. The decrease of LVEDD and LVESD
Both LVEDD and LVESD are the indexes of cardiac function that obviously increase in patients with HF secondary to ICM. In this study, a total of 8[16,18,20–21,23,26,30–31] studies with 748 subjects investigated measurements of LVEDD, and a total of 5[23,24,28,30,33] trials with 356 patients assessed those of LVESD between the experimental and control groups. There was no heterogeneity of these 2 indexes, and the fixed effect model was performed for analysis (LVEDD, P = .20, I2 = 29%; LVESD, P = .80, I2 = 0%). The results showed that SBP combined with trimetazidine significantly decreased LVEDD (MD = −6.18, 95% CI, [−6.80, −5.56], P < .00001) (Fig. 6A) and LVESD (MD = −7.96, 95% CI, [−9.66, −6.25], P < .00001) compared with the control group (Fig. 6B). These 2 indicators represented the improvement in cardiac function and indicated that SBP combined with trimetazidine ameliorated cardiac function in patients with ICM-induced HF.
Figure 6.
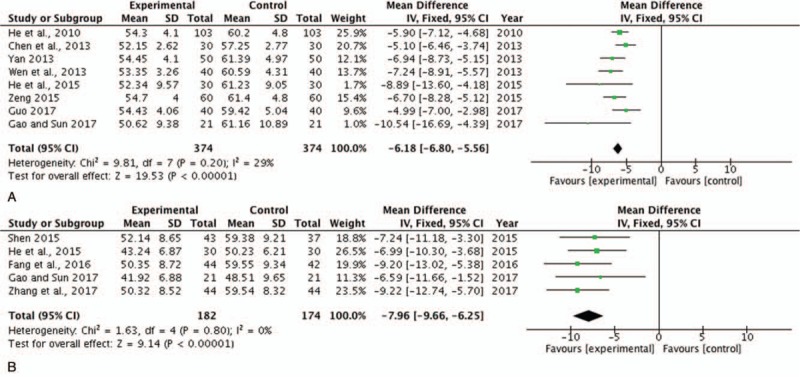
Forest plot of LVEDD and LVESD comparing the experimental and control groups. (A) Forest plot of LVEDD; (B) forest plot of LVESD. P and I2 are the criteria for the heterogeneity test. ♦ pooled mean difference, –▪– mean difference and 95% CI. LVEDD = left ventricular end-diastolic diameter, LVESD = left ventricular end-systolic diameter.
3.7. The comparison of 6-MWD
The 6-MWD was used as a supplemental parameter of hemodynamics. In this systematic review, 5 studies[16,24,26,28,30] with 534 patients reported the level of 6-MWD. There was no heterogeneity found among individual trials (P = .81, I2 = 0%) (Fig. 7) and fixed effect model was conducted for analysis. The pooled analysis suggested that SBP combined with trimetazidine therapy significantly improved 6-MWD compared with the control group (MD = 62.07, 95% CI [54.43, 69.71], P < .00001). Meanwhile, the exercise endurance of HF patients was observably increased.
Figure 7.
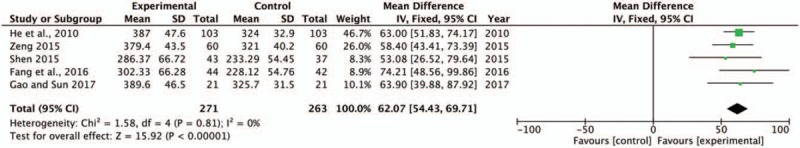
Forest plot of 6-MWD comparing the experimental group and control group. P and I2 are the criterion for the heterogeneity test. ♦ pooled mean difference, –▪– mean difference and 95% CI. 6-MWD = 6-minute walk distance.
3.8. Subgroup analysis of different control groups
Twelve trials[17,19–23,25–27,30–31,33] contained trimetazidine plus conventional treatment as a control group, and 6 trials[16,18,24,28–29,32] included conventional treatment alone as a control group. Therefore, a subgroup analysis was performed to identify whether there was any influence between these 2 schemes. Notably, 2 trials[18,24] with a conventional treatment alone did not report the clinical efficacy of SBP combined with trimetazidine compared with a control group. The subgroup analysis of these 2 schedules of control groups demonstrates no obvious heterogeneity in either the trimetazidine plus conventional treatment (P = .68, I2 = 0%) group or the conventional treatment alone (P = .90, I2 = 0%) group. The pooled analysis suggested that there was a significant difference between these 2 schemes. The pooled analyses indicated that trimetazidine plus conventional treatment (RR, 1.27; 95% CI, 1.19–1.35; P < .00001) (Fig. 8A) showed greater improvement in the total efficacy rate compared with the conventional treatment alone (RR, 1.22; 95% CI, 1.12–1.34; P < .00001) (Fig. 8B).
Figure 8.
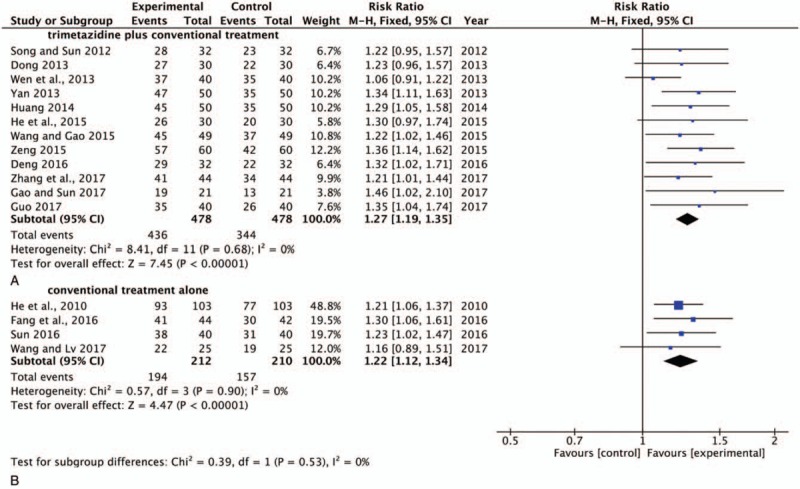
Forest plot of conventional treatment with or without combining with trimetazidine in control group in total efficacy rate. A: Control group was trimetazidine plus conventional treatment; B: Control group was conventional treatment alone. SBP = Shexiang Baoxin Pill; RR = risk ratio; CI = confidence interval. ♦ pooled mean difference, –▪– mean difference and 95% CI.
3.9. Adverse reactions
Adverse events were not mentioned in 7[19–20,23,25–26,30,32] of the studies (38.9%), while 11[16–18,21–22,24,27–29,31,33] (61.1%) reported that adverse reactions were observed during the treatment. Re-hospitalizations or adverse reactions and death in the SBP plus trimetazidine group and the control group were listed in Table 2. Neither of the 2 groups experienced any serious adverse reactions during the treatment in the 2 studies.[28,33]
Table 2.
The adverse reactions and mortality of included trials.
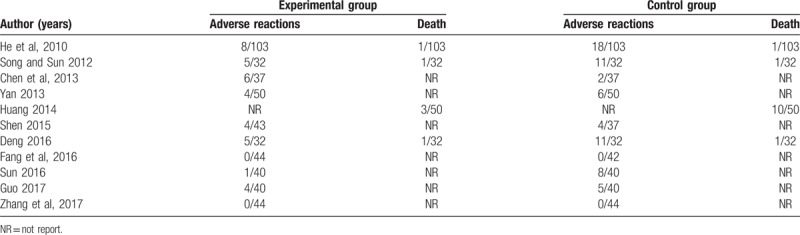
The side-effects included digestive symptoms such as abnormal bowel sounds and slight diarrhea, as well as headache, dizziness[18,24,31]. Impairment of liver and kidney function was also observed.[21] Though mortality is a very important indicator in the assessment of side-effects of administered medicine, these trials only reported the number of deaths rather than the cause and gave very inaccurate descriptions of the cause of death. Moreover, the studies were not designed to evaluate mortality as primary outcome measure. In summary, the incidence of adverse events in the treatment group was significantly lower than that in the control group. Thus, the use of SBP combined with trimetazidine represents a better therapy for patients with HF secondary to ICM.
3.10. Other outcomes
Plasma brain natriuretic peptide (BNP) and serum hypersensitive C-reactive protein (hs-CRP) were selected as outcome measures in 2 trials[23,28] with 146 patients. Only 1 trial[23] reported the level of N-terminal pro-brain natriuretic peptide (NT-ProBNP), and 1 trial[25] evaluated heart rate. For these 4 indicators, no significant differences were found between the 2 groups before treatment, while all of the levels in the SBP plus trimetazidine and CT groups were significantly lower than those in the control group after therapy.
3.11. Publication bias
Publication bias was expressed by the use of a funnel plot based on the data for clinical efficacy. Sixteen trials[16–17,19–23,25–33] were included in the funnel plot and are detailed in Fig. 9. The funnel plot indicated that there was no asymmetry in clinical efficacy improvement.
Figure 9.
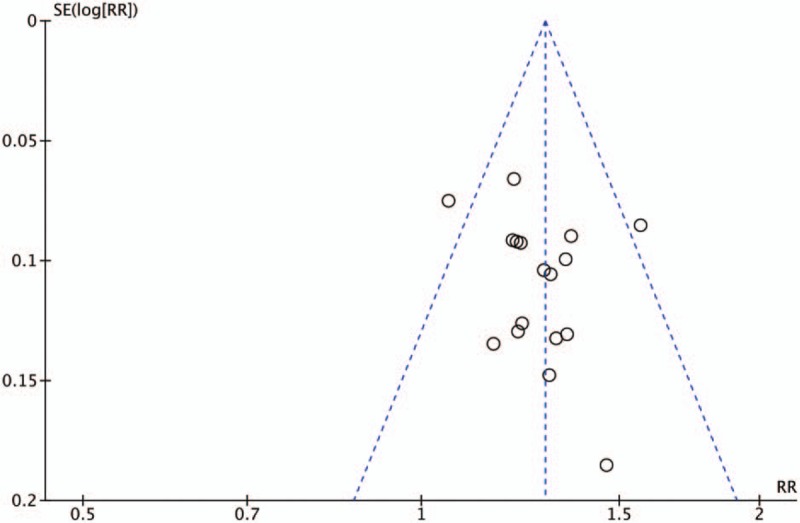
Funnel plot for the publication bias of the clinical efficacy.
4. Discussion
Heart failure (HF) is a global epidemic with increasing prevalence due to an ageing worldwide population with increasing comorbidities.[35] Ischaemic cardiomyopathy due to heart failure is one of the comorbidities. ICM has been widely recognized as one of the primary causes of death and disability with a high mortality and morbidity and is a serious threat to patient health and quality of life.[36] Worse still, the prognosis of patients with heart failure is quite poor.[37] Currently, several therapeutic medicines are available to treat HF that have improved survival, including ACE-I, beta-blockers (BB), angiotensin receptor blockers (ARB), diuretics, antiarrhythmic medications, vasodilators, calcium channel blockers (CCB), and statins. However, for the other types of HF, only a novel blocker of the funny channel and a combination of hydralazine/isosorbide dinitrate improved survival, while the previously listed agents only improved signs and symptoms.[38] For patients with HF due to ICM, the use of drugs is aimed at improving blood flow, balancing the electrolyte disturbance, and regulating the heart rate. Nevertheless, the long-term use of conventional agents[39] is associated with adverse reactions and side-effects. Therefore, more effective agents for treating patients with ICM due to HF are desirable.
TCM has been playing a significantly important role in treating cardiovascular diseases in the elderly for the past 2000 years. As mutually complementary, TCM combined with western medicine will have potential benefits for elderly patients with cardiovascular diseases with fewer side effects.[40] A previous meta-analysis comprehensively evaluated the clinical efficacy of SBP combined with trimetazidine in the treatment of ICM and HF in elderly patients.[13] This study indicated that SBP combined with trimetazidine had potentially increased clinical efficacy, LVEF, and 6-MWD, while LVEDD decreased significantly when compared with conventional treatment. In this systematic review, we further assessed the effect of SBP combined with trimetazidine on ICM due to HF and provided more extensive findings. First, the clinical efficacy of SBP combined with trimetazidine was evaluated according to the “Nomenclature and criteria for diagnosis of diseases of the heart and great vessels” developed by the criteria committee of the New York Heart Association, 9th ed[34] and was reported based on the NYHA classification, which were directly related to the improvement of patients with ICM-associated HF. Second, since an individual patient's long-term prognosis and lower mortality rate is related primarily to his or her cardiac function,[41] the cardiac function indexes such as LVEF, LVEDD, LVESD were systematically evaluated. Moreover, trimetazidine is commonly used in treating cardiovascular diseases and improves myocardial energy metabolism and protects both myocardial cells and blood vessel endothelia.[42] Thus, trimetazidine may be used in conventional therapy especially for patients with angina. SBP combined with trimetazidine has certain clinical effect in treating elderly patients with HF secondary to ICM and the mechanisms may be related to reduce the plasma BNP level and serum hs-CRP level.[43] In this study, subgroup analysis was performed because the control group was comprised of patients taking conventional treatment with and without trimetazidine. Furthermore, we also assessed the medication-associated adverse reactions of SBP/trimetazidine combination therapy as well as publication bias.
In this study, the overall meta-analysis demonstrated that, compared with the control group, SBP combined with trimetazidine therapy significantly improved the total efficacy rate in the treatment of ICM-related HF in elderly patients. In addition to the positive influence in the control group, a more significant increase in LVEF and 6-MWD as well a reduction in LVEDD and LVESD were observed in the experimental group with an estimated mean. Subgroup analysis indicated that conventional treatment with trimetazidine for the control group had greater clinical efficacy compared with conventional treatment alone. However, more rigorous and well-designed RCTs should be performed owing to the small samples in this systematic review. Furthermore, no serious adverse events were observed in any of the included trials. Though the plasma level of BNP, hs-CRP, and NT-ProBNP were only reported in 2, 2, 1 trial, respectively, their levels were all significantly lower in the SBP plus trimetazidine and CT than in the conventional treatment group. Additional trials would provide a better database for further clinical research.
Even though the clinical efficacy and safety of SBP combined with trimetazidine were comprehensively analyzed with a large number of trials and strict methodologies, the existence of potential publication bias indicated that there were still limitations of this study still. First, characteristics of the included trials were not reported in full detail, such as the age of patients, course of disease, and NYHA classification. Second, with regard to methodological quality, it must be noted that both the blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias) were not reported in any of the trials. Generally, independent ethics committees at each study site should approve the study protocol, and all patients should provide written informed consent before initiation of study-specific procedures. However, there were a seriously low number of trials that reported this statement of ethics. All the included trials were published in Chinese. In addition, the presence of publication bias indicated that this may influence the results of this systematic review. Finally, due to small sample size among the included studies, subgroup analysis was not performed in this study. Because of these limitations, more rigorous and well-designed RCTs are needed to confirm these findings.
5. Conclusion
Overall, this systematic review suggested that SBP combined with trimetazidine provides an obvious clinical efficacy for the treatment of HF secondary to ICM, indicating that the combination therapy has some clinical potential. However, due to the small samples and generally lower quality studies included in this review, we expect more evidence from high quality trials to confirm advantages of the extensive clinical use of SBP for elderly patients with ICM-related HF.
Acknowledgments
The authors would like to thank all authors of references.
Author contributions
JXW and XM performed the search and wrote the manuscript. LZ and XHL analyzed the data. JXW and YXY performed the data extraction. JXW, JW, and YLZ designed the study and amended the paper. All authors have approved the manuscript is agree with submission to journal.
Conceptualization: Yuxue Yang.
Formal analysis: Jianxia Wen.
Funding acquisition: Jian Wang, Yanling Zhao.
Investigation: Lu Zhang, Yuxue Yang.
Methodology: Lu Zhang.
Software: Xiao Ma.
Supervision: Yuxue Yang, Jian Wang.
Validation: Xiaohua Lu, Yanling Zhao.
Visualization: Yanling Zhao.
Writing – original draft: Jianxia Wen.
Writing – review & editing: Jianxia Wen, Xiaohua Lu, Jian Wang, Yanling Zhao.
Footnotes
Abbreviations: 6-MWD = 6-minute walk distance, BNP = brain natriuretic peptide, CBM = Chinese Biomedical Database, CI =confidence intervals, CNKI = China National Knowledge Infrastructure, CT = conventional therapy, HF = heart failure, hs-CRP = hypersensitive C-reactive protein, I2 = I-square, ICM = ischemic cardiomyopathy, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, LVESD = left ventricular end-systolic diameter, MD = mean difference, NT-ProBNP = N-terminal pro-brain natriuretic peptide, NYHA = New York Heart Association, RCTs = randomized controlled trials, RR = risk ratio, SBP = Shexiang Baoxin Pill, TCM = Traditional Chinese medicine, VMIS = VIP medicine information system.
JXW and XM have contributed equally to the study.
This work was financially supported by grants from National Natural Science Foundation of China [grant number: 81873023, 81473371 and 81573631].
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- [1].Isomura T, Hirota M, Hoshino J, et al. Strategy of treatment for ischemic cardiomyopathy. J Jpn Coron Assoc 2013;19:339–46. [Google Scholar]
- [2].Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee Stroke Statistics Subcommittee Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 2014;129:e28–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mentz RJ, Broderick S, Shaw LK, et al. Persistent angina pectoris in ischemic cardiomyopathy: increased rehospitalization and major adverse cardiac events. Eur J Heart Fail 2014;16:854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li GQ, Wang F, Wang SH, et al. Whole medical systems in lung health and sleep: Focus on Traditional Chinese Medicine. Integrat Therap Lung Health Sleep 2011;4:269–303. [Google Scholar]
- [5].Wei D, Zheng NN, Zheng LY, et al. Shexiang Baoxin Pill corrects metabolic disorders in a rat model of metabolic syndrome by targeting mitochondria. Front Pharmacol 2018;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tan B, Guo HH, Jiang J, et al. A Meta-analysis of effectiveness evaluation between Shexiang Baoxin Pill and isosorbide mononitrate tablet. J Pharm Biomed Sci 2017;7:122–7. [Google Scholar]
- [7].Liu Q, Lv C, Zhang WD, et al. Advance in modern studies on Shexiang Baoxin Pill. Chin Tradit Herbs Drug 2016;47:1409–17. [Google Scholar]
- [8].Fang HY, Zeng HW, Lin LM, et al. A network-based method for mechanistic investigation of Shexiang Baoxin Pill's treatment of cardiovascular diseases. Sci Rep 2017;7:43632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu XY, Wang YZ, Han Z. Effect of shexiang baoxin pill on myocardial fibrosis of diabetic rats. Pharmacol Clin Chin Materia Medica 2012;28:28–31. [Google Scholar]
- [10].Zhang QZ, Bu PL, Yu WQ, et al. The Influence of shexingbaoxinwan pill on myocardial fibrosis in spontaneously hypertensive rats. Chin J Integrat Med Cardio-/Cerebrovasc Dis 2008;6:546–8. [Google Scholar]
- [11].Marazzi G, Gebara O, Vitale C, et al. Effect of trimetazidine on quality of life in elderly patients with ischemic dilated cardiomyopathy. Advanc Ther 2009;26:455–61. [DOI] [PubMed] [Google Scholar]
- [12].Zhang KJ, Zhu JZ, Bao XY, et al. Shexiang Baoxin Pills for coronary heart disease in animal models: preclinical evidence and promoting angiogenesis mechanism. Front Pharmacol 2017;8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lin XD, Wang JN, Tang JM, et al. Clinical efficacy of Shexiang Baoxin Wan combining trimetazidine in treatment of ischemic cardiomyopathy and heart failure in elderly patients: a meta-analysis. Chin J Evid Cardiovasc Med 2016;10:1162–6. [Google Scholar]
- [14].Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu F, Shen C, Yao L, et al. Acupoint massage for managing cognitive alterations in older adults: a systematic review and meta-analysis. J Altern Complement Med 2018;24:532–40. [DOI] [PubMed] [Google Scholar]
- [16].He HY, Yue L, Li XX. Observation on the therapeutic effect of Shexiang Baoxin Pill combined with trimetazidine on senile ischemic cardiomyopathy with Heart Failure. Chin Gen Pract 2010;13:2034–5. [Google Scholar]
- [17].Song ZW, Sun YP. Clinical effect of Shexiang Baoxin Pill combined with trimetazidine on senile Ischemic cardiomyopathy heart failure. Nei Mongol J Trad Chin Med 2012;31:24. [Google Scholar]
- [18].Chen YQ, Lu X, Li WJ, et al. Clinical effect of Shexiang Baoxin Pill combined with trimetazidine on senile ischemic cardiomyopathy heart failure. Guide China Med 2013;11:288–9. [Google Scholar]
- [19].Dong J. Clinical effect of Shexiang Baoxin Pill combined with trimetazidine on senile ischemic cardiomyopathy heart failure. Chin J Mod Drug Appl 2013;7:123–4. [Google Scholar]
- [20].Wen ZP, Zhong SC, Fang XS, et al. Observation on the therapeutic effect of Shexiang Baoxin Pill combined with trimetazidine on senile ischemic cardiomyopathy with heart failure. Med Innov China 2013;10:161–2. [Google Scholar]
- [21].Yan CM. Clinical effect and safety of Shexiang Baoxin Pill combined with trimetazidine in treating senile ischemic cardiomyopathy heart failure. China Health Ind 2013;10:67–8. [Google Scholar]
- [22].Huang MK. Clinical observation of Shexiang Baoxin Pills combined with trimetazidine in treating senile ischemic cardiomyopathy with heart failure. Mod Diagn Treat 2014;25:5330–1. [Google Scholar]
- [23].He YL, Luo GQ, Luo TM. Curative observation of using Shexiang Baoxin Pill combined with trimetazidine dihydrochloride to treat elderly patients with ischemic cardiomyopathy and heart failure. J Sichuan Trad Chin Med 2015;33:49–52. [Google Scholar]
- [24].Shen Z. Clinical effect and safety of Shexiang Baoxin Pill combined with trimetazidine in treating senile ischemic cardiomyopathy heart failure. Strait Pharma J 2015;27:106–7. [Google Scholar]
- [25].Wang XG, Gao SM. Observation on the therapeutic effect of Shexiang Baoxin Pill combined with trimetazidine on senile ischemic cardiomyopathy with heart failure. J Clin Med 2015;2:5524–5. [Google Scholar]
- [26].Zeng XQ. Clinical observation on 60 cases of senile ischemic cardiomyopathy heart failure treated by Shexiang Baoxin Pill combined with trimetazidine. Chin J Ethnomed Ethnopharm 2015;24:84–5. [Google Scholar]
- [27].Deng LX. Clinical analysis of Shexiang Baoxin Pills combined with trimetazidine in treating senile ischemic cardiomyopathy with heart failure. World Latest Med Inform 2016;16:132–3. [Google Scholar]
- [28].Fang TT, Sun YL, Li F, et al. Impact of trimetazidine combined with Shexiang-baoxin Pills on plasma BNP level and serum hs-CRP level of aged patients with heart failure caused by ischemic cardiomyopathy. Pract J Card Cereb Pneumal Vasc Dis 2016;24:88–90. [Google Scholar]
- [29].Sun Y. Efficacy of Shexiang Baoxin Pills combined with trimetazidine in senile ischemic cardiomyopathy heart failure. Cardiovasc Dis J Integra Trad Chin Western Med 2016;4:196. [Google Scholar]
- [30].Gao HY, Sun W. Effect of trimetazidine combined with Shexiang Baoxin Pill on 42 cases of senile patients with ischemic cardiomyopathy heart failure. Clin Res Pract 2017;2:12–3. [Google Scholar]
- [31].Guo P. Clinical efficacy of Shexiang Baoxin Pill combined with trimetazidine in senile patients with heart failure caused by ischemic cardiomyopathy. J China Prescrip Drug 2017;15:105–6. [Google Scholar]
- [32].Wang HF, Lv L. Analysis of Shexiang Baoxin Pill combined with trimetazidine on clinical efficacy in senile patients with heart failure caused by ischemic cardiomyopathy. World Latest Med Inform 2017;17:58. [Google Scholar]
- [33].Zhang JZ, Yang D, Zhang YZ, et al. Analysis of curative effect of heart pill of musk and trimetazidine in senile patients with ischemic cardiomyopathy heart failure. China Foreign Med Treat 2017;36:125–6. +129. [Google Scholar]
- [34].Dolgin M. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels The Criteria Committee of the New York Heart Association. 9th ed1994;Boston Mass: Little Brown & Co, 253–256. [Google Scholar]
- [35].Orso F, Fabbri G, Maggioni AP. Epidemiology of heart failure. Handb Exp Pharmacol 2017;243:15–33. [DOI] [PubMed] [Google Scholar]
- [36].Chen JF, Xue SF, Duan KD, et al. Clinical controlled study on danhong injection in the treatment to acute heart failure caused by ischemic cardiomyopathy. World Chin Med 2017;12:1065–7. [Google Scholar]
- [37].Maggioni AP, Dahlstr MU, Filippatos G, et al. EUR observational research programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 2013;15:808–17. [DOI] [PubMed] [Google Scholar]
- [38].Papadimitriou L, Hamo CE, Butler J. Heart failure guidelines on pharmacotherapy. Handb Exp Pharmacol 2017;243:109–29. [DOI] [PubMed] [Google Scholar]
- [39].Miura M, Sugimura K, Sakata Y, et al. Prognostic impact of loop diuretics in patients with chronic heart failure - effects of addition of renin-angiotensin-aldosterone system inhibitors and β-blockers. Circ J 2016;80:1396–403. [DOI] [PubMed] [Google Scholar]
- [40].Luo J, Xu H, Chen KJ. Potential benefits of Chinese Herbal Medicine for elderly patients with cardiovascular diseases. J Geriatr Cardiol 2013;10:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Suzanne JB, Suzanne VA, Howard CH, et al. Impact of ejection fraction and aortic valve gradient on outcomes of transcatheter aortic valve replacement. J Am Coll Cardiol 2016;67:2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu H, Chen LP, Li HJ, et al. Atorvastatin/trimetazidine combination therapy in patients with chronic cardiac failure. Trop J Pharma Res 2017;16:2013–8. [Google Scholar]
- [43].Fang TT, Sun YL, Li F, et al. Impact of trimetazidine combined with Shexiang—baoxin pills on plasma BNP level and serum hs-CRP level of aged patients with heart failure caused by ischemic cardiomyopathy. Pract J Card Cereb Pneumal Vascu Dis 2016;24:88–90. [Google Scholar]


