Abstract
To investigate the efficacy and toxicity of intensity-modulated radiotherapy (IMRT) combined with induction-adjuvant cisplatin and fluorouracil (PF) in locoregionally advanced nasopharyngeal carcinoma (NPC).
A total of 91 biopsy-proven NPC patients treated with IMRT were retrospectively analyzed. All patients received induction chemotherapy (IC) consisting of cisplatin 25 mg/m2 on day 1 to 3, and 5-Fu 2500 mg/m2 as an intravenous infusion over 120 hours every 3 weeks for 2 cycles. Adjuvant chemotherapy of the same regime was given 28 days after the end of IMRT.
A total of 87 patients completed 2 cycles of IC. During adjuvant chemotherapy phase, 74.7% patients received at least 1 cycle. With a median follow-up time of 45 months (10–123 months), the 5-year local control, regional control, distant metastasis-free (DMF) and overall survival (OS) rates were 84.1%, 86.9%, 81.3%, and 74.4%, respectively. The 5-year local control rates for patients with Stage T1-2 and T3-4 was 94.6% and 76.5%, respectively (P = .045). The 5-year DMF rates for patients with N0-1 and N2-3 diseases were 90.6% and 73.3%, respectively (P = .072). During radiotherapy (RT), 24.2% patients suffered severe acute mucositis (grade 3–4). Severe late toxicities included cranial nerve palsy in 1 patient and grade 3 hearing impairment in 1 patient.
IMRT combined with induction-adjuvant chemotherapy consisting of PF regimen is well tolerated and provides satisfactory local-regional control for locoregionally advanced NPC. Further treatment strategies to control distant metastasis are needed in the future.
Keywords: cisplatin and fluorouracil, intensity-modulated radiotherapy, nasopharyngeal carcinoma, sequential chemotherapy
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor with unique patterns of epidemiologic and geographic distributions.[1] Due to its high radiosensitivity and anatomic constraints, radiotherapy (RT) is the main treatment modality for non-metastatic NPC patients. Concurrent chemoradiotherapy (CCRT) with or without adjuvant chemotherapy has been deemed the standard treatment for locally advanced NPC, since the publication of Intergroup 0099 trial and several subsequent studies from endemic areas.[2–4] However, in the Intergroup 0099 trial,[2] 37% patients did not complete protocol CCRT due to high incidence of acute toxicity. All of these trials were based on 2-dimensional radiotherapy (2DRT). With the high-speed development of radiation technology, intensity-modulated radiotherapy (IMRT) has become the standard RT technique for NPC because of its superiority of dosimetry. To our knowledge, no published results of randomized trials are available to confirm the role of CCRT for locally advanced NPC in the IMRT era.
With the improved local and regional control, distant metastasis is the predominant failure pattern in locoregionally advanced NPC after IMRT.[5,6] More potent systemic therapy needs to be investigated to control distant metastasis. Induction chemotherapy (IC) may reduce the risk of locoregional recurrence and eradicate micro-metastases. A randomized trial from Xu et al demonstrated that the induction–adjuvant modality using the cisplatin and fluorouracil (PF) regimen (5-FU plus cisplatin) produced similar outcomes compared with concurrent chemoradiation plus adjuvant chemotherapy.[7] In this study, we aimed to address the treatment outcomes and to evaluate the efficacy of induction–adjuvant chemotherapy using the PF regimen plus IMRT for advanced NPC patients.
2. Methods
2.1. Patients
Between June 2005 and December 2014, a total of 91 patients with histologically diagnosed non-metastatic NPC treated by definitive IMRT were enrolled in this study. Pretreatment evaluations consisted of a medical history and physical examination, blood chemistry tests, chest X-ray/computed tomography (CT), abdominal ultrasound/CT, enhanced magnetic resonance imaging (MRI) of the nasopharynx, and neck, nasopharyngoscopy and bone emission computed tomography (ECT). Other tests were performed for those with suspicious findings. Dental extraction, if deemed necessary, was performed before RT. All participants underwent disease staging according to the American Joint Committee on Cancer (AJCC) staging system. All patients provided informed written consent before treatment.
2.2. RT
Patients were immobilized in the supine position with thermoplastic masks. Intravenous contrast-enhanced CT planning scans were performed and contiguous slices 3 to 5 mm thick were obtained from the vertex to 2 cm below the clavicle. An 88-cm aperture CT was used for analog positioning, and the CT images were transferred to the treatment planning system through LAN.
Gross tumor volume (GTV) included the primary tumor and metastatic lymph nodes found in clinical and imaging examination. The clinical target volume (CTV) included the nasopharynx, retropharyngeal lymph node, skull base, anterior one-third of the clivus, pterygoid fossae, parapharyngeal space, inferior sphenoid sinus, posterior one-third of the nasal cavity and maxillary sinus, and drainage of the upper neck (levels II, III, and Va in N0 patients and levels IV-Vb in N1-N3 patients). For T3 and T4 patients, the whole clivus and sphenoid sinus were covered. Critical normal structures, including the brainstem, spinal cord, optic nerves, chiasm, lens, eyeballs, temporal lobes, and parotid glands, larynx were carefully delineated.
A total dose of 66-70.4 Gy/30-32 fractions was prescribed to the planning target volume (PTVg), defined as the GTV with a 0.5 cm margin. PTV60 (high-risk clinical target volume) covering the CTV and a 0.5 cm margin was prescribed 60 Gy/30 fractions. PTV54 (low-risk clinical target volume with 0.5 cm margin) was prescribed 54 Gy/30 fractions. All patients were treated with 1 fraction daily, for 5 days per week.
2.3. Chemotherapy
Patients received 2 cycles of IC: cisplatin 25 mg/m2 days 1 to 3, and 5-Fu 2500 mg/m2 as an intravenous infusion over 120 hours every 21 days. Four weeks after the completion of IMRT, adjuvant chemotherapy as the same regimen was administered every 3 weeks. Chemotherapy was postponed due to the disqualification of hematological index. Moreover, grade 4 hematological toxicity required a 20% dose reduction for the following cycle of chemotherapy. Complete blood count and liver and renal function were tested before each chemotherapy cycle.
2.4. Assessment and follow-up
Patients were evaluated weekly during radiation therapy. After treatment completion, follow-ups occurred every 3 months for the first 2 years, every 6 months from the third through the fifth year and annually thereafter. Each follow-up included medical history, physical examination, and nasopharyngoscopy. Enhanced MRI of the nasopharynx and neck areas was performed every 6 to 12 months after treatment. Chest X-ray/CT and ultrasonography of the abdomen were conducted once yearly. Additional tests were ordered whenever there was any clinical indication. Adverse events related to chemotherapy were graded by National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. Acute and late RT-related toxicities were graded according to the Radiation Therapy Oncology Group (RTOG).
2.5. Statistics
The times to local/regional failure and distant metastases were calculated from the start of treatment to the dates of recurrence and metastases, respectively. Statistical analyses were performed using SPSS software, version 20 (SPSS Inc., Chicago, IL). The Kaplan–Meier method was used to calculate local control, regional control, distant metastasis-free (DMF) and overall survival (OS) rates. Differences in survival curves were calculated with log-rank tests. A 2-sided P <.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
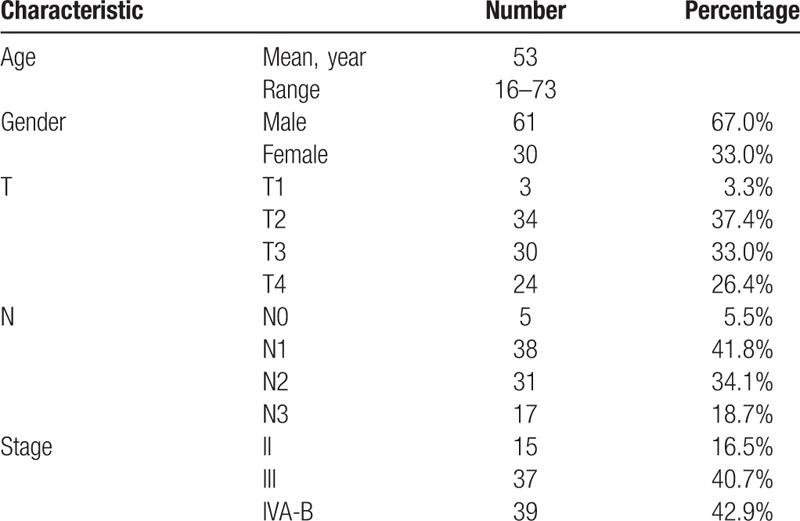
Between June 2005 and December 2012, 91 consecutive non-metastatic NPC patients treated in our institution were enrolled in this study. All patients were histologically proven to have World Health Organization (WHO) type II/III NPC. The study included 61 male (67.0%) and 30 female (33.0%). The numbers of patients with stage II (lymph node measured 4 cm or more in diameter), stage III and stage IV (A–B) disease were 15 (16.5%), 37 (40.7%) and 39 (42.9%), respectively. Characteristics of all the patients were illustrated in Table 1.
Table 1.
Characteristics of patients.
3.2. Treatment and compliance
All 91 patients underwent radical radiotherapy (IMRT). The median total dose of GTV was 66 Gy (range, 66–70.4 Gy). Duration of radiation was 45 days (range, 39–63 days). At the end of RT, MRI scans of nasopharynx and neck were conducted. Based on clinical and radiologic examinations, nasopharyngeal, and neck nodal residual diseases were determined at the discretion of the attending radiation oncologist. Boosts to cervical lymph nodes were 4.4Gy/2Fx (1 case) and 4Gy/2Fx (1 case) by using x-ray and electron beam. Boosts to retropharyngeal lymph nodes were 4.4Gy/2Fx (2 cases) by using x-ray. Four patients received boost doses at the primary site of nasopharynx by boost external irradiation of x-ray (4.4 Gy/ 2F: 3 cases, 6.6 Gy/3F: 1 case).
Eighty-seven patients (95.6%) completed 2 cycles of IC, and 4 patients only completed 1 cycle because of severe bone marrow suppression (1 patient), liver function damage (2 patients) and pneumonia (1 patient). Sixty-eight patients (74.7%) received adjuvant chemotherapy after IMRT. Twenty-3 patients did not receive adjuvant chemotherapy for various reasons, most due to bone marrow suppression and refusal of chemotherapy by patients.
3.3. Survival analysis
With the median follow-up of 45 months (range: 10 to 123 months), thirteen patients exhibited nasopharyngeal recurrence. Among these patients, 2, 5, and 6 had T2, T3, and T4 diseases, respectively. The 5-year local control rates for all patients were 84.1%, respectively. The 5-year local control rates for patients with Stage T1-2 and T3-4 was 94.6% and 76.5%, respectively (P = .045) (Fig. 1). Eleven patients had regional recurrence from 8 to 39 months after IMRT. The 5-year regional control rates for all patients were 86.9%, respectively. Thirteen patients developed distant metastasis with N1 (3/38), N2 (6/31), or N3 (4/17) disease. Six patients developed distant metastasis in a single organ: 3 cases in bone, 1 case in liver, 2 cases in lung. Seven patients had multiple metastases (≥2 sites). The most common site for distant metastases was bone. The 5-year DMF rates for patients with N0-1 and N2-3 diseases were 90.6% and 73.3%, respectively (P = .072) (Fig. 2). All patients who had treatment failure are listed in Table 2.
Figure 1.
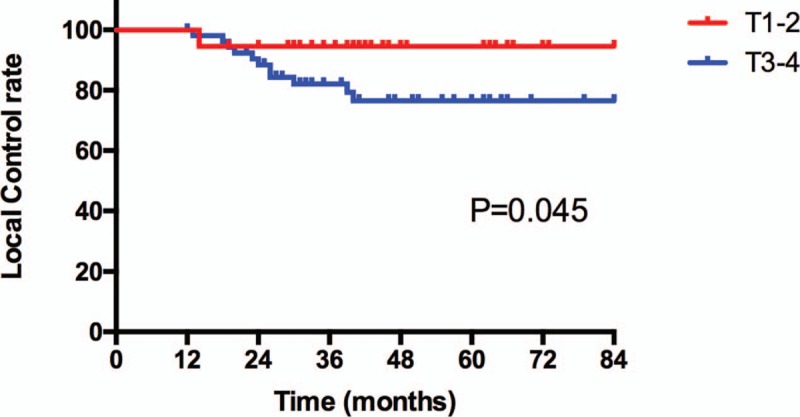
Local control rate for all patients according to T stage.
Figure 2.
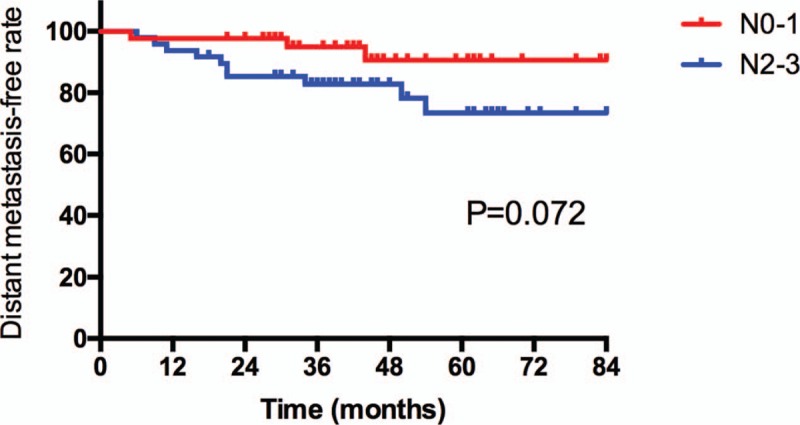
Distant metastasis-free rate for all patients according to N stage.
Table 2.
Treatment failure patterns of all patients.

At the last follow-up visit, a total of 16 patients died: 7 patients died of distant metastasis, 2 of distant metastases accompanied by recurrence in the regional lymph nodes, 2 of distant metastases accompanied by recurrence in nasopharynx, 2 of recurrence in nasopharynx and regional lymph nodes, 1 of recurrence in nasopharynx, 1 of recurrence in regional lymph nodes and 1 of non-neoplastic disease. The 5-year local control, regional control, DMF and OS rates were 84.1%, 86.9%, 81.3%, and 74.4%, respectively.
3.4. Toxicities
There were no treatment-related deaths. The acute toxicities were mainly grade 1/2 hematologic toxicities in patients treated with PF, but severe toxicities were uncommon. During the RT phase, mucositis, and weight loss were the most common acute treatment toxicities in our study. Among all the patients, 4 (4.4%), 65 (71.4%), 21 (23.1%), and 1 (1.1%) had grade 1, 2, 3, and 4 mucositis, respectively. Fifty-three patients needed intravenous nutritional support for mucosal reaction, and the median duration was 6 days (range: 2-21days). None of these patients required tube-feeding support. A total of 86 patients suffered weight loss, while the median weight loss was 9.1% in all patients.
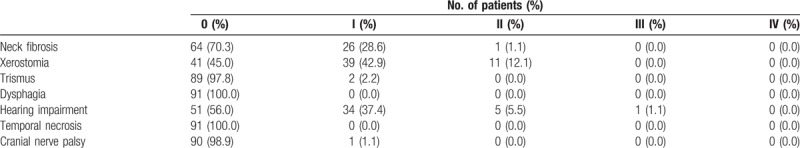
Table 3 shows the late toxicities in all patients. Overall, most late injuries were assessed as grades 0 to 1. There were 39 patients (42.9%) and 11 patients (12.1%) developing grade 1 and 2 xerostomia, respectively. Grade 2 and 3 hearing loss occurred in 5 patients (5.5%) and 1 patient (1.1%), respectively. Only 1 patient had cranial nerve palsy, and the possibility of recurrent disease was excluded by a series of MRI scans and physical examination. No cases of radiation-induced dysphagia and temporal necrosis were observed.
Table 3.
The late toxicities.
4. Discussion
NPC is a highly chemosensitive malignancy. Combining chemotherapy with RT is 1 of the crucial evolutions in the treatment of locoregionally advanced NPC. The current standard of care in locoregionally advanced NPC is CCRT. However, CCRT increased the occurrence of severe acute toxicity. Moreover, adjuvant chemotherapy after CCRT is poorly tolerated. In the IMRT era, the results from several large clinical reports revealed that CCRT did not improve survival rates in locoregionally advanced NPC treated with IMRT.[5,6,8] With the application of this modern RT technique, the value of CCRT improving locoregional control might be not so notable. Hence, CCRT with or without adjuvant chemotherapy may not always be the only option for locoregionally advanced NPC. To explore other optional chemotherapy sequences would be a reasonable attempt.
IC before RT is a valid treatment strategy for the eradication of micro-metastasis and improving clinical outcomes in locoregionally advanced NPC. A phase II trial by Hui et al demonstrated that compared with concurrent cisplatin-radiotherapy (CRT) alone, IC followed by CRT improved the 3-year OS rates significantly (hazard ratio (HR) = 0.24; 95% CI, 0.078–0.73; P = 0.012).[9] Additionally, the results of 2 meta-analyses indicated IC followed by CCRT was associated with reduced distant failure as compared with CCRT alone.[10,11] Recently, Cao and his colleagues reported the clinical outcomes of a phase III multicenter randomized controlled trial. In this trial, 476 patients were randomly assigned into IC with PF chemotherapy followed by CCRT (n = 238) or CCRT alone (n = 238). The addition of IC to CCRT achieved a 7.9% improvement in the 3-year disease-free survival (DFS) rate compared with the control arm (82.0% vs 74.1%, P = .028).[12]
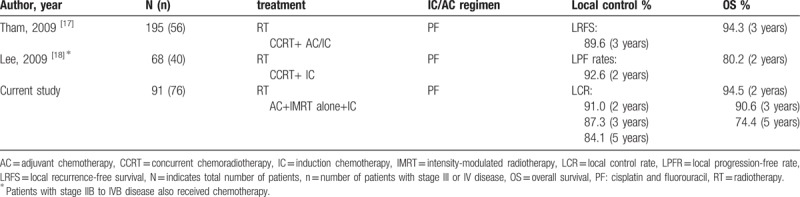
For the past decades, the value of adjuvant chemotherapy has been one of the highlights of research in locoregionally advanced NPC. Adjuvant chemotherapy plays an important role in reducing the subsequent occurrence of distant metastases. The combined analyses of NPC-9901 and NPC-9902 trials demonstrated that additional adjuvant chemotherapy with a fluorouracil-containing regimen contributed to the improvement of distant control.[13] However, the poor compliance of adjuvant chemotherapy after CCRT was always the major obstacle of limiting its broader application. Du et al reported that induction–adjuvant chemotherapy using cisplatin, fluorouracil, plus docetaxel (TPF) obtained encouraging outcomes with good compliance and well-tolerated toxicities in locoregionally advanced NPC. Fifty-seven patients (95%) received 2 courses of adjuvant chemotherapy. The 2-year estimated locoregional failure-free survival, distant failure-free survival, progression-free survival, and OS were 96.6%, 93.3%, 89.9%, and 98.3%, respectively.[14] Recently, some prospective clinical trials indicated that taxanes-based chemotherapy combined with IMRT could provide superior outcomes.[15,16] Nevertheless, there were certain risks for patients with diabetes, gastric ulcer due to patients received premedication with oral dexamethasone before docetaxel, and alopecia caused mainly by docetaxel adds the psychological stress of patients. The PF regimen has been generally considered as the standard of care for the induction/adjuvant chemotherapy and widely used in patients with locoregionally advanced NPC for years. In our study, patients received induction-adjuvant chemotherapy with PF regimen plus IMRT. Sixty-eight patients (74.7%) received adjuvant chemotherapy after IMRT. With the median follow-up of 45 months, we achieved excellent local control and satisfactory OS (Table 4). The 5-year local control, regional control, DMF, and OS rates were 84.1%, 86.9%, 81.3%, and 74.4%, respectively.
Table 4.
List of several studies reporting use of PF combining with for nasopharyngeal carcinoma.
To best of our knowledge, CCRT increased the grade and incidence of acute and late toxicities, including severe mucositis, weight loss, and hearing impairment.[19–21] Mucositis and weight loss were most common acute toxicities during CCRT, which were associated with poorer tolerance and unfavorable efficacy of treatment.[22] A study from Sun Yat-sen University Cancer Center reviewed 322 NPC patients to explore the potential influence factors of weight loss during RT. The results showed that the acute radiation toxicities in skin (P = .001), mucosal (P <.001), pharynx, and esophagus (P <.001), and upper gastrointestinal tract (P = .003) were associated with weight loss (%) during RT. In addition, the vicious circle of acute radiation toxicities and weight loss resulted in poor prognosis in NPC patients.[23] Zheng et al investigated the late toxicities of 208 NPC patients who achieved long-term (more than 5 years) survival after IMRT. With the multifactorial analysis, the occurrences of xerostomia and hearing loss were related to chemotherapy. A recent published meta-analysis by Du et al evaluated the incidence and risk of severe late toxicity with CCRT in NPC patients. The results showed that the use of concurrent chemotherapy was associated with an increased risk of severe late toxicities, with a relative risk (RR) of 1.349 (95% CI, 1.108–1.643; P = .005). Especially, CCRT significantly increased the risk of ear deafness/otitis compared with RT (RR = 1.567; 95% CI, 1.192–2.052).[24] In the present study, acute toxicities during IMRT were mild and well tolerated, leading to excellent compliance with adjuvant chemotherapy. Moreover, a few severe late toxicities were observed. Only 1 patient had grade 3 hearing loss and 1 patient exhibited cranial nerve palsy.
Distant metastasis has outnumbered the locoregional recurrence and become the main treatment failure in NPC patients treating with IMRT. Tumor-nodal-metastasis (TNM) system for NPC is critical in predicting prognosis and facilitating treatment planning. Thus, N categories have been reported to be one of the most important predictors for distant control.[25,26] Zhang et al retrospectively analyzed a cohort of 449 patients with biopsy-proven NPC treated with RT or chemoradiotherapy. The results showed the 3-year DMFS decreased with increasingly higher N category (N0 96%, N1 93.2%, N2 81.1%, and N3 71.7%, P <.001). However, advanced N-classification (N2-3 vs N0-1) was also associated with an increased risk of distant metastasis (HR = 2.570; 95% CI, 1.422–4.579; P = .001).[27] The similar results were observed in the current cohort. Compared with N0-1 patients, N2-3 patients were revealed to have a trend to develop distant metastasis (5-year DMF rates: 73.3% vs 90.6%, P = .072). Novel systemic therapy for cases that are at high risk for metastases needs further investigation. Several limitations in our study should be considered. First, the study population was relatively small; although the PF regimen has been widely used in patients with locoregionally advanced NPC for years, induction-adjuvant PF chemotherapy combined with IMRT for locoregionally advanced NPC is uncommon. Second, this is a retrospective study with the lack of a control group. Third, the data were obtained exclusively from a single institution. Therefore, well-designed phase 3, multicenter, randomized controlled trials are needed for further investigation.
5. Conclusions
In summary, IMRT with sequential induction-adjuvant PF chemotherapy provides satisfactory local-regional control with well tolerance and acceptable toxicities in patients with locoregionally advanced NPC. The main cause of treatment failure was distant metastasis. New therapies concerning distant metastasis need further investigation.
Acknowledgments
We acknowledge the support of Department of Radiation Oncology. The views expressed in this publication are those of the authors.
Author contributions
Conceptualization: Xiayun He.
Data curation: Xiayun He, Chaosu Hu.
Formal analysis: Mingyao Wu.
Software: Mingyao Wu.
Supervision: Xiayun He, Chaosu Hu.
Writing – original draft: Mingyao Wu.
Writing – review & editing: Mingyao Wu, Xiayun He, Chaosu Hu.
Xiayun He orcid: 0000-0001-9875-7885.
Footnotes
Abbreviations: 2DRT = 2-dimensional radiotherapy, CCRT = concurrent chemoradiotherapy, CT = computed tomography, CTV = clinical target volume, DMF = distant metastasis-free, GTV = gross tumor volume, IC = induction chemotherapy, IMRT = intensity-modulated radiotherapy, MRI = magnetic resonance imaging, NPC = nasopharyngeal carcinoma, OS = overall survival, PF = cisplatin and fluorouracil, PTV = planning target volume, RT = radiotherapy.
The authors have no conflicts of interest to disclose.
References
- [1].Chua ML, Wee JT, Hui EP, et al. Nasopharyngeal carcinoma. Lancet 2016;387:1012–24. [DOI] [PubMed] [Google Scholar]
- [2].Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310–7. [DOI] [PubMed] [Google Scholar]
- [3].Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American joint committee on cancer/international union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005;23:6730–8. [DOI] [PubMed] [Google Scholar]
- [4].Lee AW, Tung SY, Chua DT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2010;102:1188–98. [DOI] [PubMed] [Google Scholar]
- [5].Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014;110:398–403. [DOI] [PubMed] [Google Scholar]
- [6].Ou X, Zhou X, Shi Q, et al. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget 2015;6:38381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu T, Zhu G, He X, et al. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: updated long-term survival outcomes. Oral Oncol 2014;50:71–6. [DOI] [PubMed] [Google Scholar]
- [8].Lin S, Lu JJ, Han L, et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer 2010;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 2009;27:242–9. [DOI] [PubMed] [Google Scholar]
- [10].Chen YP, Wang ZX, Chen L, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol 2015;26:205–11. [DOI] [PubMed] [Google Scholar]
- [11].OuYang PY, Xie C, Mao YP, et al. Significant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trials. Ann Oncol 2013;24:2136–46. [DOI] [PubMed] [Google Scholar]
- [12].Cao SM, Yang Q, Guo L, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: A phase III multicentre randomised controlled trial. Eur J Cancer 2017;75:14–23. [DOI] [PubMed] [Google Scholar]
- [13].Lee AW, Tung SY, Ngan RK, et al. Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur J Cancer 2011;47:656–66. [DOI] [PubMed] [Google Scholar]
- [14].Du C, Ying H, Zhou J, et al. Experience with combination of docetaxel, cisplatin plus 5-fluorouracil chemotherapy, and intensity-modulated radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Int J Clin Oncol 2013;18:464–71. [DOI] [PubMed] [Google Scholar]
- [15].Kong L, Zhang Y, Hu C, et al. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: Final results of 2 parallel phase 2 clinical trials. Cancer 2017;123:2258–67. [DOI] [PubMed] [Google Scholar]
- [16].Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 2016;17:1509–20. [DOI] [PubMed] [Google Scholar]
- [17].Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys 2009;75:1481–6. [DOI] [PubMed] [Google Scholar]
- [18].Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009;27:3684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Atasoy BM, Dane F, Yumuk PF, et al. Toxicity and feasibility analysis for cisplatin-based concomitant chemoradiotherapy in locally advanced nasopharyngeal carcinoma. J BUON 2008;13:43–50. [PubMed] [Google Scholar]
- [20].Qiu C, Yang N, Tian G, et al. Weight loss during radiotherapy for nasopharyngeal carcinoma: a prospective study from northern China. Nutr Cancer 2011;63:873–9. [DOI] [PubMed] [Google Scholar]
- [21].Zeng L, Tian YM, Sun XM, et al. Late toxicities after intensity-modulated radiotherapy for nasopharyngeal carcinoma: patient and treatment-related risk factors. Br J Cancer 2014;110:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163–71. [DOI] [PubMed] [Google Scholar]
- [23].Li G, Jiang XY, Qiu B, et al. Vicious circle of acute radiation toxicities and weight loss predicts poor prognosis for nasopharyngeal carcinoma patients receiving intensity modulated radiotherapy. J Cancer 2017;8:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Du CR, Ying HM, Kong FF, et al. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta-analysis based on randomized controlled trials. Radiat Oncol 2015;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li AC, Xiao WW, Shen GZ, et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: long-term results and benefits of chemotherapy. Oncotarget 2015;6:24511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 2009;75:1071–8. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Y, Li WF, Mao YP, et al. Establishment of an integrated model incorporating standardised uptake value and N-classification for predicting metastasis in nasopharyngeal carcinoma. Oncotarget 2016;7:13612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


