Abstract
Rationale:
Insulin autoimmune syndrome (IAS) is a rare endocrine disease characterized by repeated fasting hypoglycemia or episodes of hypoglycemia late after meals, elevated serum insulin, and positivity for insulin autoantibody (IAA) or insulin receptor antibody (IRA). We summarize the clinical manifestations and treatment experiences of 3 patients with IAS.
Patient concerns:
One patient with >20-year history of type 2 diabetes mellitus had irregular episodes of hypoglycemia 2 years of after treatment with insulin. Another patient with a 6-year history of type 2 diabetes mellitus presented irregular episodes of hypoglycemia after 6 months of treatment with insulin. One patient with a history of Graves’ disease showed hypoglycemia after administration of thiamazole.
Diagnosis:
Serum islet cell antibody (ICA) and glutamic acid decarboxylase antibody (GADA) were negative, while antibody insulin autoantibodies were positive in all the 3 patients. Two patients demonstrated diabetes mellitus after an oral glucose tolerance test, while one had normal glucose tolerance. Furthermore, serum insulin levels significantly elevated and did not matched C peptide levels. No abnormalities were found on enhanced MRI of the pancreas, and all 3 patients were clinically diagnosed with IAS.
Interventions:
In case one, insulin aspart 30 injection was withdrawn after admission. In addition, the patient was prescribed sublingual acarbose 3 times daily. Two weeks after admission, prednisone acetate was administered orally once daily at night. In case 2, insulin aspart 30 injection was withdrawn after admission, the patient was prescribed sublingual acarbose 3 times daily with a meal. Five days after admission, oral prednisone acetate was administered once daily at night. In case 3, oral propylthiouracil was prescribed and thiamazole withdrawn after admission, and the patient consumed an extra meal before sleeping.
Outcomes:
At the 3-month follow-up visit, the hypoglycemic episodes had disappeared, serum insulin levels were significantly decreased, and insulin antibody (IA) levels were no longer detectable in all 3 patients.
Lessons:
For those patients with high-insulin hypoglycemia, IAA should be evaluated if serum insulin concentrations are inconsistent with C peptide levels. Therapeutically, a lower dose of glucocorticoids with more appropriate medication timing can be used to achieve good results.
Keywords: hypoglycemia, insulin autoantibody, Insulin autoimmune syndrome
1. Introduction
Insulin autoimmune syndrome (IAS), or autoimmune hypoglycemia (AIH), also called Hirata disease, was first reported by Hirata et al in 1970.[1] IAS is a rare endocrine disease characterized by repeated fasting hypoglycemia or episodes of hypoglycemia after meals, with elevated serum insulin, and positivity for IAA or insulin receptor antibody (IRA).[2] In this study, we summarize the clinical manifestations and diagnostic and therapeutic experiences of 3 patients with IAS of different etiologies who were evaluated at the Department of Endocrinology, People's Hospital of China Three Gorges University. We used a lower dose of glucocorticoids with more appropriate medication timing to achieve good results.
2. Case description
Case 1: A 76-year-old man was referred to the Neurology Department on August 14, 2013 with a complaint of recurrent abnormal behavior for 6 months, which had recurred 5 hours prior to presentation, and he was referred to Endocrinology Department due to frequent hypoglycemia at night and in the early morning. This patient has a history of type 2 diabetes mellitus (T2DM) for more than 2 decades and was treated with 18 U of subcutaneous insulin aspart 30 injection before breakfast for the previous 2 years. His fasting blood glucose was maintained at approximately 4 mmol/L. He had no history of smoking, drinking or use of other drugs, and he had a history of hypertension but no other diseases. On physical examination at admission, his temperature was 36.2°C, his pulse rate was 82 beats per min, his respiratory rate was 20 beats per min, his blood pressure was 153/83 mm Hg, and he was conscious and had clear speech. Both pupils were equal in size and round with a diameter of approximately 2.5 mm, and both were sensitive to light. The muscular force and tension of his limbs were normal with symmetrical tendon reflexes. The patient was diagnosed with T2DM and hypoglycemia.
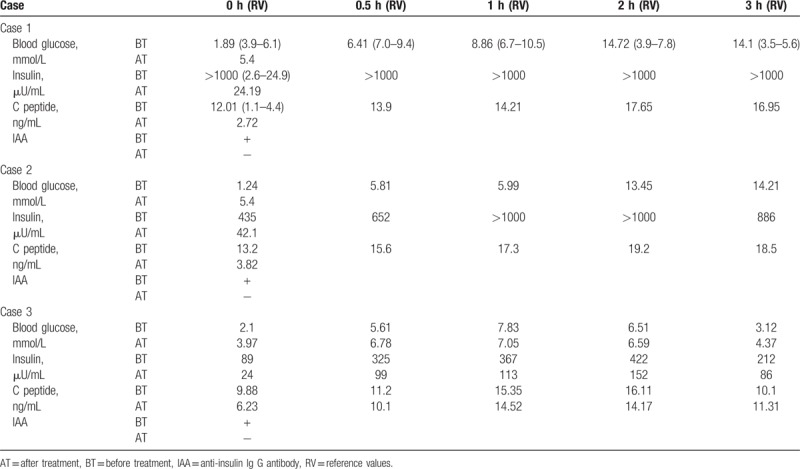
An examination after admission showed that routine blood counts, hepatorenal function, and electrolyte levels were normal. A random blood glucose level was 20.63 mmol/L during the day, his fasting blood glucose was 1.89 mmol/L, and his glycosylated hemoglobin was 5.8%. On August 18, the results of an OGTT and insulin/C peptide release test were as follows: low-fasting plasma glucose and high-plasma glucose after administration of oral glucose solution. His insulin levels were significantly elevated and not proportional to the elevated levels of C peptide (Table 1). His cortisol levels at 12 am, 8 am, and 4 pm were 149.00 nmol/L, 235.00 nmol/L, and 182.00 nmol/L, respectively, indicating a secretory rhythm disorder. Fasting adrenocorticotropic hormone levels, thyroid function testing, and electrocardiography were normal. Chest radiography and cerebral CT demonstrated no obvious abnormalities. No abnormalities were found on enhanced MRI of the pancreas. The patient's blood glucose level was 2.12 mmol/L on the night of August 28. Furthermore, additional test results showed an insulin level of more than 1000.00 μU/mL, a C peptide level of 16.47 ng/mL and positivity for IAA, while viral hepatitis were negative. Insulin aspart 30 injection was withdrawn after admission. In addition, the patient was prescribed sublingual acarbose tablets (50 mg) 3 times daily with a meal. The patient's blood glucose was controlled well during the daytime, but repeated episodes of hypoglycemia occurred at night and in the early morning. Two weeks after admission, prednisone acetate tablets (7.5 mg) were administered orally once daily at night. The patient was discharged when no recurrence of hypoglycemia was found during 1 week of observation. He continued to take prednisone acetate tablets (7.5 mg) orally after discharge, and there was no recurrence of hypoglycemia during 3 months of follow-up. Hypoglycemia did not occur later after the withdrawal of medication. Five months later, the patient was re-evaluated at our hospital, and his test results showed a fasting blood glucose level of 5.4 mmol/L, a fasting insulin of 88.06 μU/mL), a C peptide level of 2.92 ng/mL, an HbA1c level of 6.4% and negativity for ICA, IAA, and GAD. Ten months later, the patient's test results revealed a fasting blood glucose level of 6.0 mmol/L, a fasting insulin level of 24.19 μU/mL, a C peptide level of 2.72 ng/mL), an HbA1c level of 6.2%), negativity for ICA and weak positivity for IAA and GAD (Table 1).
Table 1.
Comparison of data before and after treatment in 3 cases.
Case 2: A 50-year-old man was admitted to the hospital on November 13, 2014 due to an elevated blood glucose for 6 years and repeated episodes of hypoglycemia for 1 month. The patient was admitted to the hospital in 2008 for polydipsia, polyphagia, and weight loss. He was diagnosed with T2DM with a fasting blood glucose 10.0 mmol/L, and subsequently, metformin and glimepiride tablets were administered. Subcutaneous injection of insulin aspart 30R (10 U at breakfast and dinner) was initiated in May 2014. His blood glucose was repeatedly <2.8 mmoL/L and was elevated after meals with a maximum of 18.9 mmol/L. He was not aware of hypertension, gout, hyperthyroidism, etc. On admission, the patient's vital signs included a blood pressure of 130/80 mm Hg, a body mass index of 24.8 kg/m2, and no obvious abnormalities of the heart, lungs, or abdomen. Muscular force and tension of the limbs were normal. The patient was diagnosed with T2DM and hypoglycemia.
Several examinations were performed after admission. Blood, urine, and stool (routine + occult blood) tests, and hepatorenal function and electrolyte levels were normal. Autoimmune antibody tests, thyroid function testing, and growth hormone and blood cortisol levels were normal. Finally, the patient had low fasting plasma glucose levels and high plasma glucose levels after administration of oral glucose solution. Insulin levels were significantly elevated, not proportional to elevated levels of C peptide (Table 1). His cortisol levels at 12 am, 8 am and 4 pm were 98.00 nmol/L, 342.00 nmol/L, and 179.00 nmol/L, respectively. IAA was positive, and ICA and GAD were negative. His glycosylated hemoglobin was 6.5%, and no abnormalities were found on enhanced MRI of the pancreas. Insulin aspart 30 injection was withdrawn after admission. In addition, the patient was prescribed sublingual acarbose tablets (100 mg) 3 times daily with a meal. His blood glucose was controlled well during the daytime, but he had repeated episodes of hypoglycemia during the early morning. Five days after admission, oral prednisone acetate tablets (5 mg) were administered once daily at night. The patient was discharged after he had no recurrence of hypoglycemia during 4 consecutive days of observation. With the same therapy after discharge, there was no recurrence of hypoglycemia during 3 months of follow-up. Furthermore, the patient had a maximum blood glucose level of 8.9 mmoL/L, a glycosylated hemoglobin level of 5.2%, a fasting blood glucose level of 5.4 mmol/L, a fasting insulin level of 42.1 μU/mL, a C peptide level of 3.82 ng/mL and negativity for ICA, IAA, and GAD (Table 1).
Case 3: A 40-year-old woman was referred to our hospital on October 10, 2016 complaining of palpitations, sweating, polyphagia, and weight loss for 6 months. She was diagnosed with Graves’ disease at our hospital on August 10, 2016, and she had a fasting blood glucose level of 5.0 mmol/L. Thiamazole tablets were prescribed for 1 month. The patient's blood glucose was repeatedly < 2.8 mmoL/L; however, her blood glucose was elevated after meals. She had no history of diabetes mellitus, hypertension, or previous exposure to insulin or oral antidiabetic agents. On physical examination at admission, her blood pressure was 110/60 mmHg, and her body mass index was 21.0 kg/m2. The thyroid was swollen to II degrees with a bumpy soft texture and no obvious palpable nodules or tenderness, and the patient had no extravascular murmur. She had no abnormalities of her heart, lungs, or abdomen, and muscular force and tension of the limbs were normal. The patient was diagnosed with Graves’ disease and hypoglycemia.
Examinations after admission revealed that blood, urine, stool (routine + occult blood) tests, and hepatorenal function and electrolyte levels were normal, and autoimmune antibody, growth hormone, and blood cortisol levels were also normal. The patient was positive for IAA but negative ICA and GAD. Low-fasting plasma glucose and high-plasma glucose levels after administration of oral glucose solution were observed. The patient's insulin levels were significantly elevated, but were not proportional to the elevation in C peptide levels (Table 1). Her glycosylated hemoglobin level was 4.8%. Thyroid function testing revealed a triiodothyronine level of 4.17 nmol/L, an increased thyroxine level of 221.80 nmol/L, a decreased thyroid-stimulating hormone level of 0.00 mIU/L, and increased free triiodothyronine level of 9.37 pmol/L, an increased free thyroxine level of 32.92 pmol/L, an elevated thyroid peroxidase antibody (TPoAb) level >1000.00 IU/mL, and an elevated thyrotropin receptor antibody (TRAb) level of 211.69 IU/mL. Thyroid iodine uptake was 42.7% at 3 hours and 61.6% at 24 hours, which is higher than normal level. Thyroid ECT showed that the thyroid lobes were mildly enlarged, with increased total technetium uptake demonstrating enhanced function. Thyroid color Doppler ultrasound depicted diffuse abnormal echo of the thyroid parenchyma and plenty of blood flow signal (demonstrating the possibility of hyperthyroidism). No abnormality was found on enhanced MRI of the pancreas. The patient was prescribed oral propylthiouracil tablets, thiamazole was withdrawn after admission, and the patient consumed an extra meal before sleeping. Hypoglycemia did not occur during 3 months of follow-up. Then, the patient returned to a normal diet, and no hypoglycemia was found during a 6-month follow-up. IAA, ICA and GAD were negative on reexamination, an OGTT was normal, and insulin and C peptide levels were significantly decreased compared with previous levels (Table 1).
3. Ethics statement
Institutional review board approval was waived due to the retrospective nature of the study. Informed consent was obtained from the patients for the publication of this case report.
4. Discussion
IAS is caused by 2 mechanisms:[3] one is the reaction between IAA and insulin (the patient may have been or may never have been treated with exogenous insulin therapy); the other is the interaction between insulin receptor autoantibody and the insulin receptor. Autoantibodies were present at higher titers in the blood of patients with IAS. Due to the combination of insulin and autoantibodies, insulin fails to bind with the receptors in the liver and peripheral tissues. Thus, the physiological function of insulin does not work appropriately, resulting in hyperglycemia. Disaggregation between autoantibody and insulin enhances the effect of insulin and then causes hypoglycemia. Therefore, patients with IAS often have abnormal glucose tolerance or diabetes and repeated hypoglycemia attacks. The half-life of insulin is significantly prolonged in patients’ blood circulation. The combination and disaggregation between insulin and autoantibodies prevents regulation of blood glucose levels, resulting in repeated episodes of hypoglycemia and hyperglycemia.
The patients in cases 1 and 2 were treated with exogenous insulin before admission. Whether hyperinsulinemia and insulin antibodies are endogenous must be determined before diagnosing IAS. First, there was evidence of endogenous hyperinsulinemia: The patient remained severely hypoglycemic after withdrawal of exogenous insulin for at least 3 days. Furthermore, the patient's insulin and C peptide levels were extremely high. Insulin aspart is generally metabolized between 15 and 18 hours. After 3 days of withdrawal, the patient's insulin levels were still abnormally elevated, and there was little possibility of exogenous hyperinsulinemia. According to the Mayo Clinic's diagnostic criteria for endogenous hyperinsulinemia hypoglycemia, a diagnosis can be confirmed when the fasting blood glucose level is lower than 2.5 mmol/L, the insulin concentration is greater than 3 U/mL, and C peptides are > 0.6 ng/mL. In contrast, C peptide levels are decreased[4] due to inhibition if hypoglycemia is caused by exogenous insulin. Second, there is some evidence suggesting that IAS in cases one and 2 was caused by endogenous anti-insulin antibodies. Affinity differs in endogenous and exogenous IAA. It has been reported[5] that insulin antibody (IA) in patients with IAS has significantly lower affinity than IA induced by exogenous insulin. It is easier for insulin to disaggregate from an antibody in this case, contributing to repeated episodes of hypoglycemia. The titers for exogenous IA are relatively lower and decrease gradually in IAS, and the symptoms of hypoglycemia symptoms have a tendency to be self-limiting. Antibody levels in case 1 were suspicious for positivity 10 months after withdrawal of insulin, which confirmed that the antibodies were endogenous anti-insulin antibodies.
Moreover, IAS can occur with autoimmune diseases.[4] Many drugs containing sulfhydryl compounds (–SH) can cause disease in individuals with a genetic predisposition. Drugs with an –SH chemical construction can induce disease and include thiamazole, α-mercaptopropionylglycine (tiopronin), α-lipoic acid, captopril, glutathione, penicillamine, etc. It is commonly believed that the interaction between –SH and S–S in the insulin molecule leads to allosterism of endogenous insulin, triggering an immunoreaction and generating IAA, which affects the combination and release of insulin.[6] However, this kind of allosterism is reversible. Once these drugs are discontinued, insulin antigen and antibody will disappear, and hypoglycemia resolve. The patient with hyperthyroidism in case 3 was prescribed thiamazole tablets for 1 month. Hypoglycemia and hyperinsulinemia occurred, she was positive for IAA, and no abnormalities were found on enhanced MRI of the pancreas. After admission, thiamazole was discontinued, and the patient was prescribed propylthiouracil tablets. Hypoglycemia did not occur during 3 months of follow-up, and IAA was negative 6 months later with a normal OGTT and significantly decreased insulin levels. IAS was considered to be induced by oral thiamazole. Some non -SH compounds have also been reported to induce IAS, such as steroids, D860, α-interferon and bone stagnation. The pathogenetic mechanism is still unknown. Furthermore, IAS can occur after some autoimmune diseases, such as Graves’ disease, which is most common, systemic lupus erythematosus, systemic scleroderma and acanthosis nigricans. Thus, IAS may be associated with immunodeficiency.
IAS is a rare cause of hypoglycemia. Decreased fasting blood glucose levels and hyperinsulinemia is commonly observed in patients with pancreatic B cell tumors. Insulinoma was also considered as a diagnosis for these 3 patients. However, no obvious abnormalities were found on pancreas CT or MRI, and blood insulin concentrations were significantly higher than those of patients with insulinomas. However, in IAS, the blood insulin concentrations associated with hypoglycemia caused by other reasons will not be 10 times higher than normal.[7] IAS could be diagnosed in these 3 patients due to repeated episodes of hypoglycemia without pancreatic masses.
The treatment for IAS is aimed at eliminating IAA and rectifying and preventing episodes of hypoglycemia. The clinical treatments include the following: Drugs that may induce IAS should be withdrawn. Few meals and a low-carbohydrate diet can gradually improve the condition for most patients within 1 to 3 months. Glucocorticoid can be prescribed for patients with repeated episodes. Oral prednisone (30–60 mg/d) is often reported.[8] Patients with IAS had been treated with a larger dose of oral prednisone (40 mg/d) during the early morning, aggravating hyperglycemia during daytime. We prescribed a small dose of oral prednisone to be taken at 8:00 pm every day, which not only improves hypoglycemia at night and in the early morning but also does not aggravate hyperglycemia after meals. The drug was gradually discontinued after IAA was negative. Glucocorticoids and immunosuppressive drugs can be used as adjuvant therapy.[9] Nursing care and close monitoring of changes in blood glucose can be strengthened. Intravenous glucose injection can be prescribed if hypoglycemia occurs. Plasmapheresis[10] or partial pancreatectomy can be performed in severe cases. All the patients had a favorable prognosis. Hypoglycemia-related sequelae may occur if severe hypoglycemia is been treated in time. Regularly detect insulin levels and IAA.
5. Conclusion
Although IAS is rare, this disease can trigger severe hypoglycemia and lead to death. Since insanity might occur during hypoglycemia, IAS is often misdiagnosed as mental or neurological disease, as described in case one, in which the patient was admitted to the Neurology Department for psychiatric symptoms. The diagnostic criteria for classic IAS are spontaneous hypoglycemia episodes without the administration of exogenous insulin.[2] The patients in cases 1 and 2 had hypoglycemia after the use of exogenous insulin, which reminds us that the possibility of IAS should be considered for hypoglycemic patients treated with insulin aspart 30 injection even though such cases have been rarely reported. Whether other types of insulin may cause IAS needs to be further evaluated, and the clinical diagnosis and treatment need to be summarized. In case 3, IAS was due to the use of thiamazole, suggesting that if patients develop spontaneous hypoglycemia during the use of thiamazole, the possibility of IAS should be considered. Therapeutically, we used a lower dose of glucocorticoids with more appropriate medication timing to achieve good results. In summary, IAA should be measured hypoglycemic patients with high insulin levels if their serum insulin concentrations are inconsistent with C peptide levels. Properly recognizing and diagnosing IAS in a time manner is of great importance to avoid misdiagnosis, undue surgery, and severe adverse effects.
Author contributions
Conceptualization: Wei You.
Data curation: Jian Ming Yang, Wei Wang.
Formal analysis: Jian Ming Yang, Wei Wang.
Investigation: Fangyuan Chen, Yanqun Liu, Jun Yang.
Methodology: Jian Ming Yang, Jun Yang.
Project administration: Wen Wang.
Resources: Jian Ming Yang, Wen Wang.
Supervision: Jian Ming Yang.
Validation: Fangyuan Chen, Yanqun Liu.
Visualization: Li Zhu.
Writing – original draft: Fangyuan Chen.
Writing – review & editing: Fangyuan Chen.
Footnotes
Abbreviations: AIH = autoimmune hypoglycemia, GADA = glutamic acid decarboxylase antibody, IA = insulin antibody, IAA = insulin autoantibody, IAS = insulin autoimmune syndrome, ICA = islet cell antibody, IRA = insulin receptor antibody, T2DM = type 2 diabetes mellitus.
FC and JY contributed equally to this article.
The authors have no conflicts of interest to disclose.
References
- [1].Hirata Y, lshizu H, Ouchi N, et al. Insulin autoimmunity in a case of spontaneous hypoglycemia. Jpn Diabetes Soc 1970;13:312–20. [Google Scholar]
- [2].Uchigata Y, Eguchi Y, Takayarna-Hasumi S, et al. Insulin autoimmune syndrome (Hirata disease):clinical features and epidemiology in Japan. Diabetes Res Clin Pract 1994;22:89–94. [DOI] [PubMed] [Google Scholar]
- [3].Bu S, Yang WY. The insulin autoimmune syndrome. Chin J Diabetes 2007;15:60–1. [Google Scholar]
- [4].Kronenberg HM, Melmed S, Polonsky KS. Williams Textbook of Endocrinology. 12th edn2012;1569. [Google Scholar]
- [5].Suzuli K, Hirayama S, Ito S. A case of a non-insulin dependent diabetic patient with regular spontaneous hypoglycemic attacks,which were due to insulin-binding antibodies induced by human insulin therapy. Tohoku J Exp Med 1997;182:163–73. [DOI] [PubMed] [Google Scholar]
- [6].Ismail AA. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med 2016;54:1715–24. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- [7].Uchigata Y, Eguchi Y, Takayama-Hasumi S, et al. Insulin autoimmune syndrome (Hirata disease): clinical features and epidemiology in Japan. Diabetes Res Clin Pract 1994;2:89–94. [DOI] [PubMed] [Google Scholar]
- [8].Chu JP, Zheng XW, Lu J, et al. Lin F.Insulin-induced autoimmune syndrome: a case report. Exp Ther Med 2016;12:3359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saxon DR, McDermott MT, Michels AW. Novel management of insulin autoimmune syndrome with rituximab and continuous glucose monitoring. J Clin Endocrinol Metab 2016;101:1931–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jassam N, Amin N, Holland P, et al. Analytical and clinical challenges in a patient with concurrent type 1diabetes, subcutaneous insulin resistance and insulin autoimmune syndrome. Endocrinol Diabetes Metab Case Rep 2014;2014:130086. [DOI] [PMC free article] [PubMed] [Google Scholar]


