Abstract
Rationale:
Colorectal liver metastasis develops in 50% of patients diagnosed with colorectal cancer, whereas concurrent intrahepatic cholangiocarcinoma (ICC) and colorectal liver metastases is extremely rare.
Patient concerns:
A 72-year-old man was referred to our hospital complaining of abdominal discomfort, diarrhea, and weakness over the last month.
Diagnoses:
Colorectal liver metastases concurrent intrahepatic cholangiocarcinoma (ICC).
Interventions:
The patient was treated with mFOLFOX6 (5-fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 85 mg/m2) plus bevacizumab 5 mg/kg every 2 weeks for 2 months. However, chemotherapy was not effective for the liver S3 lesion in our case. The possibility of ICC was considered based on the multidisciplinary team (MDT) mode, together with an anomalous increase in cancer antigen 19-9 and a history of hepatolithiasis.
Outcomes:
Simultaneous resection of the colon cancer and liver tumors was performed at 6 weeks after discontinuing bevacizumab. Colorectal liver metastases concurrent ICC was confirmed by postoperative pathology. The patient's disease-free survival time is currently >14 months.
Lessons:
This is the first case report of the diagnosis and timely treatment of colorectal liver metastases harboring ICC. These results suggest that multiple primary tumors should be considered as a differential diagnosis when imaging or laboratory test results are abnormal.
Keywords: cancer antigen 19-9, colorectal liver metastases, intrahepatic cholangiocarcinoma, multidisciplinary team
1. Introduction
Colorectal liver metastasis develops in 50% of patients diagnosed with colorectal cancer and is a very common malignancy worldwide.[1,2] However, the coexistence of liver metastases from colorectal cancer and intrahepatic cholangiocarcinoma (ICC) is extremely rare. One case of a combined cholangiocarcinoma-hepatocellular carcinoma housing a metastasis from colorectal cancer, and which occurred after radical resection of sigmoid colon cancer, has been reported.[3] Another author reported ICC coinciding with colorectal liver metastasis, but missed the diagnosis and the best opportunity for surgery.[4] We herein report a case of concomitant colorectal liver metastases and ICC that was treated successfully. We successfully resected colorectal liver metastases and the ICC based on multidisciplinary team (MDT) mode, where one of liver tumors developed gradually during chemotherapy. This is the first case report of the diagnosis and timely treatment of colorectal liver metastases harboring ICC.
2. Case report
A 72-year-old man was referred to our hospital complaining of abdominal discomfort, diarrhea, and weakness over the last month. He had an allergic constitution and a history of drinking small amounts of Shaoxing wine. His medical history was unremarkable with the exception of hepatolithiasis, which was found incidentally during a routine ultrasonography examination 8 years ago. Two years ago, he developed upper abdominal pain with fever and was diagnosed with acute cholangitis. After anti-inflammation and other treatments, his condition improved but without further treatment. Serum biochemistry was as follows: carcinoembryonic antigen (CEA), 51.6 ng/mL (0–5 ng/mL); cancer antigen 19-9 (CA 19-9), 428.7 U/mL (0–37 U/mL); total bilirubin, 24 μmol/L (0–21 μmol/L); direct bilirubin, 9 μmol/L (0–5 μmol/L), and indirect bilirubin, 15 μmol/L (3–14 μmol/L). All other tests were normal, including aspartate aminotransferase, aspartate aminotransferase, alkaline phosphatase, and gamma glutamyl transpeptidase. He had no history of hepatitis B or C. An endoscopic examination revealed a 3 cm diameter sigmoid non-obstructing neoplasm located 25 cm distant from the anal verge. A biopsy of the lesion established a diagnosis of moderately differentiated adenocarcinoma. Genetic testing of the biopsy revealed the KRAS gene mutation in exon 3, with no mutated NRAS or BRAF gene. Whole abdominal computed tomography (CT) revealed sigmoid cancer with multiple liver metastases (S3, S4–8), hepatolithiasis, and dilatation of the hepatic biliary ducts and common bile duct (Fig. 1). Liver magnetic resonance imaging (MRI) showed multiple liver metastases in liver segment 3 (S3, 1.2 cm in diameter) and segments 4–8 (S4–8, 6.5 cm in diameter; Fig. 1). He was diagnosed with colon cancer and synchronous liver metastases (2 metastases). His preoperative clinical stage was T3N1M1a stage IV (AJCC Cancer Staging Manual 2017). The primary lesion was resectable while the metastatic lesions were difficult to complete resection. After the first MDT meeting, the therapeutic strategy was decided as systemic chemotherapy including bevacizumab (Bev) to convert unresectable colorectal-liver metastases to complete resection. The patient was treated with mFOLFOX6 (5-fluorouracil 2400 mg/m2, leucovorin 400 mg/m2, and oxaliplatin 85 mg/m2) plus bevacizumab 5 mg/kg every 2 weeks. After 2 months of therapy, his CEA tumor marker level decreased dramatically, from 51.6 to 5.6 ng/mL, but CA19-9 continued to rise, from 428.7 to 2682.1 U/mL. A whole abdominal computed tomography scan and liver MRI revealed that the S4 to S8 tumor had shrunk from 6.5 to 3.6 cm, but the S3 tumor had grown from 1.1 to 1.7 cm. A second MDT meeting was held immediately due to the abnormal imaging examination and laboratory tests. Chemotherapy was ineffective for the S3 lesion, so the possibility of primary malignancy of the liver was considered. Simultaneous resection of the colon cancer and liver tumors was performed at 6 weeks after discontinuing bevacizumab. The postoperative pathological examination showed that the S3 lesion was moderately differentiated ICC. The carcinoma cells were positive for CK7 and CK19 and negative for CK20 (Fig. 2). He recovered well and was discharged on postoperative day 10. The patient completed perioperative chemotherapy using mFOLFOX6 with bevacizumab and was followed up every 3 months. The tumor marker examination and imaging evaluation showed no signs of recurrence. The patient's disease-free survival time is currently >14 months.
Figure 1.
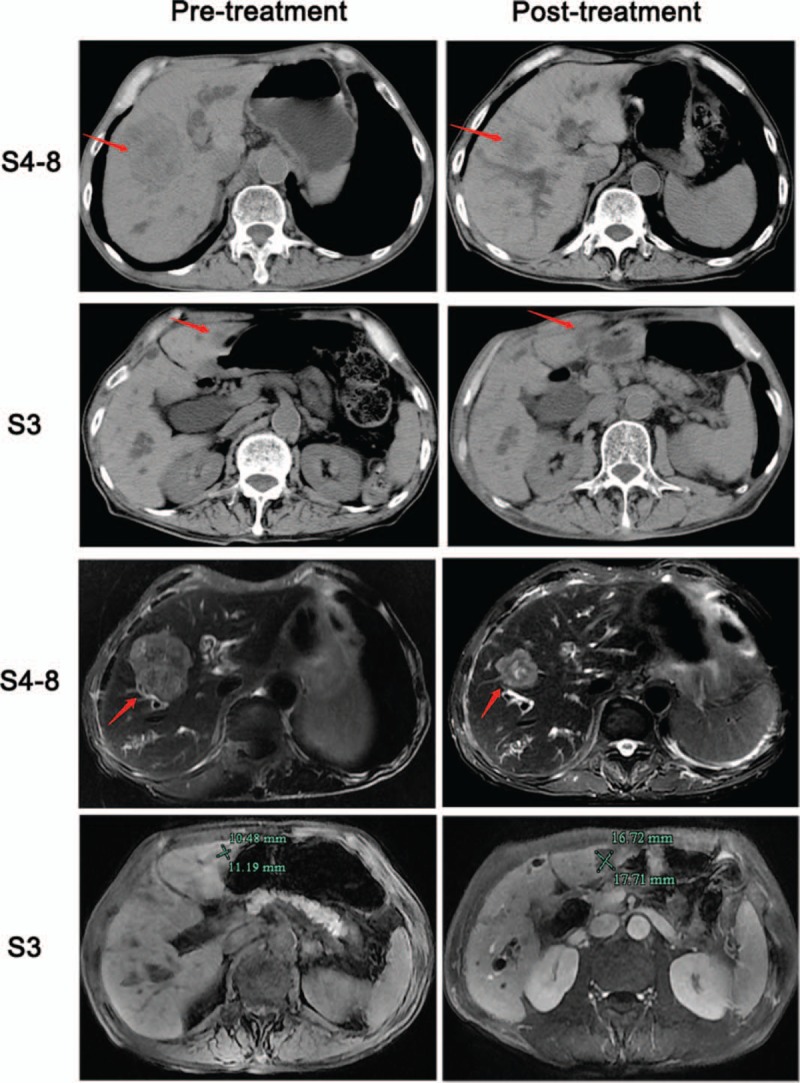
Computed tomography (CT) and magnetic resonance imaging (MRI) showed the liver tumor's radiographic response to chemotherapy. The liver tumor S4 to S8 had shrunk distinctly after the chemotherapy, but the S3 tumor had slightly grown on the MRI scan (1.1–1.7 cm).
Figure 2.
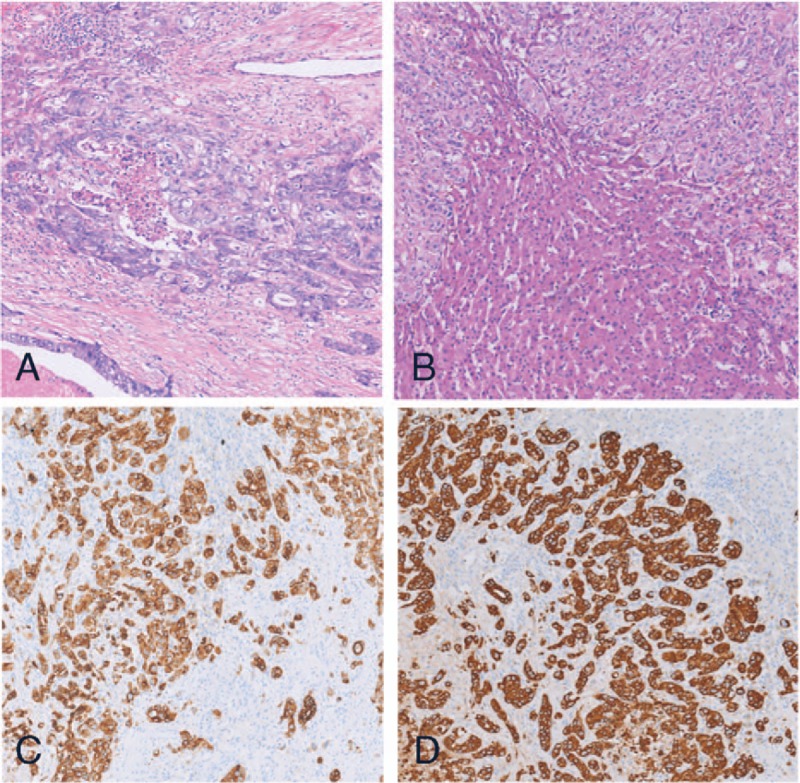
(A) Microscopic findings of the S4 to S8 tumor show moderately differentiated adenocarcinoma (H&E staining ×100); (B) microscopic findings of the S3 tumor show moderately differentiated intrahepatic cholangiocarcinoma (H&E staining ×100); (C) S3 tumor carcinoma cells are positive for CK7 (on immunohistochemistry, ×100); (D) S3 tumor carcinoma cells are positive for CK19 (on immunohistochemistry, ×100).
3. Discussion
This is the first report of successful diagnosis and treatment of concomitant colorectal liver metastases and ICC. The successful diagnosis and treatment of this case depended on the sincere cooperation of a MDT. Although the coexistence of 2 benign primary liver lesions, or a benign and a malignant one, is not infrequent, the finding of 2 unrelated malignant liver tumors is extremely rare. ICC occasionally coexists with other cancers, such as lymphoma,[5] renal cell carcinoma,[6] hepatocellular carcinoma,[7–9] thyroid cancer,[10] and gastrointestinal stromal tumors.[11] Only 2 reports are available in the literature about colorectal metastases with ICC; however, both diagnoses were delayed.[3,4]
Cholangiocarcinoma is a relatively infrequent type of primary hepatic malignancy, which accounts for only 5% to 10% of liver cancers.[12] The cause of ICC remains unknown.[13] Although there is no clear evidence directly linking ICC with hepatolithiasis, the presence of stones and contaminated bile may denote malignant changes leading to the development of cholangiocarcinoma.[14,15] Hepatolithiasis is an established risk factor for ICC in Asian countries, about 2% to 10% of patients with hepatolithiasis developing ICC.[16–18] A south Korean study suggested that a strong association between hepatolithiasis and ICC, with an OR of 50.0 (95% CI 21.2–117.3).[19] Zhou et al[20] also showed a significant association in Chinese patients, with the odds ratio (OR) at 5.8 (95% confidence interval [CI] 1.97–16.9). Although there is less data on the relationship between hepatolithiasis and ICC in Western countries, studies in Italy and the United States also showed a significant association.[21,22] A meta-analysis study showed the presence of pre-existing bile duct stones (choledocholithiasis alone or choledocholithiasis accompanied by hepatolithiasis) was associated with the risk of ICC (OR 17.64, 95% CI 11.14–27.95).[23] In summary, the association between hepatolithiasis and ICC has been increasingly identified recently.
The diagnosis of ICC is challenging because of its nonspecific presentation and low incidence.[24] Serum marker CA19–9, the most widely used serum marker for ICC, tends to have high specificity and low sensitivity, because it can increase in response to other benign or malignant diseases.[25,26] Similarly, MRI and CT rarely reveal the pathognomonic features of ICC to a greater extent compared with other liver lesions.[27,28] In our case, it was difficult to initially diagnose the ICC before chemotherapy because an increase in CA199 is also very common in colorectal liver metastasis, and CT/MRI showed that the 2 tumors in the liver were similarly shaped. However, chemotherapy was ineffective for the S3 lesion and, combined with the anomalous increase in CA 19-9 and a history of hepatolithiasis, the possibility of primary malignancies of the liver, such as ICC, was considered based on the MDT mode. The MDT approach is not only beneficial to treat difficult diseases, but is also helpful to discover occult diseases.
The patient prognosis of ICC is relatively poor, with 3- and 5-year overall survival rates of 30% and 18%, respectively.[29] Surgery is currently the only curative form of treatment that offers a chance for long-term survival in patients with ICC. A positive resection margin is strongly associated with a poor prognosis, so every effort should be made to achieve a wide surgical margin during hepatic resection of ICC.[30,31] In our case, the patient underwent resection of the S3 lesion, with a wider surgical margin compared with classic parenchyma-sparing hepatectomy for the S4 to S8 liver metastasis. Systematic treatment should target the tumor with the poorest prognosis when simultaneous neoplasms are diagnosed. However, there have been no randomized trials confirming the efficacy of adjuvant chemotherapy for ICC.[32] A targeted approach and immune therapy using programmed cell death 1 inhibitors have reinforced the potential role of therapy in this disease.[33] The postoperative chemotherapy in our case required that attention be paid to the colorectal liver metastasis, and use of mFOLFOX6 and bevacizumab to complete the perioperative chemotherapy.
In conclusion, we experienced an instructive case of colorectal liver metastasis, from which we learned to suspect the possibility of ICC, particularly when chemotherapy proved ineffective for some hepatic lesions. The possibility of multiple primary tumors should be considered as a differential diagnosis when abnormal imaging examination and laboratory tests occur. We should strive for accurate diagnosis and appropriate treatment based on the MDT mode when simultaneous neoplasms are diagnosed.
Author contributions
Data curation: Dong Chen, Weixiang Zhong.
Investigation: Feng Zhao.
Visualization: Piao Yang.
Writing – original draft: Xiaofei Cheng, Xiangming Xu.
Writing – review & editing: Weilin Wang.
Footnotes
Abbreviations: CA 19-9 = cancer antigen 19-9, CEA = carcinoembryonic antigen, CT = computed tomography, ICC = intrahepatic cholangiocarcinoma, MDT = multidisciplinary team, MRI = magnetic resonance imaging.
This work was supported by Zhejiang Province Natural Science Foundation of China (LY19H160040), Chinese traditional medicine Foundation of Zhejiang Province (2017ZA077), and Zhejiang education department scientific research project (Y201636334).
Informed consent statement: Consent was obtained from relatives of the patient for publication of this report and any accompanying images.
The authors declare that they have no conflicts of interest concerning this article.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Pernot S, Artru P, Mithieux F, et al. Complete pathological response of unresectable liver metastases from colorectal cancer after trans-arterial chemoembolization with drug-eluting beads loaded with irinotecan (DEBIRI) and concomitant systemic FOLFOX: a case report from the FFCD 1201 trial. Clin Res Hepatol Gastroenterol 2015;39:e73–7. [DOI] [PubMed] [Google Scholar]
- [3].Pintea B, Di Tommaso L, Destro A, et al. Combined hepatocellular carcinoma - cholangiocarcinoma harboring a metastasis of colon adenocarcinoma. J Gastrointestin Liver Dis 2013;22:341–3. [PubMed] [Google Scholar]
- [4].Akabane S, Ohira M, Kobayashi T, et al. Intrahepatic cholangiocarcinoma coinciding with a liver metastasis from a rectal carcinoma: a case report. Surg Case Rep 2016;2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fwu CW, Chien YC, You SL, et al. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology 2011;53:1217–25. [DOI] [PubMed] [Google Scholar]
- [6].Levy BF, Nisar A, Karanjia ND. Cholangiocarcinoma, renal cell carcinoma and parathyroid adenoma found synchronously in a patient on long-term methotrexate. HPB (Oxford) 2006;8:151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fuji N, Taniguchi H, Amaike H, et al. Synchronously resected double primary hepatic cancer, hepatocellular carcinoma and cholangiocarcinoma. J Gastroenterol Hepatol 2005;20:967–9. [DOI] [PubMed] [Google Scholar]
- [8].Inaba K, Suzuki S, Sakaguchi T, et al. Double primary liver cancer (intrahepatic cholangiocarcinoma and hepatocellular carcinoma) in a patient with hepatitis C virus-related cirrhosis. J Hepatobiliary Pancreat Surg 2007;14:204–9. [DOI] [PubMed] [Google Scholar]
- [9].Wu C, Bai DS, Jiang GQ, et al. Synchronous double cancers of primary hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a case report and review of the literature. World J Surg Oncol 2014;12:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang QL, Li XJ, Zhao K, et al. Synchronous double primary cancer - intrahepatic cholangiocarcinoma with bone metastases and thyroid carcinoma: a case report. Oncol Lett 2015;10:3799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nam SJ, Choi HS, Kim ES, et al. Synchronous occurrence of gastrointestinal stromal tumor and intrahepatic cholangiocarcinoma: a case report. Oncol Lett 2015;9:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nature reviews. Clin Oncol 2018;15:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koga A, Ichimiya H, Yamaguchi K, et al. Hepatolithiasis associated with cholangiocarcinoma. Possible etiologic significance. Cancer 1985;55:2826–9. [DOI] [PubMed] [Google Scholar]
- [15].Okugawa T, Tsuyuguchi T, K CS, et al. Peroral cholangioscopic treatment of hepatolithiasis: long-term results. Gastrointest Endosc 2002;56:366–71. [DOI] [PubMed] [Google Scholar]
- [16].Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis 2008;12:131–50. ix. [DOI] [PubMed] [Google Scholar]
- [17].Kubo S, Kinoshita H, Hirohashi K, et al. Hepatolithiasis associated with cholangiocarcinoma. World J Surg 1995;19:637–41. [DOI] [PubMed] [Google Scholar]
- [18].Lesurtel M, Regimbeau JM, Farges O, et al. Intrahepatic cholangiocarcinoma and hepatolithiasis: an unusual association in Western countries. Eur J Gastroenterol Hepatol 2002;14:1025–7. [DOI] [PubMed] [Google Scholar]
- [19].Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716–20. [DOI] [PubMed] [Google Scholar]
- [20].Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2008;14:632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Donato F, Gelatti U, Tagger A, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control 2001;12:959–64. [DOI] [PubMed] [Google Scholar]
- [22].Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cai H, Kong WT, Chen CB, et al. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: a meta-analysis of observational studies. BMC Cancer 2015;15:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nature reviews. Gastroenterol Hepatol 2016;13:261–80. [DOI] [PubMed] [Google Scholar]
- [25].Chen CY, Shiesh SC, Tsao HC, et al. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepatogastroenterology 2002;49:616–20. [PubMed] [Google Scholar]
- [26].Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol 2000;26:474–9. [DOI] [PubMed] [Google Scholar]
- [27].Zhang Y, Uchida M, Abe T, et al. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr 1999;23:670–7. [DOI] [PubMed] [Google Scholar]
- [28].Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis 2004;24:155–64. [DOI] [PubMed] [Google Scholar]
- [29].Sakamoto Y, Kokudo N, Matsuyama Y, et al. Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer 2016;122:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ribero D, Pinna AD, Guglielmi A, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg 2012;147:1107–13. [DOI] [PubMed] [Google Scholar]
- [31].Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg 2008;15:417–22. [DOI] [PubMed] [Google Scholar]
- [32].Guro H, Kim JW, Choi Y, et al. Multidisciplinary management of intrahepatic cholangiocarcinoma: current approaches. Surg Oncol 2017;26:146–52. [DOI] [PubMed] [Google Scholar]
- [33].Abdel-Rahman O, Elsayed Z, Elhalawani H. Gemcitabine-based chemotherapy for advanced biliary tract carcinomas. Cochrane Database System Rev 2018;4:CD011746. [DOI] [PMC free article] [PubMed] [Google Scholar]


