Abstract
Background:
Numerous studies have investigated the associations between RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation and clinical progression of patients with breast cancer, however the results remained uncertain due to the small sample size. Therefore, we performed a meta-analysis to explore the role of RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation in the susceptibility and clinical progression of breast cancer.
Methods:
Eligible studies were obtained by searching Medicine, Embase, Web of knowledge, and Chinese National Knowledge Infrastructure (CNKI) databases. The odds ratios (OR) and 95% confidence intervals (CI) were calculated to evaluate the associations of RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation with breast cancer pathogenesis. Trial sequential analysis (TSA) was applied to observe the reliability of pooled results of RARβ2 gene, and obtain a conservative required information size (RIS).
Results:
In primary screened 445 articles, 39 literatures with 4492 breast cancer patients were finally enrolled in the final meta-analysis. The results indicated that the frequency of RARβ2 promoter hypermethylation in case group was significantly higher than the frequency of control group (OR = 7.21, 95% CI = 1.54–33.80, P < .05). The RARβ2 promoter hypermethylation had a significant association with lymph node metastasis of breast cancer (OR = 2.13, 95% CI = 1.04–4.47, P < .05). And, the RARβ2 promoter hypermethylation was more common in the breast cancer patients of TNM III–IV stage than those patients of TNM I–II stage (OR = 1.85, 95% CI = 1.33–2.57, P < .05). In addition, the promoter hypermethylation of DAPK, hMLH1, and p14 genes were significantly associated with the susceptibility of breast cancer (for DAPK, OR = 4.93, 95% CI = 3.17–7.65; for hMLH1, OR = 1.84, 95% CI = 1.26–1.29; for p14, OR = 22.52, 95% CI = 7.00–72.41; for p15, OR = 2.13, 95% CI = 0.30–15.07).
Conclusions:
Our findings revealed that the RARβ2 promoter hypermethylation significantly increased the risk of breast cancer. In the meantime, the meta-analysis demonstrated that there were significant associations of RARβ2 promoter hypermethylation with lymph node metastasis and TNM-stage of breast cancer patients. In addition, DAPK, hMLH1, and p14 genes promoter hypermethylation were significantly associated with the susceptibility of breast cancer.
Keywords: breast cancer, DAPK, hMLH1, meta-analysis, p14, p15, promoter hypermethylation, RARβ2
1. Introduction
Although the improvements of early diagnosis and treatment significantly decrease the mortality rate of breast cancer, breast cancer is still the most common cancer in woman.[1] In addition, early detection of breast cancer provided more options of treatment, which included surgical treatment, radiotherapy, hormonal therapy, and chemotherapy.[2] As is known to all, environmental factors and genetic events played an important role in the development of breast cancer. Recently, the advances of early detection and treatment benefited from the finding of many biomarkers such as: estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), BRCA1, and BRCA2. PR and ER, typical tumor markers of breast cancer, had a great impact on the therapy of breast cancer. Although the 2 biomarkers had poor prognostic, they were considered as strong predictive factors of response to the treatment of hormonal therapy in breast cancer.[3,4] HER2, oncogenic protein, was a transmembrane protein, which was encoded by ERBB2.[5] Under normal situation, low expression level of HER2 gene was presented in normal epithelia of breast. It was reported that 15% to 20% of breast cancer patients had an abnormal amplification and high expression of HER2.[6] HER2 was often considered as a predictive factor for the chemotherapeutic of breast cancer. If some single chemotherapeutics were used or these drugs were combined to treat breast cancer, HER2-targeted drugs had a significant effect on chemotherapy.[7] However, HER2 status had a significant heterogeneity among individuals. Previous reports have found that 5% but no more than 50% nonclustered tumor cells presented HER2 heterogeneity.[8] In addition, BRCA1 and BRCA2, benefitting the early discovery of breast cancer, were the most notable biomarkers of breast cancer patients.[9] Although many other biomarkers were also applied to find breast cancer, a large proportion of breast cancer patients were found in late stage. Thus, in order to research the associations between genetic alterations and breast cancer pathogenesis, numerous biomarkers should be found and studied.
RARβ2 (retinoic acid receptor beta), a member of retinoic acid receptor subfamily and encoded by RARβ2 gene, primarily mediated the retinoic acid (RA) activity. RA was a major bioactive metabolite of vitamin A, which had an important influence in cell growth and differentiation.[10] The abnormity of RA signaling pathways might result in occurrence of disease, such as abnormal embryo development and cancers. Of note, previous studies have suggested that the RARβ2 gene hypermethylation was presented in several cancers such as: skin cancer, head/neck cancer, lung cancer, ovarian cancer, prostate cancer, renal cell carcinoma, pancreatic cancer, and liver cancer.[11] In addition, some clinical studies were also performed to explore the relationships between DAPK, hMLH1, p14, and p15 promoter hypermethylation and breast cancer risk and clinical progression. But the results were unconvincing due to some factors such as sample size and race. So, the sensibility and specificity of one gene might not be so strong to accurately diagnose the occurrence of breast cancer. However, if several genes were applied to diagnose the breast cancer or assess the prognosis of breast cancer together, the accuracy of results should be increased greatly. To find more relevant genes and clarify the relationship between promoter hypermethylation of these genes and susceptibility and clinical progression of breast cancer, we performed this meta-analysis.
2. Materials and methods
2.1. Publication search
On the basis of PRISMA guideline, PubMed, Embase, Web of knowledge, and Chinese National Knowledge Infrastructure (CNKI) databases were retrieved to search eligible articles, investigating the associations of RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation with susceptibility and clinical progression of breast cancer.[12] The following terms were used: “Breast Neoplasms,” “breast cancer,” “breast tumor,” “retinoic acid receptor beta,” “RARβ2,” “Hypermethylation,” “methylation,” “DAPK,” “hMLH1,” “p14,” “p15,” and “Epigenomics.” The references of included articles and related reviews were checked to identify additional articles. All relevant articles were searched up to June 2018.
2.2. Inclusion criteria and exclusion criteria
The eligible studies were selected using the following inclusion criteria: the studies investigating the associations of RARβ2, DAPK, hMLH1, p14, and p15 genes promoter hypermethylation with susceptibility and clinical progression of breast cancer. Case–control studies or cohort studies; the studies which contained full data of RARβ2, DAPK, hMLH1, p14, and p15 hypermethylation frequency; English or Chinese publications. The exclusion criteria were as follows: reviews or meta-analysis; studies which were conducted in cells or animals; articles that contained duplicate data.
2.3. Data collection and quality assessment
According to inclusion criteria, 2 reviewers independently searched eligible studies. The following studies’ characteristics were extracted: the name of first author, race, methylation frequency of control and case, detection method of gene methylation, source of control, cancer type, and clinical information. The information of breast tumor differentiation, age, and distant metastasis were not extracted since the information was too few. Furthermore, Newcastle-Ottawa Scale (NOS, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) was applied to assess the methodological quality of included studies.[13] Two investigators assessed the included studies and scored them according to the NOS table. If some divergences existed, 2 authors discussed these divergences and reached a consensus score. Methodological qualities of included studies were estimated according to 3 parts such as: sample selection, sample comparability, and sample exposure. The score of quality assessment ranged from 0 to 9 stars.
2.4. Trial sequential analysis
To observe the stable of results and estimate the required information size, TSA was performed on the basis of the RARβ2 promoter hypermethylation frequency of control group and case group. TSA 0.9 software (Copenhagen Trial Unit, Center for Clinical Intervention Research, Denmark, http://www.ctu.dk/tsa/downloads.aspx) was applied to assess statistical significance, using type I errors of 5% and type II errors of 10%.[14] If the Z-curve did not cross any boundary, the result suggested that no significant association existed. In addition, the Z-curve crossed the traditional boundary and the trial sequential monitoring boundary, which indicated the sample size was enough large and a significant association was observed. However, if the Z-curve only crossed the traditional boundary, it showed a lack of firm evidence. When new studies were added or repeating tests were performed, a meta-analysis commonly leaded to type I errors or type II errors. To detect and minimize the risk type I errors and type II errors, TSA was a powerful statistical method.
2.5. Statistical analysis
All statistical analysis were conducted, using Stata 12.0 (StataCorp LP, College Station, TX) software and TSA 0.9 software. The ORs and 95% CIs were used to evaluate the strength of associations between RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation and breast cancer pathogenesis.[15]I2 statistics and Cochrane Q were calculated to assess the interstudy heterogeneity, while P-value of <.05 or I2 value of >50% indicated a significant heterogeneity.[16,17] The fixed effects model was utilized when heterogeneity was detected; otherwise, random effects model was used.[18,19] Further, subgroup analysis based on race was conducted to evaluate the strength of associations in different population. In addition, Begg test and Egger test were performed to observe publication bias of included studies.[20,21] Forest plot and funnel plot were drawn to visually observe differences among included studies.
2.6. Ethnical statement
The consent of ethics committee or institutional review board was not required in the meta-analysis, because the study was a review article.
3. Results
3.1. Literature selection
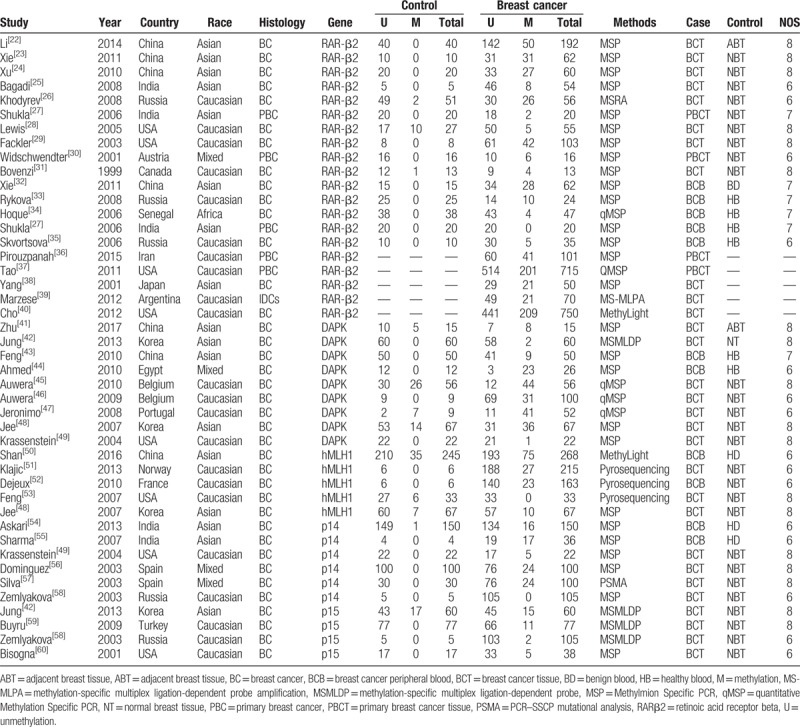
A total of 39 articles with 4492 breast cancer patients were finally included. Medicine, Embase, Web of knowledge, and Chinese National Knowledge Infrastructure (CNKI) databases were all retrieved to search eligible studies.[22–60] Initially, 445 articles were remained after repeated retrievals were excluded. Then, 312 articles were eliminated after title and abstract were read. Further, 53 literatures were removed because they were not related with the RARβ2, DAPK, hMLH1, p14, and p15 genes hypermethylation. Moreover, 41 articles were excluded since they had no enough data to conduct a meta-analysis. Finally, 39 articles which compared the frequency of RARβ2, DAPK, hMLH1, p14, and p15 genes promoter hypermethylation in control group with those in case group were included.[21–59] In these included studies, 19 studies were involved in RARβ2 gene promoter hypermethylation, 9 studies were about DAPK gene promoter hypermethylation, hMLH1 gene promoter hypermethylation were studied in 5 studies, 6 studies were about p14 gene promoter hypermethylation, and 4 studies were about p15 gene promoter hypermethylation. The included studies were mainly carried out in Asians and Caucasians in the present meta-analysis. Figure 1 illustrates the flow diagram of literature selection (Table 1).
Figure 1.
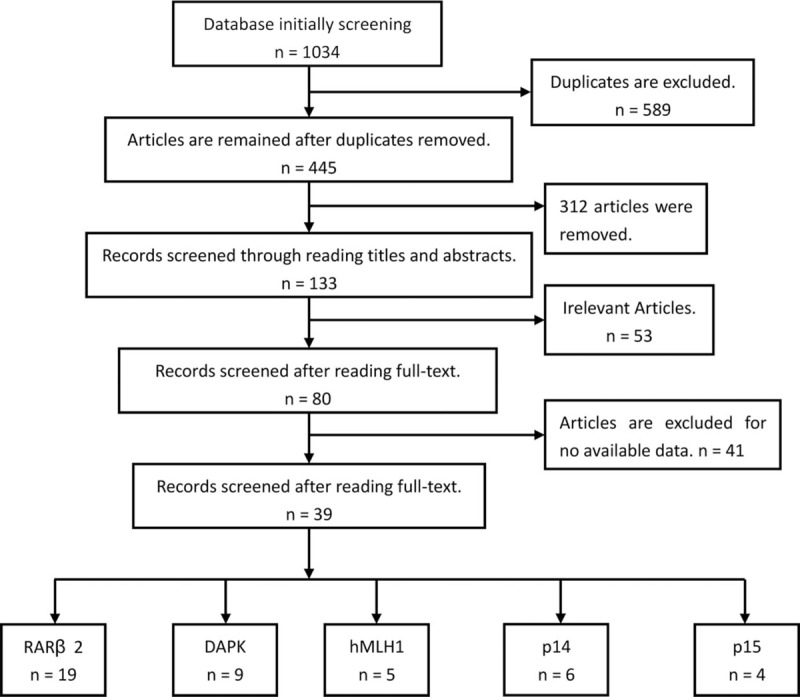
Flow chart of included studies searching.
Table 1.
Characteristics of included studies evaluating the associations of RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation with breast cancer susceptibility.
3.2. Quality assessment
The NOS score of included studies was from 6 to 8 stars. The results showed that studies of moderate or high quality were included in this meta-analysis, which greatly improve the power of statistics.
3.3. Results of meta-analysis and trial sequential analysis about RARβ2 promoter hypermethylation
According to results of the meta-analysis, RARβ2 promoter hypermethylation significantly associated with the risk of breast cancer (OR = 7.21, 95% CI = 1.54–33.80, P < .05). TSA showed that the sample size has exceeded the required information size (RIS = 528), while the Z-curve has crossed conventional boundary and trial sequential monitoring boundary. Therefore, the result was stable and no further studies were conducted. In addition, we found that the frequency of RARβ2 promoter hypermethylation in tissue sample was lower than the frequency of RARβ2 promoter hypermethylation in blood sample. The subgroup analysis based on race was conducted due to significant heterogeneity. The results showed that heterogeneity among studies in Asians disappeared, and the result still presented a significant association in Caucasians (OR = 12.51, 95% CI = 3.39–46.15, P < .05). From the analysis of clinical progression of breast cancer, significant associations of RARβ2 promoter hypermethylation with lymph node metastasis and TNM-stage of breast cancer were detected in Caucasians (for node metastasis, OR = 2.13, 95% CI = 1.04–4.47, P < .05; for TNM-stage, OR = 1.85, 95% CI = 1.33–2.57, P < .05). At the same time, no heterogeneity among studies was observed on breast cancer pathogenesis. In addition, no associations of RARβ2 promoter hypermethylation ER status, PR status, and menopause of breast cancer were observed (Table 2, Fig 2, 3, 4).
Table 2.
Meta-analysis results of associations between RARβ2, DAPK, hMLH1, p14, and p15 promoter hypermethylation and clinical features of breast cancer.
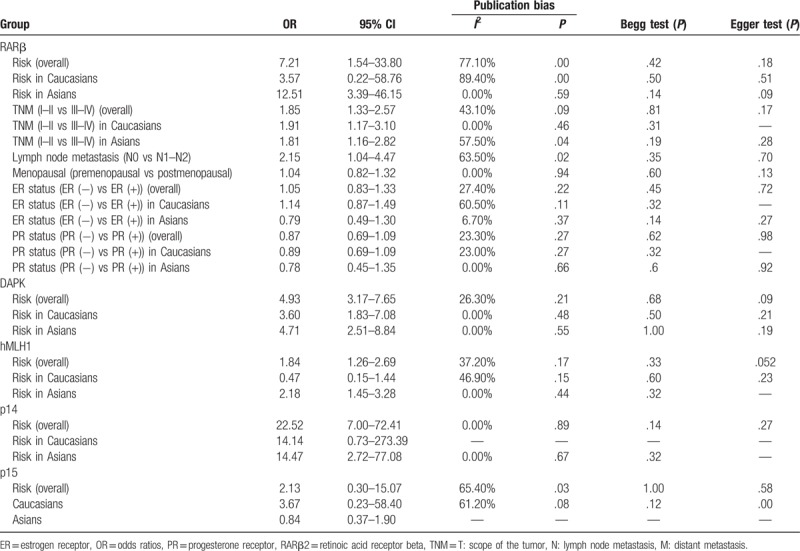
Figure 2.
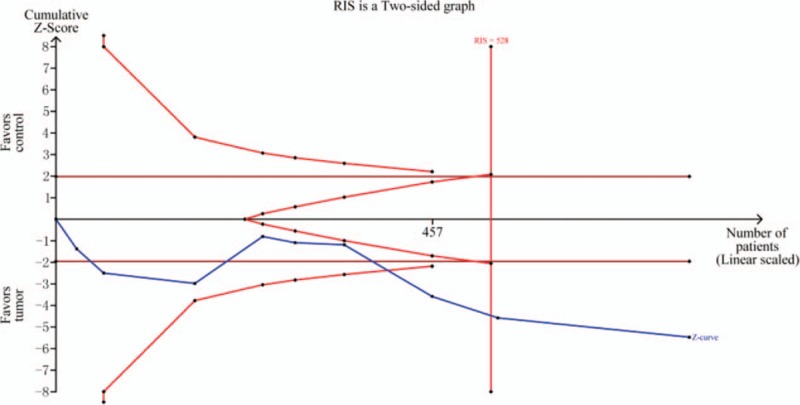
TSA on pooled results for effects of RARβ2 promoter hypermethylation on risk of breast cancer. RIS = required information size.
Figure 3.
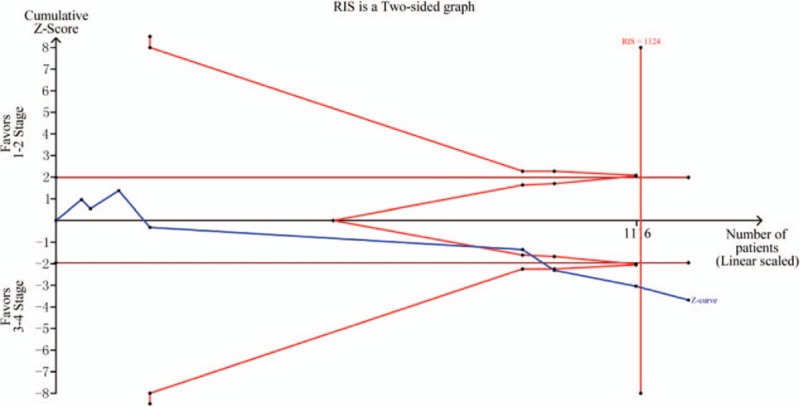
TSA on pooled results for effects of RARβ2 promoter hypermethylation on TNM-stage of breast cancer. RIS = required information size.
Figure 4.
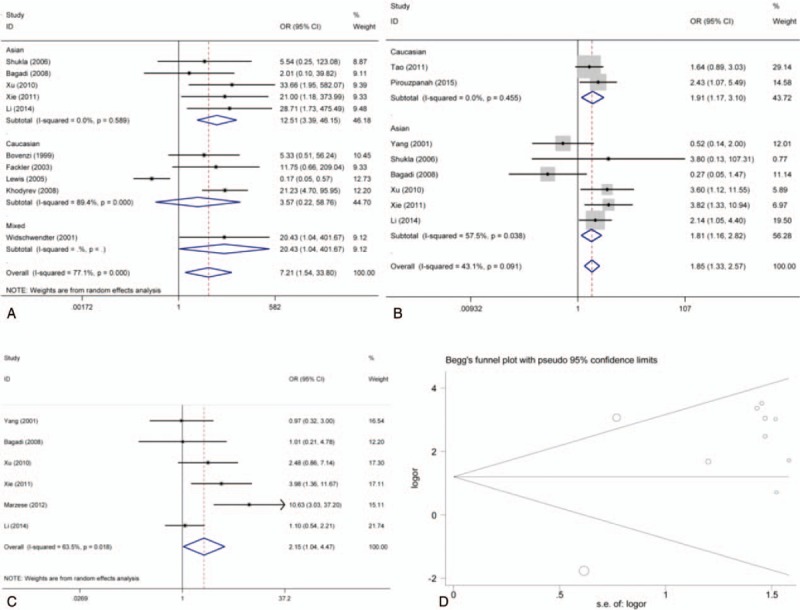
Forest plot and funnel plot for associations of RARβ2 promoter hypermethylation with risk, TNM-stage, and lymph node metastasis of breast cancer. (A) Forest plot for risk; (B) forest plot for TNM-stage; (C) forest plot for lymph node metastasis; (D) funnel plot for risk. CI = confidence intervals, Log OR = log odds ratio, OR = odds ratio, s.e. of logOR = standard error of log odds ratio.
3.4. Results of DAPK, hMLH1, p14, and p15 promoter hypermethylation in susceptibility of breast cancer
In order to observe the strength of associations between DAPK, hMLH1, p14, and p15 promoter hypermethylation and breast cancer risk, we drawed the forest plots to acquire the overall OR and 95% CI. The results showed that there were significant associations of DAPK, hMLH1, and p14 gene promoter hypermethylation with breast cancer risk, but not p15 gene promoter hypermethylation (DAPK, OR = 4.93, 95% CI = 3.17–7.65; for hMLH1, OR = 1.84, 95% CI = 1.26–1.29; for p14, OR = 22.52, 95% CI = 7.00–72.41; for p15, OR = 2.13, 95% CI = 0.30–15.07). Moreover, no significant heterogeneity was found in the analysis of DAPK, hMLH1, and p14 gene promoter hypermethylation. However, significant heterogeneity was found in the analysis about p15 gene promoter hypermethylation. In the sensitivity analysis, we found the study of Jung et al contributed a lot to the heterogeneity. But there was still no significant association between p15 gene promoter hypermethylation and breast cancer risk after the study of Jung was eliminated (OR = 3.67, 95% CI = 0.23–58.40) (Table 2, Fig. 5).
Figure 5.
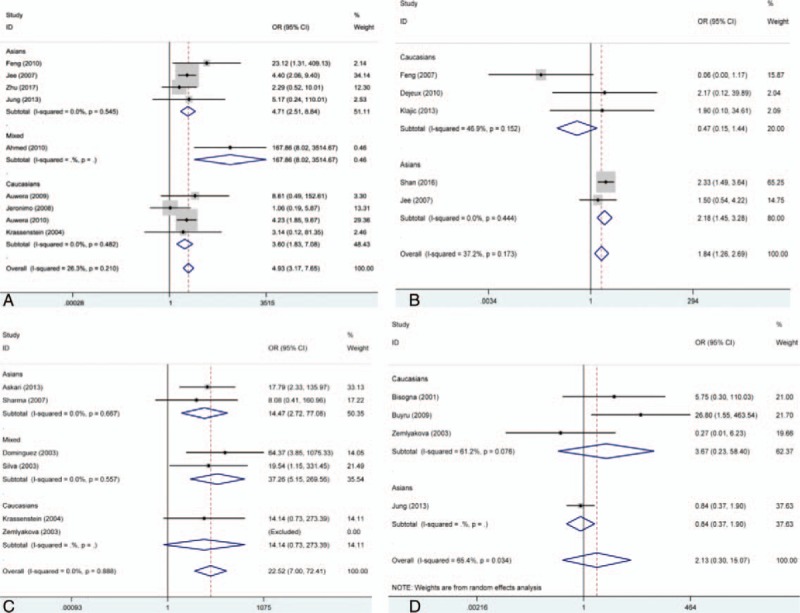
Forest plot for associations of DAPK, hMLH1, p14, and p15 promoter hypermethylation with risk of breast cancer. (A) Forest plot for DAPK; (B) forest plot for hMLH1; (C) forest plot for p14; (D) forest plot for p15. CI = confidence intervals, OR = odds ratio.
3.5. Publication bias and sensitivity analysis
According to the results of Begg test and Egger test, no obvious publication bias among studies was found in the analysis (Table 2, Fig. 6). Sensitivity analysis revealed that the pooled ORs did not have a significant change by eliminating each study in the analysis of RARβ2, DAPK, hMLH1, and p14 genes promoter hypermethylation. Therefore, it showed a robust result of the meta-analysis.
Figure 6.
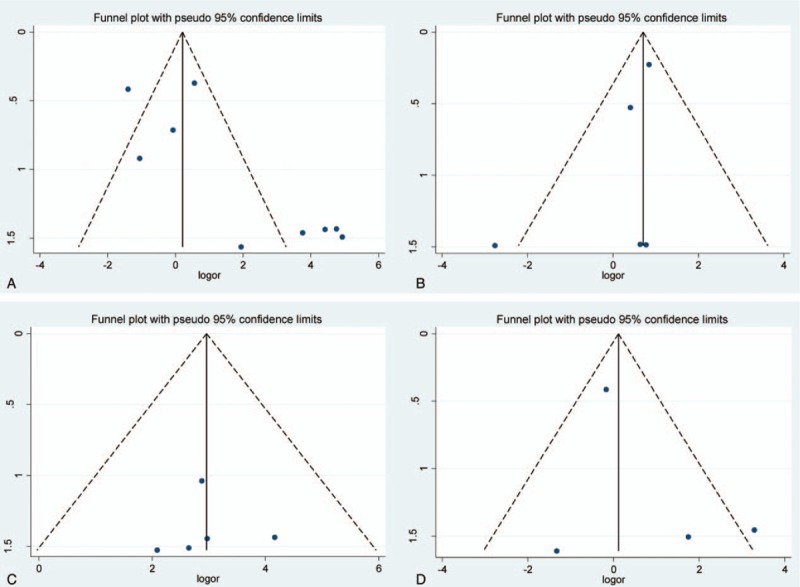
Funnel plot for associations of DAPK, hMLH1, p14, and p15 promoter hypermethylation with risk of breast cancer. (A) DAPK; (B) hMLH1; (C) p14; (D) p15. Log OR = log odds ratio, s.e.of logOR = standard error of log odds ratio.
4. Discussion
Although digital mammography and magnetic resonance imaging have screened numerous breast cancer patients, these physical methods lacked specificity and sensitivity.[61] As alternative biomarkers, DNA methylation was extensively studied in many tumor suppressor genes, especially in gene promoter region. DNA methylation usually was detected in the early stage of breast cancer and had a significant association with clinical characteristics of breast cancer, which was helpful for early detection and treatment of breast cancer.[62] Many biomarkers, benefitting the classification of clinical features in breast cancer, were identified through genome-wide approach, using tissue sample and blood sample.[63] For example, hyaluronoglucosaminidase 2 (HYAL2) had a lower methylation frequency in breast cancer group than control group. And the hypomethylation of HYAL2 was different with the methylation frequency of HYAL2, which the HYAL2 methylation was significant higher than control group. Therefore, these results demonstrated that abnormity of HYAL2 methylation might not originate from the tumor cycle DNA.[64] In this meta-analysis, the data of RARβ2 promoter hypermethylation in blood sample was extracted and analyzed, which indicated the frequency of RARβ2 promoter hypermethylation was higher than those in control group (OR = 15.04, 95% CI = 3.59–63.00, P < .05).[32–35] Given the condition of HYAL2, the hypermethylation of RARβ2 promoter hypermethylation in blood sample might not originate from tumor cells or tumor cycle DNA. Thus, the association of RARβ2 promoter hypermethylation with breast cancer pathogenesis was evaluated on the basis of the results of tissue sample.
According to results of tissue sample, the RARβ2 promoter hypermethylation had a significant association with the susceptibility of breast cancer, in which the breast cancer group had a higher frequency of RARβ2 promoter hypermethylation than normal tissue. These results were only indicated in Asians. In these included studies, 5 studies obtained negative results,[25,27–29,31] while other studies found significant associations.[22–24,26,30,32–35] Trial sequential analysis was also performed and the result suggested that the sample size has reached the required information size (RIS = 528) and the significant result was found. The trial sequential analysis was carried out according to the incidence of breast cancer group and control group. On the basis of the cumulative data, the average incidence rate of control group and breast cancer group were 6.19% and 31.85%. Furthermore, no significant associations of RARβ2 promoter hypermethylation with ER status, PR status, and menopause of breast cancer were observed by the present meta-analysis (P > .05). Of these studies for TNM-stage of breast cancer, Pirouzpanah et al,[38] Xu et al[24], Xie et al,[32] and Li et al[38] found that the frequency of RARβ2 promoter hypermethylation in III–IV stage was higher than those patients in I–II stage, but contrary results was indicated in the study of Tao et al,[37] Yang et al,[38] Shukla et al,[27] and Bagadi et al.[25] One thing to note was that no significant heterogeneity was found among included studies for the meta-analysis of TNM-stage. The required information size of studies regarding TNM-stage of breast cancer was 1124 based on TSA, but Z-curve has crossed the futility boundary, which showed an inapparent association. According to the data of lymph node metastasis, this epigenetic change of RARβ2 was significantly associated with the lymph node metastasis of breast cancer. In addition, no significant association between RARβ2 promoter hypermethylation and menopausal was presented in Caucasians and Asians. However, considering the limited number and sample sizes of studies both in Caucasians and Asians, more studies were still needed to demonstrate these findings of the meta-analysis. In addition, we did not find that RARβ2 promoter hypermethylation had significant associations with the status of ER and PR in Caucasians and Asians. As is well known, the expression of ER had an important role in predicting the response to endocrine therapy and recurrence of breast cancer.[61] Based on these results, RARβ2 promoter hypermethylation might not be associated with the ER status and PR status. RARβ2 promoter methylation might be a single biomarker to predict the risk and pathogenesis of breast cancer. However, further studies with large sample size and more clinical information were needed to be conducted to clarify these findings. Furthermore, a previous meta-analysis which assessed the association between RARβ2 promoter hypermethylation and breast cancer risk was conducted by Fang et al, and the results were consistent with the meta-analysis.[65] However, no clinical information was included in the previous meta-analysis. Therefore, this meta-analysis demonstrated RARβ2 promoter hypermethylation was significantly associated with the clinical progression of breast cancer.
On the other hand, significant heterogeneity was only found in the analysis of breast cancer risk and RARβ2 promoter hypermethylation in Caucasians. The result of subgroup analysis based on race indicated that race was not the mainly source of heterogeneity because significant heterogeneity still exist. Perhaps, other clinical information or tumor heterogeneity contributed a lot to the significant heterogeneity. Furthermore, no significant heterogeneity was found among studies about lymph node metastasis and TNM-stage of breast cancer. According to the results of sensitivity analysis, overall results were stable.
In many studies, many genes were used to detect breast cancer, including RASSF1A, APC, RARβ2, GSTP1, BRCA1, HOXA5, HIC-1, E-cadherin, p16, CyclinD2, HIN1, and TWIST genes promoter hypermethylation.[25,28,29,34–38] So the doctor diagnosed the breast cancer and estimated the clinical progression of breast cancer on the basis of the results of the status of methylation in these genes. In addition aberrant hypomethylation of genes such as: RASSF1A, APC, p16, p14, p15 were often detected in cancers. Thus the status of genes aberrant methylation might have a strong influence in the production of tumor cells. In the literature searching, we found that no meta-analysis was conducted to summarize the published data and discuss the relationship of DAPK, hMLH1, p14, and p15 hypermethylation and breast cancer. In the present meta-analysis, we have observed that DAPK, hMLH1, and p14 genes promoter hypermethylation were significantly associated with the susceptibility of breast cancer, and no obvious heterogeneity was found. However, the p15 gene promoter hypermethylation did not have a significant relationship with breast cancer risk. In the pooled analysis about p15 gene promoter hypermethylation, 4 studies with 280 breast tumor samples and 159 control samples were included in the meta-analysis. This might be partly explain the significant heterogeneity among studies (I2 = 65.4%, P = .034).
Several potential limitations should be noted in the present meta-analysis: the small sample size might be the mainly restrictions; no enough and detailed clinical information was included in this analysis, which might affect the evaluation of associations between RARβ2, DAPK, hMLH1, p14, and p15 gene promoter hypermethylation and susceptibility and clinical progression of breast cancer; heterogeneity was mainly found in Caucasians, therefore more studies in Caucasians should be further performed in the analysis of RARβ2; the studied population of included studies was mostly from Asians and Caucasians, thus caution should be taken in studied populations, and more studies in other races should be performed to clarify these results. The detailed molecular mechanisms of the abnormal hypomethylation of these genes should be studied. In addition, the expression of DNMT1 (DNA methyltransferase1), DNMT3a, DNMT3b, and TET double oxygenase in cancer or other disease, regulating genes methylation levels, were often lower than the control group.[66,67] However, it has been reported that some molecules might accurately regulate the methylation of certain target gene such as ncRNA in plants.[65] Published studies often altered the protein expression levels of DNMT1 (DNA methyltransferase1), DNMT3a, DNMT3b, and TET double oxygenase, and then observed relevant genes methylation, which might cause unpredictable influences.[66–68] In arabidopsis, the study of Zhou et al suggested that CLASSY family could control the locus-specific methylation of de novo DNA. And authors of the study speculated that similar regulating system of DNA methylation might exist in a broad range of organisms. Therefore, this might be a new direction to explore the DNA methylation.[69]
5. Conclusion
In conclusion, this meta-analysis demonstrated that RARβ2, DAPK, hMLH1, and p14 promoter hypermethylation was significantly associated with breast cancer risk. At the same time, significant associations of RARβ2 promoter hypermethylation with lymph node metastasis and TNM-stage of breast cancer were found. Considering the heterogeneity among studies, further studies with larger sample size, more clinical information, and environmental factors should be performed to validate these findings.
Author contributions
Data curation: Ming Qi.
Formal analysis: Ming Qi.
Funding acquisition: Xiang Xiong.
Investigation: Ming Qi.
Methodology: Ming Qi, Xiang Xiong.
Software: Ming Qi, Xiang Xiong.
Writing – original draft: Ming Qi.
Writing – review & editing: Xiang Xiong.
Footnotes
Abbreviations: CI = confidence intervals, CNKI = Chinese National Knowledge Infrastructure, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HYAL2 = hyaluronoglucosaminidase 2, NOS = Newcastle-Ottawa Scale, OR = odds ratios, PR = progesterone receptor, RA = retinoic acid, RARβ2 = retinoic acid receptor beta, RIS = required information size, TSA = trial sequential analysis.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [2].Rosa M. Advances in the molecular analysis of breast cancer: pathway toward personalized medicine. Cancer Control 2015;22:211–9. [DOI] [PubMed] [Google Scholar]
- [3].Diaz LK, Sneige N. Estrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracy. Adv Anat Pathol 2005;12:10–9. [DOI] [PubMed] [Google Scholar]
- [4].Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol 2008;21suppl 2:S8–15. [DOI] [PubMed] [Google Scholar]
- [5].Chang MC, Malowany JI, Mazurkiewicz J, et al. ’Genetic heterogeneity’ in HER2/neu testing by fluorescence in situ hybridization: a study of 2,522 cases. Mod Pathol 2012;25:683–8. [DOI] [PubMed] [Google Scholar]
- [6].Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- [7].Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733–43. [DOI] [PubMed] [Google Scholar]
- [8].Vance GH, Barry TS, Bloom KJ, et al. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arch Pathol Lab Med 2009;133:611–2. [DOI] [PubMed] [Google Scholar]
- [9].Mavaddat N, Antoniou AC, Easton DF, et al. Genetic susceptibility to breast cancer. Mol Oncol 2010;4:174–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mattei MG, de The H, Mattei JF, et al. Assignment of the human hap retinoic acid receptor RAR beta gene to the p24 band of chromosome 3. Hum Genet 1988;80:189–90. [DOI] [PubMed] [Google Scholar]
- [11].Uray IP, Dmitrovsky E, Brown PH. Retinoids and rexinoids in cancer prevention: from laboratory to clinic. Semin Oncol 2016;43:49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [14].Kulinskaya E, Wood J. Trial sequential methods for meta-analysis. Res Synth Methods 2014;5:212–20. [DOI] [PubMed] [Google Scholar]
- [15].Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet 1955;19:251–3. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [18].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [19].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [20].Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L, Gerson L, Maluf-Filho F. Systematic review and meta-analysis in GI endoscopy: why do we need them? How can we read them? Should we trust them? Gastrointest Endosc 2018;88:139–50. [DOI] [PubMed] [Google Scholar]
- [22].Li SY, Li R, Mai HF, et al. RARβ gene methylation could be used in prognosis of breast cancer patients with lymphatic metastasis. Cancer Res Prev Treat 2014;41:443–6. [Google Scholar]
- [23].Xie F, Xiang XY, Zhang JC, et al. Methylation of retinoic acid receptorβ2 promoter in sporadic breast cancer tissue. Cancer Res Prev Treat 2011;38:408–15. [Google Scholar]
- [24].Xu X, Sun JY, Gu L, et al. Epigenetic regulation of retinoic acid receptorβ2 gene in the carcinogenesis of breast cancer. Chin J Clin Oncol 2010;37:1326–33. [Google Scholar]
- [25].Bagadi SA, Prasad CP, Kaur J, et al. Clinical significance of promoter hypermethylation of RASSF1A, RARbeta2, BRCA1 and HOXA5 in breast cancers of Indian patients. Life Sci 2008;82:1288–92. [DOI] [PubMed] [Google Scholar]
- [26].Khodyrev DS, Loginov VI, Pronina IV, et al. Methylation of promoter region of RAR-beta2 gene in renal cell, breast, and ovarian carcinomas. Genetika 2008;44:1126–32. [PubMed] [Google Scholar]
- [27].Shukla S, Mirza S, Sharma G, et al. Detection of RASSF1A and RARbeta hypermethylation in serum DNA from breast cancer patients. Epigenetics 2006;1:88–93. [DOI] [PubMed] [Google Scholar]
- [28].Lewis CM, Cler LR, Bu DW, et al. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin Cancer Res 2005;11:166–72. [PubMed] [Google Scholar]
- [29].Fackler MJ, McVeigh M, Evron E, et al. DNA methylation of RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and invasive lobular breast carcinoma. Int J Cancer 2003;107:970–5. [DOI] [PubMed] [Google Scholar]
- [30].Widschwendter M, Berger J, Muller HM, et al. Epigenetic downregulation of the retinoic acid receptor-beta2 gene in breast cancer. J Mammary Gland Biol Neoplasia 2001;6:193–201. [DOI] [PubMed] [Google Scholar]
- [31].Bovenzi V, Le NL, Cote S, et al. DNA methylation of retinoic acid receptor beta in breast cancer and possible therapeutic role of 5-aza-2’-deoxycytidine. Anticancer Drugs 1999;10:471–6. [DOI] [PubMed] [Google Scholar]
- [32].Xie F, Zhang AB, Ouyang JL. Analysis of retinoic acid receptorβ2 promoter in blood plasma of patients with breast cancer. J Hubei Inst Med 2011;30:371–3. [Google Scholar]
- [33].Rykova EY, Tsvetovskaya GA, Sergeeva GI, et al. Methylation-based analysis of circulating DNA for breast tumor screening. Ann N Y Acad Sci 2008;1137:232–5. [DOI] [PubMed] [Google Scholar]
- [34].Hoque MO, Feng Q, Toure P, et al. Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. J Clin Oncol 2006;24:4262–9. [DOI] [PubMed] [Google Scholar]
- [35].Skvortsova TE, Rykova EY, Tamkovich SN, et al. Cell-free and cell-bound circulating DNA in breast tumours: DNA quantification and analysis of tumour-related gene methylation. Br J Cancer 2006;94:1492–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pirouzpanah S, Taleban FA, Mehdipour P, et al. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB, BRCA1, and RASSF1A genes in breast cancer patients. J Mol Med (Berl) 2015;93:917–34. [DOI] [PubMed] [Google Scholar]
- [37].Tao MH, Marian C, Nie J, et al. Body mass and DNA promoter methylation in breast tumors in the Western New York Exposures and Breast Cancer Study. Am J Clin Nutr 2011;94:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang Q, Sakurai T, Yoshimura G, et al. Hypermethylation does not account for the frequent loss of the retinoic acid receptor beta2 in breast carcinoma. Anticancer Res 2001;21:1829–33. [PubMed] [Google Scholar]
- [39].Marzese DM, Hoon DS, Chong KK, et al. DNA methylation index and methylation profile of invasive ductal breast tumors. J Mol Diagn 2012;14:613–22. [DOI] [PubMed] [Google Scholar]
- [40].Cho YH, Shen J, Gammon MD, et al. Prognostic significance of gene-specific promoter hypermethylation in breast cancer patients. Breast Cancer Res Treat 2012;131:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhu Y, Li S, Wang Q, et al. Quantitative and correlation analysis of the DNA methylation and expression of DAPK in breast cancer. PeerJ 2017;5:e3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jung EJ, Kim IS, Lee EY, et al. Comparison of methylation profiling in cancerous and their corresponding normal tissues from Korean patients with breast cancer. Ann Lab Med 2013;33:431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jing F, Yuping W, Yong C, et al. CpG island methylator phenotype of multigene in serum of sporadic breast carcinoma. Tumour Biol 2010;31:321–31. [DOI] [PubMed] [Google Scholar]
- [44].Ahmed IA, Pusch CM, Hamed T, et al. Epigenetic alterations by methylation of RASSF1A and DAPK1 promoter sequences in mammary carcinoma detected in extracellular tumor DNA. Cancer Genet Cytogenet 2010;199:96–100. [DOI] [PubMed] [Google Scholar]
- [45].Van der Auwera I, Bovie C, Svensson C, et al. Quantitative methylation profiling in tumor and matched morphologically normal tissues from breast cancer patients. BMC Cancer 2010;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Van der Auwera I, Bovie C, Svensson C, et al. Quantitative assessment of DNA hypermethylation in the inflammatory and non-inflammatory breast cancer phenotypes. Cancer Biol Ther 2009;8:2252–9. [DOI] [PubMed] [Google Scholar]
- [47].Jeronimo C, Monteiro P, Henrique R, et al. Quantitative hypermethylation of a small panel of genes augments the diagnostic accuracy in fine-needle aspirate washings of breast lesions. Breast Cancer Res Treat 2008;109:27–34. [DOI] [PubMed] [Google Scholar]
- [48].Sung-Bae J, Woo-Chan P, Kee-Whan K, et al. Methylation patterns of cancer-associated genes in breast cancer. J Breast Cancer 2007;10:51–8. [Google Scholar]
- [49].Krassenstein R, Sauter E, Dulaimi E, et al. Detection of breast cancer in nipple aspirate fluid by CpG island hypermethylation. Clin Cancer Res 2004;10(1 Pt 1):28–32. [DOI] [PubMed] [Google Scholar]
- [50].Shan M, Yin H, Li J, et al. Detection of aberrant methylation of a six-gene panel in serum DNA for diagnosis of breast cancer. Oncotarget 2016;7:18485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Klajic J, Fleischer T, Dejeux E, et al. Quantitative DNA methylation analyses reveal stage dependent DNA methylation and association to clinico-pathological factors in breast tumors. BMC Cancer 2013;13:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dejeux E, Ronneberg JA, Solvang H, et al. DNA methylation profiling in doxorubicin treated primary locally advanced breast tumours identifies novel genes associated with survival and treatment response. Mol Cancer 2010;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Feng W, Shen L, Wen S, et al. Correlation between CpG methylation profiles and hormone receptor status in breast cancers. Breast Cancer Res 2007;9:R57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Askari M, Sobti RC, Nikbakht M, et al. Promoter hypermethylation of tumour suppressor genes (p14/ARF and p16/INK4a): case-control study in North Indian population. Mol Biol Rep 2013;40:4921–8. [DOI] [PubMed] [Google Scholar]
- [55].Sharma G, Mirza S, Prasad CP, et al. Promoter hypermethylation of p16INK4A, p14ARF, CyclinD2 and Slit2 in serum and tumor DNA from breast cancer patients. Life Sci 2007;80:1873–81. [DOI] [PubMed] [Google Scholar]
- [56].Dominguez G, Silva J, Garcia JM, et al. Prevalence of aberrant methylation of p14ARF over p16INK4a in some human primary tumors. Mutat Res 2003;530:9–17. [DOI] [PubMed] [Google Scholar]
- [57].Silva J, Silva JM, Dominguez G, et al. Concomitant expression of p16INK4a and p14ARF in primary breast cancer and analysis of inactivation mechanisms. J Pathol 2003;199:289–97. [DOI] [PubMed] [Google Scholar]
- [58].Zemliakova VV, Zhevlova AI, Strel’Nikov VV, et al. [Abnormal methylation of several tumor suppressor genes in sporadic breast cancer]. Mol Biol (Mosk) 2003;37:696–703. [PubMed] [Google Scholar]
- [59].Buyru N, Altinisik J, Ozdemir F, et al. Methylation profiles in breast cancer. Cancer Invest 2009;27:307–12. [DOI] [PubMed] [Google Scholar]
- [60].Bisogna M, Calvano JE, Ho GH, et al. Molecular analysis of the INK4A and INK4B gene loci in human breast cancer cell lines and primary carcinomas. Cancer Genet Cytogenet 2001;125:131–8. [DOI] [PubMed] [Google Scholar]
- [61].Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001;19:924–30. [DOI] [PubMed] [Google Scholar]
- [62].Moulton T, Crenshaw T, Hao Y, et al. Epigenetic lesions at the H19 locus in Wilms’ tumour patients. Nat Genet 1994;7:440–7. [DOI] [PubMed] [Google Scholar]
- [63].Clark GM, McGuire WL. Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol 1988;15:20–5. [PubMed] [Google Scholar]
- [64].Yang R, Pfutze K, Zucknick M, et al. DNA methylation array analyses identified breast cancer-associated HYAL2 methylation in peripheral blood. Int J Cancer 2015;136:1845–55. [DOI] [PubMed] [Google Scholar]
- [65].Fang C, Jian ZY, Shen XF, et al. Promoter methylation of the retinoic acid receptor beta2 (RARbeta2) Is associated with increased risk of breast cancer: a PRISMA compliant meta-analysis. PLoS ONE 2015;10:e0140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Coluccio A, Ecco G, Duc J, et al. Individual retrotransposon integrants are differentially controlled by KZFP/KAP1-dependent histone methylation, DNA methylation and TET-mediated hydroxymethylation in naïve embryonic stem cells. Epigenetics Chromatin 2018;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yan F, Shen N, Pang JX, et al. A vicious loop of fatty acid-binding protein 4 and DNA methyltransferase 1 promotes acute myeloid leukemia and acts as a therapeutic target. Leukemia 2018;32:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Koch A, Joosten SC, Feng Z, et al. Analysis of DNA methylation in cancer: location revisited. Nat Rev Clin Oncol 2018;15:459–66. [DOI] [PubMed] [Google Scholar]
- [69].Zhou M, Palanca AMS, Law JA. Locus-specific control of the de novo DNA methylation pathway in Arabidopsis by the CLASSY family. Nat Genet 2018;50:865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


