Abstract
Background:
Diagnosing schizophrenia is primarily based on the presentation of defined signs and symptoms, none of which is pathognomonic for this group of syndromes. However, few significant genome-wide associations between schizophrenia and individual have detected. Protein profiling of candidate serum biomarkers in schizophrenia is therefore an area of great interest.
Methods:
In the present study, we used a combination of 7% polyethylene glycol (PEG) enrichment of immune complexes and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to separate abnormal band, then analyse the band with liquid chromatography mass spectrometry (LC-MS).
Results:
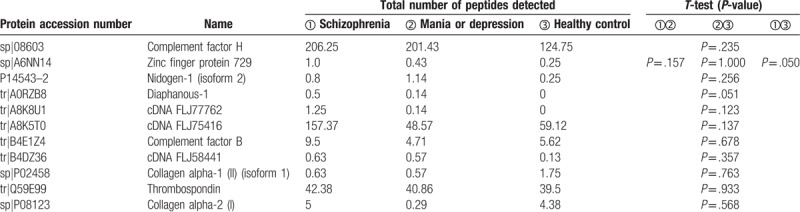
There is a special 150-kD electrophoretic band in patients with schizophrenia, bipolar disorder, or depression relative to healthy controls (each 30 samples). Analysis of the band using LC-MS resulted in the identification of 11 serum proteins whose abundance was altered between patients and controls. Among them, 8 proteins (CFH, CFB, cDNA FLJ75416, zinc finger protein 729, isoform 2 of nidogen-1, diaphanous-1, cDNA FLJ77762, and cDNA FLJ58411) were up regulated, while one protein (isoform 1 of collagen alpha-1 (II) was down regulated in patients with schizophrenia, but only zinc finger protein 729 has statistics significance (P < .05). No differences were noted with regard to thrombospondin-1 or collagen alpha-2 (I) among the 3 groups. These proteins take part in several biological functions such as focal adhesion, complement cascades, ECM-receptor interaction, and Staphylococcus aureus infection.
Conclusions:
The 150-kD electrophoretic band or zinc finger protein 729 may become biomarkers in patients with schizophrenia. In the future increasing sample size and function research of zinc finger protein 729 should be executed continuously.
Keywords: biomarkers, protein, schizophrenia
1. Introduction
Schizophrenia is a severe, complex neuropsychiatric condition that affects approximately 0.7% of the population worldwide.[1] The current standards for diagnosis are based on criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders IV or the International Statistical Classification of Diseases and Related Health Problems,10th revision (ICD-10).[2,3] Such rigid definitions leave clinicians with the pervasive problem of diagnosing schizophrenia primarily based on the presentation of defined signs and symptoms, none of which is pathognomonic for this group of syndromes.[4]
Given these issues, the availability of tests for molecular biomarkers based on the underlying pathology would greatly facilitate disease diagnosis and stratification. Several studies have been conducted in the search for genetic mutations linked to schizophrenia. However, few studies have detected significant genome-wide associations between schizophrenia and individual single nucleotide polymorphisms (SNPs).[5,6] Protein profiling of candidate serum biomarkers in schizophrenia is therefore an area of great interest and potential with regard to diagnosis and individualized patient treatment. Recent studies have indicated that some patients with schizophrenia exhibit proteomic or metabolomic alterations. Such studies have reported altered levels of phosphorylated proteins, selected apolipoproteins, and plasma and serum proteins as well as abnormal immune system function in patients with schizophrenia.[7–10] However, attempts to utilize these biomarkers to secure a diagnosis in clinical settings have been largely unsuccessful.
In the present study, we used a combination of 7% PEG enrichment of immune complexes and SDS-PAGE in order to identify potential biomarker proteins in the 150-kD band of samples obtained from patients with schizophrenia.
The results were expected to find an objective index for diagnosis, therapy, and effect of schizophrenia. They are also explored to explain the pathogenesis of schizophrenia.
2. Materials and methods
The Ethical Committee of the Longgang Central Hospital reviewed and approved the protocol of the present study as well as the procedures for sample collection and analysis. All study participants provided written informed consent.
2.1. Clinical samples
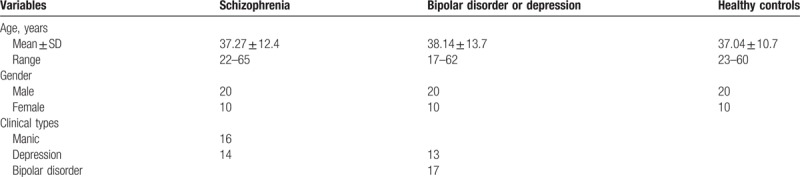
Thirty patients with paranoid schizophrenia (20 men/10 women, mean age: 37.27 ± 12.4 years) diagnosed according to ICD-10 criteria and thirty patients with bipolar disorder (n = 17) or depression (n = 13) (20 men/10 women, mean age: 38.14 ± 13.7 years) were recruited from Kangning Hospital in Shenzhen, China. In addition, 30 healthy, age- and sex-matched control participants (20 men/10 women, mean age: 37.04 ± 10.7 years) with no family history of schizophrenia or other detectable psychiatric, neurological, or medical history were recruited from among hospital staff. No participants had been diagnosed with diabetes mellitus, hyperlipidemia, or other severe physical diseases (see Table 1).
Table 1.
Basic characteristics of subjects in schizophrenia, bipolar disorder, or depression and healthy controls.
Venous blood was drawn from each participant in the morning prior to breakfast at least 8 hours, collected in 7.5 mL serum S-Monovette tubes (BD, UK), and allowed to clot for 2 hours at 21°C. Centrifugation (4000 × g for 5 minutes at 21°C) was performed to remove any remaining clotted or cellular material. The supernatants were transferred to 1.5 mL microcentrifuge tubes (Eppendorf, UK) and stored at −70°C until use.
2.2. Preparation and separation of immune complex
A total 100 μL of serum was mixed with an equal volume of 7% PEG6000 (Sigma-Aldrich) in PBS (Phosphate Buffered Saline, Gibco) and vortex mixed for 1 minute. The sample was then incubated overnight at 4°C. Centrifugation was then performed at 1500 rpm for 20 minutes at 4°C in order to produce a clear supernatant with precipitate at the bottom. The supernatant was discarded, and the sample was then resolubilized in 50 μL deionized water and vortex mixed for 1 minute.[11]
Electrophoresis was conducted at 40 mA per gel for 1 hour in buffer containing 24 mM Tris (Sigma), 0.2 M glycine (Sigma), 0.1% w/v SDS (Sigma) (pH 8.6). The concentration of the separation gel was 10%. Following electrophoresis, the gel was placed directly into the stain solution (0.2% CBB R-250, 83% methanol/17% acetic acid).[12] The gel was stained for 4 hours under gentle agitation, and then destained with 50% methanol/10% acetic acid for 4–8 hours under gentle agitation and rinsed with deionized water for at least 15 minutes prior to image acquisition.
Following imaging, we analyzed differences between the patient and control groups with regard to the electrophoretic bands observed. In cases of significant difference, the molecular weight of the protein was determined and compared to known protein markers. The appropriate area was then extracted, including the 150 kD band and related negative area, placed in a sterilized Eppendorf tube, and stored at −70°C for mass spectrometry analysis.
2.3. Component analysis of the 150 kD electrophoretic band by using LC-MS
LC-MS was performed by BGI-Tech (Shenzhen, China). Proteins were digested using trypsin (Pierce, US) at a ratio of 1:50 (w/w, trypsin/protein) for 20 hours at 37°C. Nano-LC-MS/MS experiments were performed on an HPLC system comprised of 2 LC-20AD nano-flow LC pumps, an aSIL-20 AC auto-sampler, and LC-20AB micro-flow LC pump (all Shimadzu, Tokyo, Japan) connected to an LTQ-Orbitrap mass spectrometer (ThermoFisher, San Jose, CA). The mass spectrometry data were matched with data simulated by the Swiss-Prot/UniProt database with Mascot 2.3.02 in order to obtain protein result. Gene ontology (GO), clusters of orthologous groups of proteins (COG), and Kyoto Encyclopedia of Genes and Genomes (KEGG) data were also retrieved and analyzed.
2.4. Molecular networks
In order to explore the biological function of proteins for which significant differences were identified, we utilized the Ingenuity Pathway Analysis (IPA) Knowledge Base (Ingenuity Systems, Redwood City, CA, www.ingenuity.com).
2.5. Statistical analysis
All statistical analyses were performed using SPSS Version 13 software (SPSS Inc., Chicago, IL). The non-parametric Kruskal–Wallis test was used to evaluate differences in the abundance of each protein among the 3 groups. The level of statistical significance was set at P < .05.
3. Results
3.1. The 150 kD electrophoretic protein band
See Figure 1.
Figure 1.
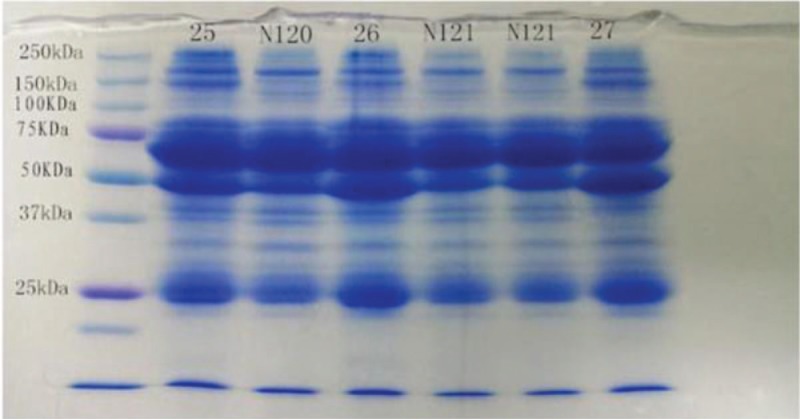
SDS-PAGE in patients with schizophrenia and healthy controls. M indicates a molecular protein marker with a scope of 10-250 kD. Samples 25, 26, 27, 41, and 42 obtained from patients with schizophrenia. Samples N120, N121 were obtained from healthy controls. SDS-PAGE = sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
3.2. Mass spectrometry of abnormal protein band
All 150 kD electrometric protein bands, including positive and negative regions, were analyzed using LC-MS. Differences in the density of each protein were noted (Table 2).
Table 2.
Density of the 11 identified proteins in patients with schizophrenia, bipolar disorder, or depression and healthy controls.
3.3. Molecular networks
In order to explore the biological function of the proteins for which significant alterations were observed, we utilized the IPA Knowledge Base. This platform enables researchers to visualize potential interactions between the molecules of interest and others that may not have been detected in a particular analysis. Using the IPA, we were able to determine that 8 of the 11 proteins identified in the schizophrenia cohort can be mapped onto a specific molecular network (see Figs. 2–4).
Figure 2.
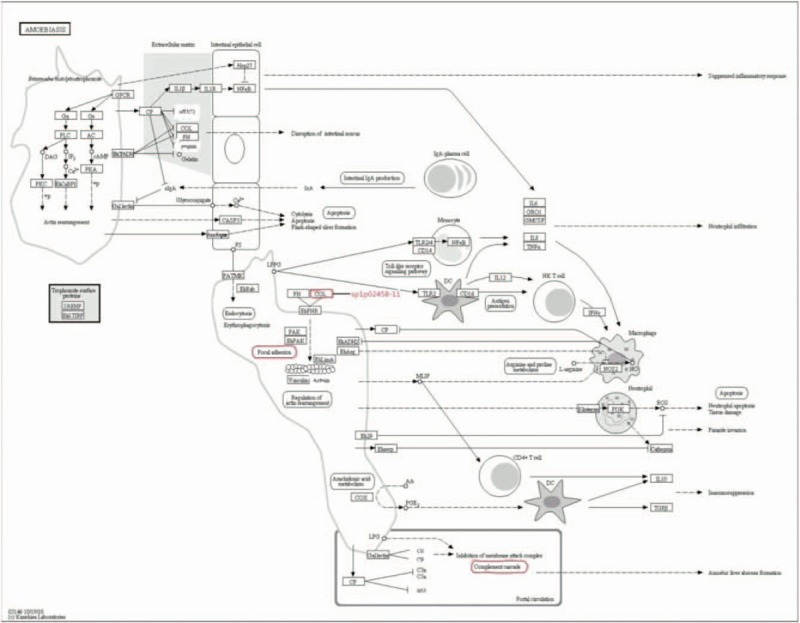
Amoebiosis. The amoebiosis included focal adhesion pathway, complement cascade pathway and isoform 1 of collagen alpha-1 (II) (sp|P02458).
Figure 4.
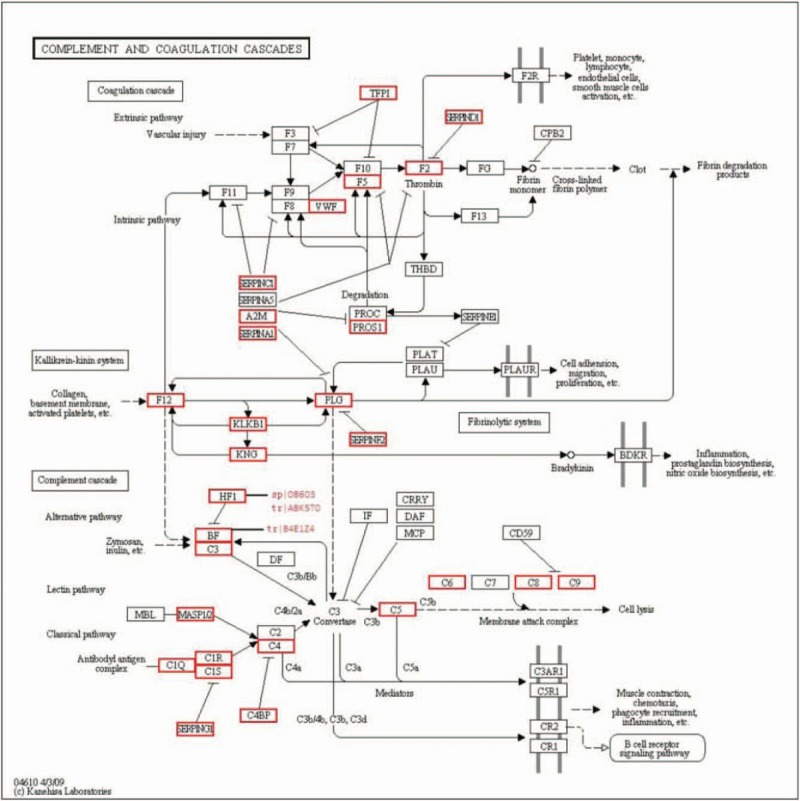
Complement cascade pathway. Complement factor H (sp|08603), cDNA FLJ75416 (tr|A8K5T0), and complement factor B (tr|B4E1Z4) are included in the pathway outlined above.
Figure 3.
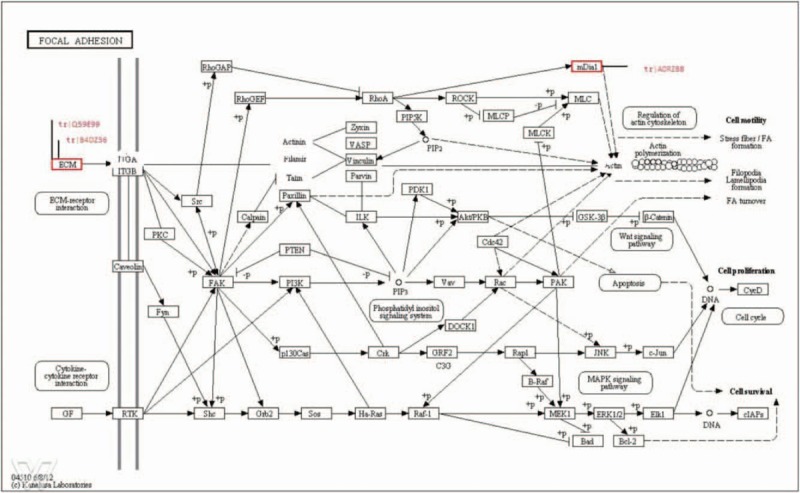
Focal adhesion pathway. The focal adhesion pathway outline above includes thrombospondin (tr|Q59E99), cDNA FLJ61587 (tr|B4DZ36), and diaphanous-1 (tr|A0RZB8).
The significantly altered proteins as well as those proteins linked to them are not part of a single biological pathway, but rather take part in several biological functions such as focal adhesion, complement cascades, ECM-receptor interaction, and Staphylococcus aureus infection.
Our analysis revealed that BF, HF, THBS-1, and diaphanous-1 are key nodes in the network. Apart from ZNF729, isoform 2 of nidogen-1, and isoform 1 of collagen alpha-1 (II), a direct link was observed between each of the identified molecules, which may indicate an interaction between them.
4. Discussion
In the present study, a PEG precipitation method was utilized for large scale enrichment of serum immune complexes. Label-free relative quantitative proteomics analyses were used to assess the serum proteomic profiles of patients with schizophrenia, bipolar disorder, depression, and healthy controls.
The present study is the first to report the identification of a special 150-kD electrophoretic band in patients with schizophrenia, bipolar disorder, or depression relative to healthy controls. Analysis of the band using LC-MS resulted in identification of 11 serum proteins whose abundance was altered between patients and controls. Among them, eight proteins (CFH, CFB, cDNA FLJ75416, zinc finger protein 729, isoform 2 of nidogen-1, diaphanous-1, cDNA FLJ77762, and cDNA FLJ58411) were up-regulated, while one protein isoform 1 of collagen alpha-1 (II) was down-regulated in patients with schizophrenia, but only zinc finger protein 729 has statistics significance (P < .05). No differences were noted with regard to thrombospondin-1 or collagen alpha-2 (I) among the 3 groups. Our findings were further supported by ELISA validation in crude serum.
4.1. Zinc finger protein 729
Chromosome 19 contains members of several different ZNF gene subfamilies, though most of the clustered genes belong to the KRAB-ZNF subtype.[13] The function of the zinc fingers primarily involves the recognition of DNA and activation of transcriptional processes. In addition to DNA binding, domains containing C2H2 zinc fingers have been implicated in nuclear localization, protein-protein interaction, and DNA bending.[14]
Currently, more than 10 families of ZNFs have been identified. Though no data with regard to ZNF729 have been reported for schizophrenia, ZNF804A has been identified as a candidate gene for Schizophrenia (SCZ), ASD (autism spectrum disorders), and BD (bipolar disorder) in replicated GWAS (genome wide association studies) and via CNV (copy number variation) analysis, although its function has not been well characterized.[15] Furthermore, Jaros et al[7] reported decreased phosphorylation of ZNF148 in patients with schizophrenia, though no changes in overall levels of ZNF148 were observed. We found that ZNF 729 obviously increased in schizophrenia than other groups. Further study will focus on ZNF729 to explore its function in schizophrenia etiology.
4.2. Complement factors B and H
Three of the 11 proteins for which significant alterations in abundance were observed (CFB, CFH, cDNA FLJ75416) belong to the complement super family. CFH, a regulator of complement activation, is involved in protection from thrombotic microangiopathies and advanced macular degeneration.[16] CFH competes with B or Bb for binding to C3b and accelerates the displacement of Bb from C3b, thereby inhibiting the formation of C3 convertase (C3bBb) in the alternative pathway.[17] Constitutive high expression of CFH has been detected in the eye, and a CFH polymorphism is strongly associated with age-related macular degeneration.[18] Individuals with a congenital deficiency of factor H are susceptible to microangiopathies,[19,20] and those with senile deficiency are susceptible to early development of advanced macular degeneration.[21,22] CFB is an enhancer of an alternative pathway of complement activation and is increased and augmented in many inflammatory diseases.
Nevertheless, the findings regarding the role of CFH and CFB in schizophrenia remain inconsistent. Singer et al[23] reported a significant increase in the frequency of the FB∗F allele of CFB among patients with paranoid schizophrenia, while Rudduck et al[24] provided contrary data supporting a decrease in the frequency of this allele in familial schizophrenia. Furthermore, Yang et al[25] and Karine[26] reported increased plasma levels of CFB in schizophrenia, while Jaros et al[7] reported unchanged levels of CFH and increased levels of CFB. In the present study, we observed increases in CFH, CFB, and cDNA FLJ75416 in patients with schizophrenia. Such conflicting findings lead us to speculate that the methods utilized for serum preparation in some studies may have resulted in the depletion of some high-abundance proteins, selectively enriching results for phosphorylated proteins.
4.3. Thrombospondin-1 (THBS-1) and Nidogen-1
THBS-1 is a protein encoded by the THBS1 gene in humans. Research has indicated that THBS-1 is involved in cell adhesion, signaling, proliferation, and angiogenesis.[27,28] Further reports suggest that THBS-1 is involved in tumorigenesis[29] and promotes the induction of immune regulatory cells.[30–32] In humans, 2 nidogen proteins, nidogen-1 (150 kD), and nidogen-2 (200 kD), have been identified. The 2 proteins share a 46% primary sequence identity and a similar three-dimensional structure, consisting of 3 globular domains (G1, G2, G3) connected by a flexible link and a rod.[33] The nidogens bind and form a ternary complex with laminin-1 and collagen type IV, connecting the 2 networks and stabilizing and maintaining the structure of the basement membrane.[34] Physiologically, nidogens have been shown to interact with cell receptor molecules and also control cell polarization, migration, and invasion.[35]
Schwarz et al[36] reported that THBS-1 was significantly increased at the time of relapse between the short- and long-term relapse groups in a study involving patients with schizophrenia. Jaros et al,[7,37] however, reported no change in levels of THBS-1 in patients with schizophrenia. In contrast, Katsel et al[38] reported that THBS-1 was significantly reduced in the anterior cingulate gyrus in patients with schizophrenia.
Though no data is available regarding serum nidogens in schizophrenia, Ning et al[39] observed that both THBS-1 and nidogen levels appear to increase over time following oxidative stress. Though we observed increases in levels of nidogens, our results indicated no changes in serum levels of THBS-1 in patients with schizophrenia, bipolar disorder, or depression when compared to healthy controls. Such differences may be due to the various tissues affected by and complex pathology of schizophrenia.
4.4. Collagen alpha-1 (II) and collagen alpha-2 (I)
Type I collagen is a heterotrimer consisting of 2 a1 (I) chains and one a2 (I) chain, encoded, respectively, by the COL1A1 and COL1A2 genes. The type I alpha 2 collagen gene, located on chromosome 7q22.1, is 38 kb in length and has 52 exons.[40,41] Collagen is the most abundant extracellular matrix protein in humans and is the major structural protein of skin, bone, tendon, ligaments, and the cornea.[42] Various studies have revealed that collagen is associated with intracranial aneurysm and its redisposition. Type II collagen forms fibrils with the same periodicity in cartilage and the vitreous humor, and is a homotrimer composed of 3 α1 (II) chains, which show a high sequence homology to α1 (I).[43]
In the present study, we observed decreases in collagen alpha-1 (II) and collagen alpha-2 (I) chains in patients with schizophrenia, bipolar disorder, and depression when compared to controls. Though no previous data have been published with regard to collagen alpha-1 (II) and collagen alpha-2 (I) chains in schizophrenia, Doknic et al[44] reported that a potential long-term consequence of asymptomatic hypogonadism due to risperidone-induced hyperprolactinemia may cause a slight rise in the C-terminal telopeptide of type I collagen (CTX), particularly in patients with schizophrenia who have been treated with long-acting injectable risperidone (LAIR) for 12 to 24 months. Liang et al[45] also reported significantly increased levels of the amisulpride group in patients with schizophrenia after 4 weeks of antipsychotic treatment.
4.5. Diaphanous-1
Mammalian diaphanous-related formin (mDia1, diaphanous-1) is a regulator of linear actin polymerization and mediator of Ras homolog family members in mammals. Loss of this protein results in aberrant myeloid differentiation and hyperactivity of the immune system.[46] In the present study, we observed significant increases in diaphanous-1 in patients with schizophrenia, though further research is required in order to elucidate the potential role of this decrease in the pathology of the disorder.
4.6. Changes in cDNA FLJ77762 and cDNA FLJ58441
Research has confirmed that cDNA FLJ77762 is highly similar to cullin-associated and neddylation-dissociated 1 (CAND1) mRNA in humans. CAND1, a negative regulator of SKP1-Cullin1-F-box (SCF) ubiquitin ligases,[47] interacts with cullin in a mutually exclusive fashion via substrate adaptor binding and neddylation.[48]
On the other hand, cDNA FLJ58441 is highly similar to attractin. Attractin has a collagen-binding domain and, similar to CD26, is co-stimulatory for T cells to recall antigens.[49] Increasing experimental evidence indicates that attractin plays a role in the regulation of immune responses.[50] Furthermore, higher levels of these proteins have been observed in patients with schizophrenia, bipolar disorder, and depression patients than in controls.[51] cDNA FLJ58441 in our research also had a higher level as above, which indicated that such patients may exhibit abnormal immune system function.
In general, a special 150-kD electrophoretic band with high positive rate in patients with schizophrenia, bipolar disorder, or depression relative to healthy controls was found, and 11serum proteins whose abundance was altered between patients and controls were analyzed with LC-MS for it.
In conclusion, a special 150-kD electrophoretic band in patients with schizophrenia, bipolar disorder, or depression relative to healthy controls was found. In this band, the abundance of 11 serum proteins were altered between patients and controls. Even though only zinc finger protein 729 has statistics significance, other proteins maybe also have, because the case is seldom and the result information was not made best of due to the statistics method. Also these proteins take part in amoebiosis pathway, which includes focal adhesion, complement cascades, ECM-receptor interaction, and Staphylococcus aureus infection. In the upcoming work, we will increase sample size and identify these protein function in animal experiments to explore the pathogenesis of schizophrenia.
5. Conclusion
The present study suggests that there is a special 150-kD electrophoretic band in patients with schizophrenia, bipolar disorder, or depression relative to healthy controls (each 30 samples) with a combination of 7% PEG enrichment of immune complexes and SDS-PAGE. Analysis of the band using LC-MS resulted in identification of 11 serum proteins whose abundance was altered between patients and controls. Among them, 8 proteins (CFH, CFB, cDNA FLJ75416, zinc finger protein 729, isoform 2 of nidogen-1, diaphanous-1, cDNA FLJ77762, and cDNA FLJ58411) were upregulated, while one protein (isoform 1 of collagen alpha-1 (II) was downregulated in patients with schizophrenia, but only zinc finger protein 729 has statistics significance (P < .05). No differences were noted with regard to thrombospondin-1 or collagen alpha-2 (I) among the 3 groups. These proteins take part in several biological functions such as focal adhesion, complement cascades, ECM-receptor interaction, and Staphylococcus aureus infection. The 150-kD electrophoretic band on zinc finger protein 729 may become biomarkers in patients with schizophrenia. In the future, increasing sample size and function research of zinc finger protein 729 should be executed continuously.
Author contributions
Conceptualization: Wenbin Yuan.
Data curation: Jing Wen Liang, Mingbang Wang, Fusheng He.
Formal analysis: Xing Wan, Kang Li.
Methodology: Yi Luo.
Project administration: Jianxia Chen, Jiaxiu Zhou.
Resources: Liguo Qi.
Software: Ze Wu, Yingjun Xie, Shaoming Zhou.
Writing – original draft: Jing Wen Liang, Wenbin Yuan.
Writing – review & editing: Ruihuan Xu.
Footnotes
Abbreviations: CFB = complement factors B, CFH = complement factors H, IPA = ingenuity pathway analysis, LC-MS = liquid chromatography mass spectrometry, PEG = polyethylene glycol, SDS-PAGE = sodium dodecyl sulfate-polyacrylamide gel electrophoresis, THBS-1 = thrombospondin-1, ZNF729 = zinc finger protein 729.
Funding sources: This project was supported by Shenzhen Science Technology Research & Development Fund (No. JCYJ20140411150159429, No. JCYJ20160427101538845, No. JCYJ20160429174706491, No. JCYJ20170413093358429) from Shenzhen Science Technology and Innovation Commission, Longgang District Science Fundamental Research fund (No. 2016606163743830) from Longgang District Science Technology and Innovation Commission of Shenzhen, the National Natural Science Foundation of China (NSFC)(No.81701351, No.81500974).
There is no competing interest to declare.
The authors have no conflicts of interest to disclose.
References
- [1].van Os J, Kapur S. Schizophrenia Lancet 2009;374:635–45. [DOI] [PubMed] [Google Scholar]
- [2].American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (4th Edition), Text Revision. DC, USA: American Psychiatric Association; 2000. [Google Scholar]
- [3].Sartorius N, Ustün TB, Korten A, et al. Progress toward achieving a common language in psychiatry, II: results from the international field trials of the ICD-10 diagnostic criteria for research for mental and behavioral disorders. Am J Psychiatry 1995;152:1427–37. [DOI] [PubMed] [Google Scholar]
- [4].Van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010;468:203–12. [DOI] [PubMed] [Google Scholar]
- [5].Shi Y, Li Z, Xu Q, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet 2011;43:1224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Duan J, Sanders AR, Gejman PV. Genome-wide approaches to schizophrenia. Brain Res Bull 2010;83:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jaros JA, Martins-de-Souza D, Rahmoune H, et al. Protein phosphorylation patterns in serum from schizophrenia patients and healthy controls. J Proteomics 2012;5:43–55. [DOI] [PubMed] [Google Scholar]
- [8].Martins-De-Souza D, Wobrock T, Zerr I, et al. Different apolipoprotein E, apolipoprotein A1 and prostaglandin-H2 Disomeraselevels in cerebrospinal fluid of chizophrenia patientsand healthy controls. World J Biol Psychiatry 2010;11:719–28. [DOI] [PubMed] [Google Scholar]
- [9].Domenici E, Willé DR, Tozzi F, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One 2010;5:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levin Y, Wang L, Schwarz E, et al. Global proteomic profiling reveals altered proteomic signature in schizophrenia serum. Mol Psychiatry 2010;15:1088–100. [DOI] [PubMed] [Google Scholar]
- [11].Prabakaran S, Wengenroth M, Lockstone HE, et al. 2-D DIGE analysis of liver and red blood cells provides further evidence for oxidative stress in schizophrenia. Proteome Res 2007;6:141–9. [DOI] [PubMed] [Google Scholar]
- [12].Nishihara Julie C, Kathleen M. Champion Quantitative evaluation of proteins in one- and two-dimensional polyacrylamide gels using a fluorescent stain. Electrophoresis 2002;23:2203–15. [DOI] [PubMed] [Google Scholar]
- [13].Grimwood J, Gordon LA, Olsen A, et al. The DNA sequence and biology of humanchromosome 19. Nature 2004;428:529–35. [DOI] [PubMed] [Google Scholar]
- [14].Gizard F, Lavall B, DeWitte F, et al. A novel zinc finger protein TReP-132 interacts with CBP/p300 to regulate human CYP11A1 gene expression. J Biol Chem 2001;276:33881–92. [DOI] [PubMed] [Google Scholar]
- [15].Chen J, Lin M, Hrabovsky A, et al. ZNF804A transcriptional networks in differentiating neurons derived from induced pluripotent stem cells of human origin. PLoS One 2015;10:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang KD, Chang WC, Chuang H, et al. Increased complement factor H with decreased factor B determined by proteomic differential displays as a biomarker of Tai Chi Chuan exercise. Clin Chem 2010;1:127–31. [DOI] [PubMed] [Google Scholar]
- [17].Makou E, Herbert AP, Barlow PN. Functional anatomy of complement factor H. Biochemistry 2013;52:3949–62. [DOI] [PubMed] [Google Scholar]
- [18].Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism image-related macular degeneration. Science 2005;308:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caprioli J, Peng L, Remuzzi G. The hemolyticuremic syndromes. Curr Opin Crit Care 2005;11:487–92. [DOI] [PubMed] [Google Scholar]
- [20].Noris M, Remuzzi G. Complement factor H gene abnormalities in haemolytic uraemic syndrome: from point mutations to hybrid gene. PLoS Med 2006;3:1719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klein R. Overview of progress in the epidemiologyof age-related macular degeneration. Ophthalmic Epidemiol 2007;14:184–7. [DOI] [PubMed] [Google Scholar]
- [22].Shastry BS. Assessment of the contribution of theLOC387715 gene polymorphism in a family with exudative age-related macular degeneration and heterozygous CFH variant (Y402H). J Hum Genet 2007;52:384–7. [DOI] [PubMed] [Google Scholar]
- [23].Singer L, Mayer S, Tongio MM, et al. Frequencies of HLA-A, C and Bf antigens in schizophrenic patients originated from Alsace. Encephale 1982;8:9–15. [PubMed] [Google Scholar]
- [24].Rudduck C, Franzén G, Hansson A, et al. Properdin factor B (Bf) types in schizophrenia. Hum Hered 1984;34:331–3. [DOI] [PubMed] [Google Scholar]
- [25].Yang Y, Wan C, Li H, et al. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem 2006;78:3571–6. [DOI] [PubMed] [Google Scholar]
- [26].Mayilyan Karine R. The complement system in schizophrenia. Drug News Perspect 2008;21:200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bao J, Xiao J, Mao Y, et al. Carboxyl terminus of ADAMTS13 directly inhibits platelet aggregation and ultra large von Willebrand factor string formation under flow in a free-thiol-dependent manner. Arterioscler Thromb Vasc Biol 2014;34:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsuchida R, Osawa T, Wang F, et al. BMP4/Thrombospondin-1 loop paracrinically inhibits tumor angiogenesis and suppresses the growth of solid tumors. Oncogene 2014;33:3803–11. [DOI] [PubMed] [Google Scholar]
- [29].Gutierrez LS. The role of thrombospondin 1 on intestinal inflammation and carcinogenesis. Biomarker Insights 2008;3:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang HP, Wu Y, Liu J, et al. TSP1-producing B cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci Rep 2013;3:3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ma Y, Qu B, Xia X, et al. Glioma-derived thrombospondin-1 modulates cd14+ cell tolerogenic properties. Cancer Invest 2015;33:152–7. [DOI] [PubMed] [Google Scholar]
- [32].Bergstrom SE, Uzunel M, Talme T, et al. Antigen-induced regulation of T-cell motility, interaction with antigen-presenting cells and activation through endogenous thrombospondin-1 and its receptors. Immunology 2014;144:687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miosge N, Sasaki T, Timpl R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol 2002;21:611–21. [DOI] [PubMed] [Google Scholar]
- [34].Timpl R, Brown J. Supramolecular assembly of basement membranes. Bioassays 1996;18:123–32. [DOI] [PubMed] [Google Scholar]
- [35].Yi XY, Wayner E, Kim Y, et al. Adhesion of cultured human kidney mesangial cells to native entactin: role of integrin receptors. Cell Adhes Commun 1998;5:237–48. [DOI] [PubMed] [Google Scholar]
- [36].Schwarz E, Guest PC, Steiner J, et al. Identification of blood-based molecular signatures for prediction of response and relapse in schizophrenia patients. Transl Psychiatry 2012;21:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jaros JA, Rahmoune H, Wesseling H, et al. Effects of olanzapine on serum protein phosphorylation patterns in patients with schizophrenia. Proteomics Clin Appl 2015;9:907–16. [DOI] [PubMed] [Google Scholar]
- [38].Katsel P, Byne W, Roussos P, et al. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology 2011;36:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ning M, Sarracino DA, Kho AT, et al. Proteomic temporal profile of human brain endothelium after oxidative stress. Stroke 2011;42:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Dijk FS, Huizer M, Kariminejad A, et al. Complete COL1A1 allele deletions in osteogenesis imperfecta. Genet Med 2010;12:736–41. [DOI] [PubMed] [Google Scholar]
- [41].Wu P, Li B, Wu A, et al. Is type I alpha 2 collagen gene responsible for intracranial aneurysm in Northeast China? Neural Regen Res 2013;8:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Péter Csomor, Balázs Liktor, Bálint Liktor, et al. No evidence for disturbed COL1A1 and A2 expression in otosclerosis. Eur Arch Otorhinolaryngol 2012;269:2043–51. [DOI] [PubMed] [Google Scholar]
- [43].An B, Abbonante V, Yigit S, et al. Definition of the native and denatured type II collagen binding site for fibronectin using a recombinant collagen system. J Biol Chem 2014;289:4941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Doknic M, Maric NP, Britvic D, et al. Bone remodeling, bone mass and weight gain in patients with stabilized schizophrenia in real-life conditions treated with long-acting injectable risperidone. Neuroendocrinology 2011;94:246–54. [DOI] [PubMed] [Google Scholar]
- [45].Liang Y, Su YA, Zhao ZG, et al. Acute effects of haloperidol, amisulpride, and quetiapine on bone turnover markers in patients with schizophrenia. J Clin Psychopharmacol 2015;35:583–6. [DOI] [PubMed] [Google Scholar]
- [46].Taylor A, Halene S. The regulatory role of serum response factor pathway in neutrophil inflammatory response. Curr Opin Hematol 2015;22:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zemla A, Thomas Y, Kedziora S, et al. CSN- and CAND1-dependent remodelling of the budding yeast SCF complex. Nature Commun 2013;4:1641–52. [DOI] [PubMed] [Google Scholar]
- [48].Zemla A, Thomas Y, Kedziora S, et al. CSN- and CAND1-dependent remodelling of the budding yeast SCF complex. Nat Commun 2013;4:1641–52. [DOI] [PubMed] [Google Scholar]
- [49].Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol 2004;25:193–2001. [DOI] [PubMed] [Google Scholar]
- [50].Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol 2004;25:193–200. [DOI] [PubMed] [Google Scholar]
- [51].Gay M, Carrascal M, Gorga M, et al. Characterization of peptides and proteins in commercial HSA solutions. Proteomics 2010;10:172–81. [DOI] [PubMed] [Google Scholar]


