Abstract
Background:
Liver fibrosis index FIB-4 has been reported to be linked with hepatocellular carcinoma (HCC) prognosis, but the results were not consistent. This study aimed to synthetically explore the relationship between FIB-4 and clinical outcomes of HCC.
Methods:
A number of online databases were searched for relevant articles published before March 1, 2018. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were calculated to assess the prognostic value of the FIB-4 index in patients with HCC using Stata SE 12.0.
Results:
Eight articles (including 10 cohort studies) with 3485 HCC patients were finally included for analysis. The pooled results showed that FIB-4 index was significantly associated with overall survival (OS) for patients with HCC (HR = 1.74, 95% CI: 1.41–2.07, P <.001). And HCC patients with higher FIB-4 score were at significantly greater risk of recurrence 1.53 (95% CI: 1.29–1.78, P <.001). Subgroup analysis based on the treatment, stage and analysis type also confirmed the prognostic values of the FIB-4 score for OS and recurrence-free survival (RFS) in HCC.
Conclusions:
FIB-4 index might be a useful predictive marker in patients with HCC.
Keywords: FIB-4 index, hepatocellular carcinoma, meta-analysis, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and over half of the new HCC cases and deaths occurred in China.[1–2] Hepatectomy is considered the most commonly curative treatment in HCC patients. However, their prognosis, especially the long-term survival rate remains unsatisfactory because of the high frequency of tumor recurrence.[3–4] Thus, predicting the survival and recurrence for HCC patients can help to guide their post-treatment management.
There are many risk factors for HCC, including viral infection and fibrosis degree, which are also closely related to HCC prognosis.[5–7] FIB-4 index, a novel scoring system, can be calculated by the following formula: age x AST/platelet count [x 103/μL] x [ALT]1/2.[8] It was previously used to assess the severity of liver fibrosis and considered to be a useful fibrosis scoring systems.[9–10] Interestingly, some studies reported that the FIB-4 score was correlated with the prognosis of HCC in recent years, and it could serve as a prognostic factor for HCC.[5,11–13] However, the prognostic value of the FIB-4 index in HCC remain inconsistent from current clinical reports.[15–18] Therefore, the aim of this meta-analysis was to systematically evaluate the relationship between the FIB-4 index and HCC patient outcomes after treatments and provide a better understanding of the impact of the FIB-4 index on HCC patient prognosis.
2. Materials and methods
2.1. Search strategy and study selection
Due to our work is a meta-analysis of the published literature, the ethical approval is not required. The following online databases were searched for eligible articles: PubMed, Web of Science, Cochrane Library, Embase, Wanfang Data, and China National Knowledge Infrastructure (CNKI). The retrieval time was updated as of March 1, 2018. The search terms were as follows: (FIB-4 OR Fibrosis-4) AND (hepatocellular OR liver OR hepatic) AND (carcinoma OR cancer OR neoplasm OR tumor). We also manually reviewed the references in relevant studies for potential studies.
Inclusion criteria:
-
(1)
an article explored the prognostic impact of FIB-4 index in primary HCC;
-
(2)
a definite cutoff value of FIB-4 was given;
-
(3)
the hazard ratio (HR) with 95% confidence intervals (95% CI) for overall survival (OS), disease-free survival (DFS) or recurrence-free survival (RFS) was available;
-
(4)
Patients were divided into 2 groups according to the FIB-4 score.
The unpublished data without peer-review was excluded and if 2 articles involving overlapping populations, the one with the largest number of cases or higher-quality was included.
2.2. Data extraction and quality assessment
According to standardized data-collection protocol, the following information was abstracted from each included study by 2 investigators, independently: the name of the first author, publication year, country, included period, study type, sample size, age, method of treatment, duration of follow-up, stage, cut-off value, cut-off selection, survival type, analysis mode, HR value, and the corresponding 95% CI. Meanwhile, the Newcastle–Ottawa scale (NOS) scores were used to assess the qualities of the studies.
2.3. Statistical analysis
In the present meta-analysis, A HR >1 indicated a worse prognosis for HCC patients with higher FIB-4, and 95% CI not including 1 was regarded as statistically significant. If a study considered cases with high FIB-4 as the reference, then the data was converted to HR estimations that considering patients with low FIB-4 as a reference group to reflect the impact of high FIB-4 levels on HCC. The heterogeneity across studies was assessed by the I2 statistic value and Q-value, I2 >50% or P <.01 as determined by the Q statistic was considered significant heterogeneity, and then the random-effect model was used.
The publication bias was examined using visible plots with Begg and Egger test for OS and RFS and influence analyses were also performed to evaluate whether the results could be significantly affected by a single study on OS and RFS.
The STATA 12.0 software (Stata, College Station, TX) was applied to analyze the data in our meta-analysis, a P value of less than .05 was considered to be statistically significant difference.
3. Results
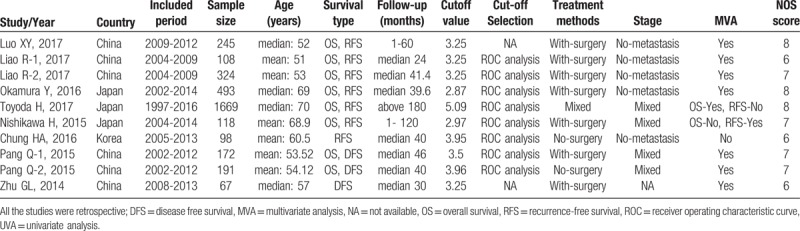
Figure 1 showed the detailed processes of the literature search. According to inclusion criteria and exclusion criteria, eventually a total of 8 articles (including 10 cohort studies) with 3485 HCC patients were included for analysis,[11–18] among those articles, 2 articles were written in Chinese,[11,18] and 6 were written in English.[12–17] All included studies were retrospective and reported the relationship between the FIB-4 value and HCC prognosis. And 6 studies were from China,[11–12,17–18] 3 came from Japan[13–15] and 1 from Korea.[16] They were all with a NOS score equal or great than 6, implying that all studies included were high-quality. The main characteristics of all included studies are summarized in Table 1.
Figure 1.
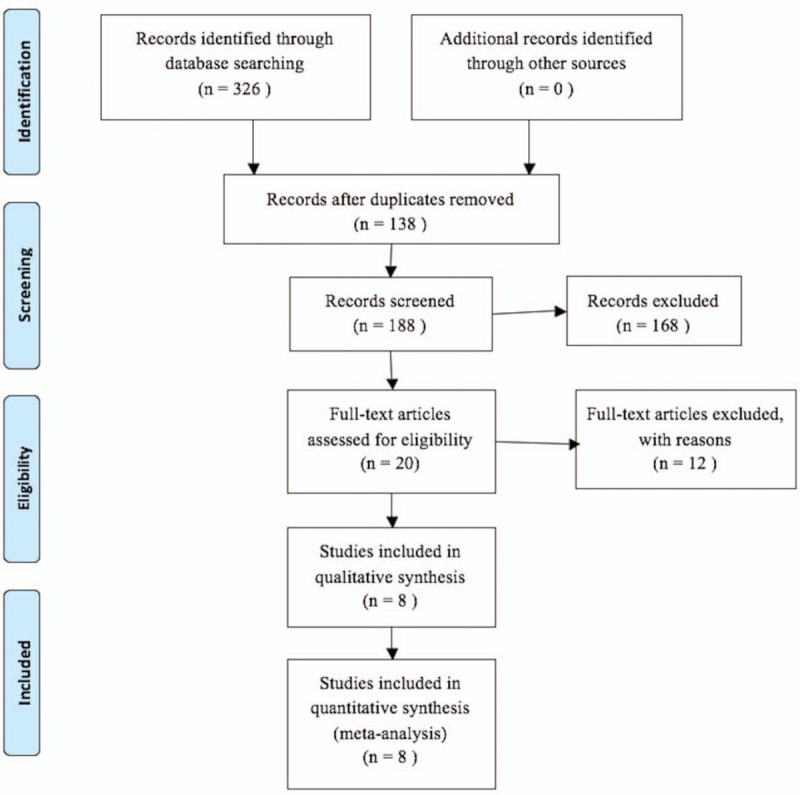
The flow chart of literature selection.
Table 1.
Main characteristics of all included studies.
3.1. FIB-4 index and OS
Eight studies with 3320 HCC patients reported the prognostic value of FIB-4 index and OS. As there was no significant heterogeneity across articles (I2 = 0.0%, P = .942), the fixed-effects model was applied. From the overall results (Fig. 2A), we found that FIB-4 was significantly associated with OS (HR = 1.74, 95% CI: 1.41–2.07, P <.001), the HCC patients with high FIB-4 score had a poor outcome for OS than those with low FIB-4 score.
Figure 2.

Forest plots for the relationships between FIB-4 and OS on patients with HCC. Overall (A) and stratified by the treatment (B), stage (C) and analysis type (D). HCC = hepatocellular carcinoma, OS = overall survival.
We also conducted subgroup analyses based on the treatment, stage and analysis type. From Figure 2B to D, it showed that the pooled HRs were significantly greater than 1.0 in these subgroup analyses. Intriguingly, the FIB-4 score could be an independent predictor of OS in patients with HCC (HR = 1.76, 95% CI: 1.42–2.10, P <.001).
3.2. FIB-4 index and RFS
A total of 7 studies with 3055 cases reported the association between FIB-4 score and RFS in HCC. As shown in Figure 3A, the pooled HR was 1.53 (95% CI: 1.29–1.78, P <.001) with no significant heterogeneity among studies (I2 = 0.0%, P = .921). The finding revealed that a high FIB-4 score was associated with HCC recurrence.
Figure 3.

Forest plot for the relationship between FIB-4 and RFS on patients with HCC. Overall (A) and stratified by the treatment (B), stage (C) and analysis type (D). HCC = hepatocellular carcinoma, RFS = recurrence-free survival.
The pooled HRs were significantly greater than 1.0 in subgroups treated with surgery (Fig. 3B), and cases with no metastasis (Fig. 3C). Notably, high FIB-4 score could be an independent unfavorable factor of RFS in HCC patients (HR = 1.54, 95% CI: 1.27–1.81, P <.001, Fig. 3D).
3.3. FIB-4 index and DFS
Only 3 studies, comprising 430 cases, reported the relationship between FIB-4 index and DFS for HCC patients. The overall results showed that there was a strong trend but no statistical difference between the FIB-4 index and DFS in HCC patients (HR = 1.53, 95% CI: 0.89–2.18) using a fixed-effect model (I2 = 35.1%, P = .214) (Fig. 4).
Figure 4.
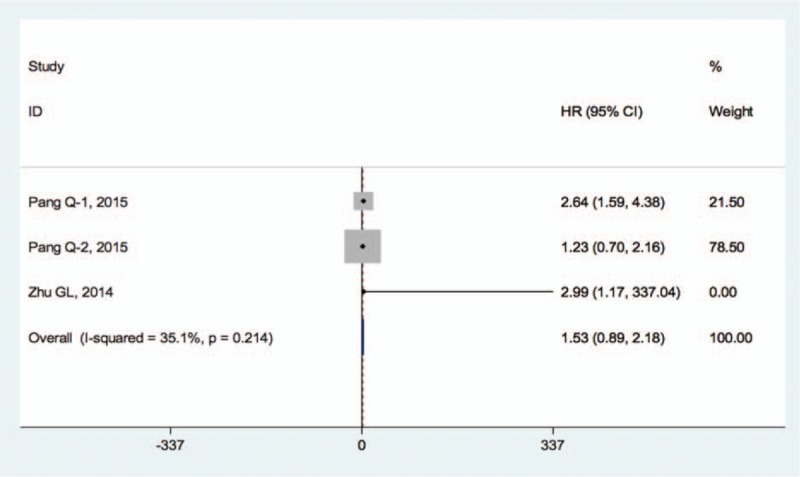
Forest plot for the relationship between FIB-4 and DFS on HCC patients. DFS = disease-free survival, HCC = hepatocellular carcinoma.
3.4. Publication bias
Begg and Egger plot were presented in Figure 5, and the test results all suggested no evidence of publication bias. (Pr Begg'stest > |z| = 0.174, P Egger'stest >|t| = 0.100 for OS; Pr Begg'stest > |z| = 0.764, P Egger'stest >|t| = 0.906 for RFS).
Figure 5.
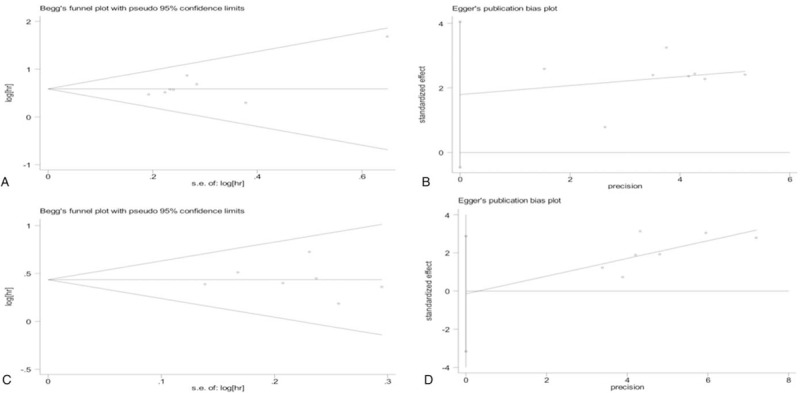
Funnel plots for publication bias test: for OS (A and B); for RFS (C and D). OS = overall survival, RFS = recurrence-free survival.
3.5. Sensitivity analysis
Sensitivity analysis showed that any individual study had little effects on the combined results (Fig. 6), indicating the robustness of our current data.
Figure 6.
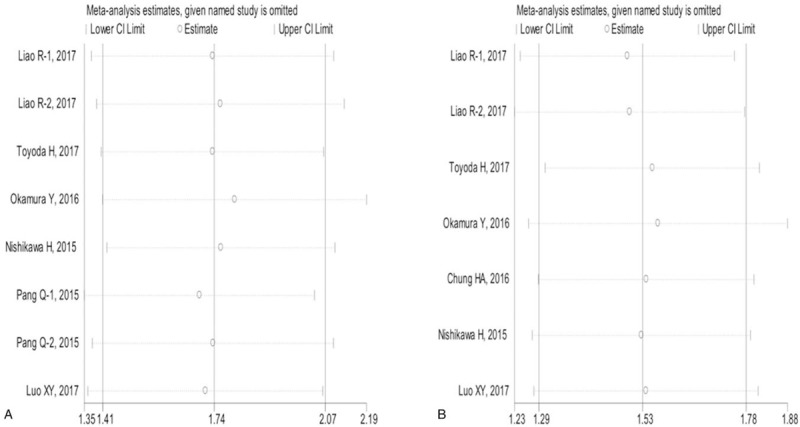
Sensitivity analysis of the relationship between FIB-4 score and HCC prognosis. for OS (A); for RFS (B). HCC = hepatocellular carcinoma, OS = overall survival, RFS = recurrence-free survival.
4. Discussion
To the best of our knowledge, no prior meta-analysis has evaluated the prognostic role of the FIB-4 in HCC patients with various treatments; this is the first meta-analysis that focused on this topic. Although a number of studies have demonstrated that the FIB-4 index was a useful marker for predicting liver fibrosis, the relationships between this serum index and the outcomes in HCC patients remain obscure. Hence, we conducted the current analyses.
In this meta-analysis, we identified 8 articles with 3485 HCC patients as qualified for pooled analysis. The combined results showed that FIB-4 score was significantly associated with OS and RFS in HCC. Furthermore, the high FIB-4 score could be an independent poor factor of OS and RFS in HCC patients. The subgroup analyses also confirmed the prognostic significance of FIB-4 in HCC patients. High FIB-4 in HCC patients indicated poorer survival outcomes. The FIB-4 score could serve as a noninvasive predictive marker for HCC cancer prognosis.
The FIB-4 index is a fibrosis-related index containing 4 common factors: age, aspartate aminotransferase (AST), platelet count and alanine transaminase (ALT).[8] As mentioned above, the finding of our study was that the FIB-4 index could serve as a promising prognostic factor for both OS and RFS in HCC patients. However, the exact mechanisms of prognostic roles of FIB-4 index in HCC remain unclear. OS usually depends on the factors related to liver function and tumor-related factors, while RFS was decided by tumor-related factors.[13] The FIB-4 index was previously identified as a liver fibrosis index that could reflect the degree of liver fibrosis, and most of HCC patients were accompanied by different degrees of liver fibrosis, which affected the normal functions and liver reserve and lead to poor survival. In addition, some studies have shown that liver fibrosis and the FIB-4 index were related to hepatocarcinogenesis,[18–20] the FIB-4 index could be a risk factor that was associated with the survival and recurrence after hepatectomy. Furthermore, AST and ALT could reflect the degree of liver disorder, and the platelet count could reflect the patient's portal hypertension status[13,21] and a low platelet count level was demonstrated to be an unfavorable factor in HCC.[22–23] Moreover, patient age was also found to be a significant prognostic factor in a nationwide follow-up survey in HCC patients.[24]
The present meta-analysis included several limitations. Firstly, all included studies were retrospective observational studies. Secondly, the total sample size enrolled in our study was relatively small. In addition, all HCC cases enrolled were from Asian countries, including China, Japan, and Korea, further studies from other countries with more ethnic groups are necessary. Furthermore, studies with negative results are generally less likely to be published than ones with positive results, this might cause selection bias. And there were some other variables that could also affect the HCC patient survival, such as post-hepatectomy chemotherapy, tumor size, and tumor differentiation. Finally, the cutoff value for high FIB-4 index varied in different studies, it was essential to be unified before it could be applied in clinical management.
In summary, our data suggest that liver fibrosis index FIB-4 could predict the clinical outcomes in HCC patients. The FIB-4 score might be helpful as a promising prognostic candidate to monitor HCC patients’ survival and recurrence after treatment. Multi-center prospective clinical studies with a larger sample are required to validate our results.
Author contributions
Conceptualization: Yi Zhang, Xianjin Yang.
Data curation: Rong Wang, Xianjin Yang.
Formal analysis: Yi Zhang, Rong Wang.
Investigation: Yi Zhang, Rong Wang, Xianjin Yang.
Methodology: Yi Zhang, Rong Wang, Xianjin Yang.
Project administration: Xianjin Yang.
Resources: Yi Zhang, Rong Wang.
Software: Yi Zhang, Rong Wang, Xianjin Yang.
Supervision: Yi Zhang, Xianjin Yang.
Validation: Yi Zhang.
Writing – original draft: Yi Zhang, Xianjin Yang.
Writing – review & editing: Yi Zhang, Xianjin Yang.
Footnotes
Abbreviations: 95% CIs = 95% confidence intervals, ALT = alanine transaminase, AST = aspartate aminotransferase, DFS = disease-free survival, HCC = hepatocellular carcinoma, HRs = hazard ratios, NOS = Newcastle–Ottawa scale, OS = overall survival, RFS = recurrence-free survival.
The authors declare no conflict of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett 2016;379:191–7. [DOI] [PubMed] [Google Scholar]
- [4].Miki D, Ochi H, Hayes CN, et al. Hepatocellular carcinoma: towards personalized medicine. Cancer Sci 2012;103:846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Toyoda H, Kumada T, Tada T, et al. Differences in the impact of prognostic factors for hepatocellular carcinoma over time. Cancer Sci 2017;108:2438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Osawa M, Akuta N, Suzuki F, et al. Prognosis and predictors of hepatocellular carcinoma in elderly patients infected with hepatitis B virus. J Med Virol 2017;89:2144–8. [DOI] [PubMed] [Google Scholar]
- [7].Wang Q, Lin L, Yoo S, et al. Impact of non-neoplastic vs intratumoural hepatitis B viral DNA and replication on hepatocellular carcinoma recurrence. Br J Cancer 2016;115:841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [9].Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46:32–6. [DOI] [PubMed] [Google Scholar]
- [10].Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546–53. [DOI] [PubMed] [Google Scholar]
- [11].Luo XY, Du CY, Yan X, et al. Relationship between preoperative FIB-4 and hepatocellular carcinoma prognosis after curative resection. Chin J Clin Oncol 2017;44:498–501. [Google Scholar]
- [12].Liao R, Fu YP, Wang T, et al. Metavir and FIB-4 scores are associated with patient prognosis after curative hepatectomy in hepatitis B virus-related hepatocellular carcinoma: a retrospective cohort study at two centers in China. Oncotarget 2017;8:1774–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Okamura Y, Ashida R, Yamamoto Y, et al. The FIB-4 index is a significant prognostic factor in patients with non-B non-C hepatocellular carcinoma after curative surgery. Langenbecks Arch Surg 2016;401:195–203. [DOI] [PubMed] [Google Scholar]
- [14].Nishikawa H, Osaki Y, Komekado H, et al. Clinical significance of the FIB-4 index for non-B non-C hepatocellular carcinoma treated with surgical resection. Oncol Rep 2015;33:88–94. [DOI] [PubMed] [Google Scholar]
- [15].Chung HA, Kim JH, Hwang Y, et al. Noninvasive fibrosis marker can predict recurrence of hepatocellular carcinoma after radiofrequency ablation. Saudi J Gastroenterol 2016;22:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pang Q, Zhang JY, Xu XS, et al. The prognostic values of 12 cirrhosis-relative noninvasive models in patients with hepatocellular carcinoma. Scand J Clin Lab Invest 2015;75:73–84. [DOI] [PubMed] [Google Scholar]
- [17].Zhu GL, Yang LK, Lin ZD, et al. Predictive value of preoperative Fib-4 index on postoperative recurrence of hepatocellular carcinoma after hepatectomy. Shandong Yiyao 2014;54:4–6. [Google Scholar]
- [18].Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med 1999;131:174–81. [DOI] [PubMed] [Google Scholar]
- [19].Tamaki N, Kurosaki M, Matsuda S, et al. Non-invasive prediction of hepatocellular carcinoma development using serum fibrosis marker in chronic hepatitis C patients. J Gastroenterol 2014;49:1495–503. [DOI] [PubMed] [Google Scholar]
- [20].Kaibori M, Kubo S, Nagano H, et al. Clinicopathological features of recurrence in patients after 10-year disease-free survival following curative hepatic resection of hepatocellular carcinoma. World J Surg 2013;37:820–8. [DOI] [PubMed] [Google Scholar]
- [21].Toyoda H, Kumada T, Tada T, et al. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery 2015;157:699–707. [DOI] [PubMed] [Google Scholar]
- [22].Pang Q, Qu K, Zhang JY, et al. The prognostic value of platelet count in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pang Q, Zhang JY, Xu XS, et al. Significance of platelet count and platelet-based models for hepatocellular carcinoma recurrence. World J Gastroenterol 2015;21:5607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Utsunomiya T, Shimada M, Kudo M, et al. Nationwide study of 4741 patients with non-B non-C hepatocellular carcinoma with special reference to the therapeutic impact. Ann Surg 2014;259:336–45. [DOI] [PubMed] [Google Scholar]


