Abstract
Rationale:
Chordoma is a relatively rare tumor that accounts for 1% to 4% of all malignant bone tumors, with an annual incidence of <0.1 per 100,000 people. Although chordoma is aligned with the axis of the spine and most commonly develops in the sacrum, to the best of our knowledge, giant sacrococcygeal chordoma is extremely rare.
Patient concerns:
A 61-year-old Chinese man presented with a massive dorsal sacral mass. The patient's primary complaint was that, during the last two months, the mass had been increasing in size and his right lower extremity was uncomfortable while he was sitting, although the discomfort was relieved when he was standing.
Diagnoses:
Based on the imaging findings, we suspected that the sacrococcygeal mass was a chordoma, and a postoperative pathological examination confirmed the diagnosis of a sacral chordoma.
Intervention:
The patient underwent extensive open surgery to achieve complete resection of the sacrococcygeal mass. An occlusion balloon catheter was used in the abdominal aorta to minimize intraoperative bleeding and maintain a clear surgical field.
Outcomes:
The patient was discharged without complications at 27 days after surgery. The 3-month follow-up revealed that the patient had recovered well, the discomfort in his right lower extremity while standing had completely resolved and that there was no evidence of recurrence.
Lessons:
The development of chordoma is not associated with clear symptoms, although early diagnosis and treatment are needed to prevent invasion of the nearby tissues and organs. Therefore, we believe that surgical treatment of sacral chordoma is effective, although care must be taken to completely remove all residual tumor tissue and reduce the risk of recurrence. Besides, This report adds to our limited understanding of the rare giant sacrococcygeal chordoma.
Keywords: chordoma, diagnosis, giant, sacrococcygeal, surgery, symptom
1. Introduction
Chordoma is a rare, slow-growing, primary bone tumor originating from notochord remnants. Chordomas only account for <5% of all primary bone tumors,[1] with the most common locations being the dorsal sacrum (40%–50%), the skull base (35%–40%), and the vertebral body (15%–20%).[2,3] Although chordoma is a histopathologically benign tumor, it exhibits aggressive clinical behavior with invasive and metastatic potential.[4] The most common symptom of a sacral chordoma is pain, which is usually characterized by dullness and poor posture.[5] Treatment typically involves excision using a posterior approach, which provides good results in many institutions as well as a lower recurrence rate relative to a combined posterior and anterior approach.[6,7] We encountered a 61-year-old man who experienced progressive enlargement of a dorsal sacral mass, which was postoperatively diagnosed as a rare sacral chordoma.
2. Case presentation
A 61-year-old male Chinese farmer presented to our clinic with a chief complaint of a gradually increased sacrococcygeal mass during the last 2 months, which was not ameliorated using traditional Chinese medicine at a local hospital. While seated, the patient experienced discomfort in his right lower extremity, although the discomfort was relieved when he was standing. He did not have any history of medical conditions, familial genetic conditions, or food and drug allergies. In addition, the patient had not recently experienced any significant change in body weight and had not had tuberculosis. The patient was able to walk to the ward without visible abnormalities in his limbs and spine; although a large right posterolateral sacrococcygeal mass was clearly visible. The local skin was smooth and not reddened, and the mass was located below the subcutaneous tissue. We did not detect any ulceration or unclear margins, although the patient reported tenderness, numbness, and decreased sensation near the right buttock and sacral areas. However, both lower limbs had normal muscle tone and sensation. A further physical examination revealed no palpable head, neck, supraclavicular, axillary, or epitrochlear lymph nodes.
Routine laboratory testing revealed normal levels of alpha-fetoprotein (4.56 ng/mL, normal: 0–7.0 ng/mL) and carcinoembryonic antigen (2.06 ng/mL, normal: 0–6.5 ng/mL), but an elevated level of neuron-specific enolase (33.75 U/mL, normal: 0–16.3 U/mL). Plain radiography revealed a large presacral tumor that had invaded the coccyx (Fig. 1A). Enhanced magnetic resonance imaging revealed destruction of the appendiceal osseous mass by an irregularly shaped lobulated mass with heterogeneous intensity and multiple signal-like low-intensity shadows (Fig. 1B). Plain helical computed tomography (CT) with three-dimensional reconstruction revealed sacrococcygeal bone destruction by a mass that was approximately 16 cm × 10 cm, which had an irregular shape, multiple high-density areas, and involved a fistula (Fig. 1C and D). Based on these findings, we suspected that the sacrococcygeal mass was a chordoma.
Figure 1.
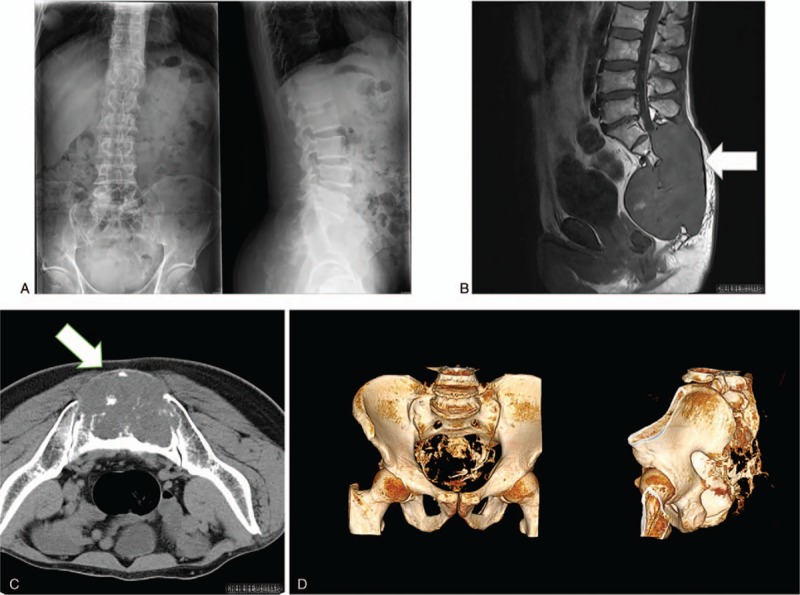
Imaging findings. A large presacral mass that had invaded the coccyx can be seen on the plain radiographs (A). Enhanced MRI revealed destruction of the appendiceal osseous mass by an irregularly shaped lobulated mass (B). Plain helical CT with three-dimensional reconstruction revealed sacrococcygeal bone destruction by the mass (C and D). CT = computed tomography, MRI = magnetic resonance imaging.
After routine preoperative evaluation, the patient was placed under general anesthesia in the prone position. A midline incision was made from the L3 level to the sacral spinous process, as well as a 15-cm left lateral oblique incision at the coccyx level (Fig. 2A). The large size of the tumor meant that the surrounding anatomy was complex and the tumor had extensive blood supply. To reduce intraoperative bleeding, a balloon catheter was used to occlude the abdominal aorta. The sacral tumor (Fig. 2B and C) was subsequently exposed and excised from the pseudocapsule membrane, with careful removal of all residual tumor and capsular tissue to reduce the likelihood of recurrence. The cavity was flushed with a large amount of normal saline, bleeding was controlled using gauze and gelatin sponges, a drainage tube was placed, and the incision was closed using sutures. A blood transfusion was required based on an estimated intraoperative blood loss of 1000 mL.
Figure 2.
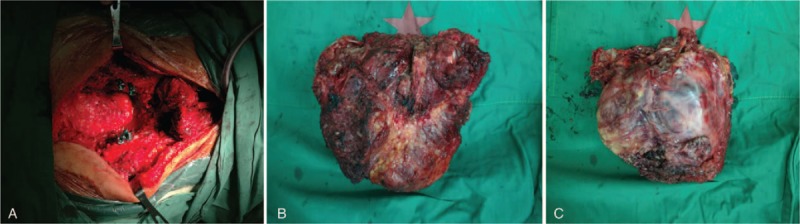
Gross findings in the operative field (A). An irregular mass of approximately 17 cm × 15 cm × 9 cm was removed (B and C).
Histological analysis of the tumor specimens revealed intracytoplasmic mucin-like substances, with large nuclei, deep staining, and an irregular matrix that resembled stroma, cartilage, and mucus with flaky necrosis (Fig. 3A and B). Immunohistochemistry revealed that the specimens were positive for cytokeratin, epithelial membrane antigen, Ki-67, S100, and vimentin (Fig. 3C–E). Based on the patient's history, the findings from the imaging and laboratory examinations, and the pathological results, the final diagnosis was sacral chordoma.
Figure 3.
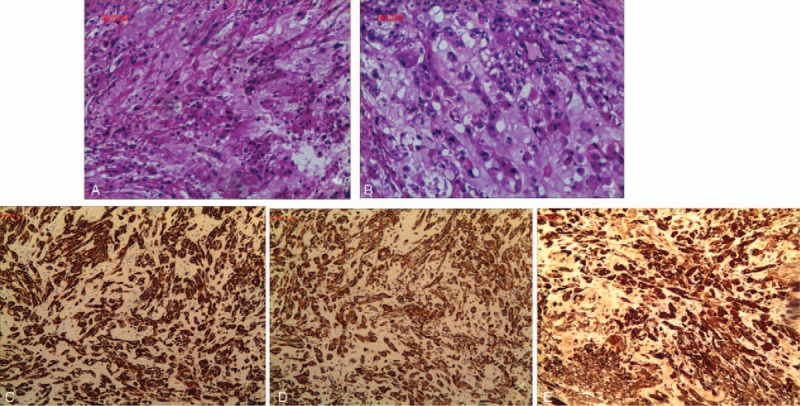
Histological analysis revealed intracytoplasmic mucin-like substances with large nuclei, deep staining, and an irregular matrix that resembled stroma, cartilage, and mucus with flaky necrosis (A and B). Immunohistochemistry revealed that the specimens were positive for cytokeratin (3+), epithelial membrane antigen (3+), Ki-67 (5%+), S100 (3+), and vimentin (3+) (C, D, and E).
The patient was discharged without any complications or adverse events at 27 days after surgery. The 3-month follow-up revealed that the patient had not undergone adjuvant treatment and that his lower extremity discomfort and numbness had resolved. Radiography was also performed, which revealed partial absence of the appendiceal area of the lumbar vertebrae and high-density posterior intraluminal fixation at L3, L4, and in the sacrum (Fig. 4A and B). CT and a clinical examination revealed no signs of tumor recurrence (Fig. 4C).
Figure 4.
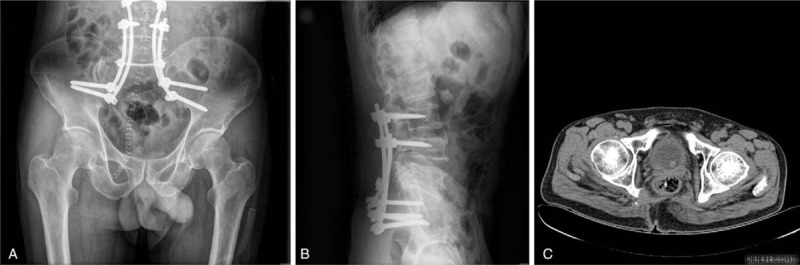
Radiography was repeated 3 months after surgery, which revealed partial absence of the appendiceal area of the lumbar vertebrae and high-density posterior intraluminal fixation at L3, L4, and in the sacrum (A and B). CT revealed no signs of tumor recurrence (C). CT = computed tomography.
3. Discussion
Chordoma accounts for 1% to 4% of all malignant bone tumors, with an annual incidence of <0.1 per 100,000 people.[8] Furthermore, the progression of chordoma is slow and insidious, with detection typical at the age of 50 to 70 years and more common among men than among women.[9–11] In those cases, the disease has progressed substantially by the time of detection, as the patients typically experience non-specific symptoms that delay the detection and diagnosis, which typically occurs when the disease has reached Enneking stage IIb.[2,8,12] We encountered a rare case of a giant chordoma in the dorsal sacrum of a 61-year-old Chinese man, whose vague symptoms also made it difficult to reach an early diagnosis. Furthermore, several conditions can mimic sacral chordoma and should be considered in the differential diagnosis. For example, chondrosarcoma has radiological and histological features that are similar to those of sacral chordoma. Giant cell tumors and plasmacytomas are also found in the sacrum, which is invasive, expandable, and may extend through the ankle joint. In those cases, radiography typically reveals large lytic lesions with unclear boundaries, which makes it difficult to differentiate them from chordomas based on imaging alone. Therefore, immunohistochemistry may be needed to reach a definitive diagnosis. In this context, chordomas are positive for epithelial membrane antigen and pan-cytokeratin,[13] with our patient's tumor being strongly positive for cytokeratin, epithelial membrane antigen, Ki-67, S100, and vimentin. Thus, the final diagnosis was sacral chordoma.
It is difficult to effectively treat chordoma, although surgical treatment is more effective than radiotherapy and chemotherapy.[14] However, there is much debate regarding the optimal approach for excising the tumor, with some authors advocating for an exclusively posterior approach[6] and others believing that a combined anterior and posterior approach helps reduce the risk of recurrence.[7] Nevertheless, the literature indicates that an exclusively posterior approach has provided good results at a number of institutions,[6] with a marginally lower rate of recurrence relative to the combined posterior and anterior approach.[7] Based on our imaging assessment of the tumor's size and degree of invasion, as well as the patient's preferences and the surgeon's experience, the posterior approach was selected for the present case.
The recurrence rates are 60% to 64% for intratumoral resection and 25% to 28% for resection of marginal tumors, which indicates that the use of inadequate surgical margins may lead to intraoperative seeding or contamination with malignant cells that leads to recurrence.[15] In addition, chordomas are encapsulated within a pseudocapsule containing occult satellite lesions,[16] and incomplete removal of the pseudocapsule can allow these occult lesions to cause recurrence. Furthermore, York et al[17] reported that radical resection provided 2.27 years of disease-free survival, relative to only 8 months for subtotal excision. Thus, we completely removed the pseudocapsule to limit the risk of recurrence. However, given the tough anterior fascia, premature invasion is not common and the rectum is usually unaffected.[18,19] Therefore, we spared the rectum and used rods and screws to achieve satisfactory mechanical support and movement in the spine and tibia.
Mavrogenis et al used targeted arterial embolization to reduce intraoperative bleeding and achieve better visibility in the surgical field and suggested that this option may be useful for patients who cannot tolerate more invasive surgery.[20] Thus, because of our case involved a large tumor with complex anatomy and a rich blood supply, we used an occlusion balloon catheter in the abdominal aorta to reduce intraoperative bleeding and facilitate complete resection. While it may be tempting to sacrifice the proximal sacral nerve root to achieve complete resection, this can produce varying degrees of bladder, bowel, and sexual dysfunction.[21–23] Thus, it would be preferable to remove the tumor while protecting the sacral nerve, although recurrence could subsequently complicate treatment and cause greater nerve root damage.
Given the typically large size of sacral chordomas, the resulting cavity and soft-tissue detects are extensive, which can lead to subsequent complications, such as sacrococcygeal skin necrosis, infection, and delayed healing or non-healing. Therefore, after resection of the sacral tumor, reconstruction is beneficial to maintain the stability of the lumbar spine and pelvis, relieve pain, support weight, and protect the pelvic internal organs. Prompt surgical intervention should also be considered to limit tumor growth and the subsequent risk of infection. Furthermore, a drainage tube should be placed in the cavity to reduce the risk of hematoma formation. Finally, adequate dressing, infection prevention, and intermittent suture removal after >2 weeks may help promote wound healing and good postoperative outcomes.
In conclusion, we encountered an extremely rare case of a giant sacrococcygeal chordoma, which has the potential to invade the neighboring tissues and organs, resulting in urination disorders, urinary incontinence, constipation, and sciatica. Early diagnosis and intervention are critical, although few reports have described this rare tumor and additional studies are needed to better understand its characteristics and diagnosis. When faced with this rare tumor, it is important for the physician to consider the patient's medical history, imaging results, and pathological findings. We believe that extensive resection is useful for treating sacral chordoma and minimizing the risk of local recurrence, as our patient was free from disease at the 3-month follow-up. Nevertheless, long-term monitoring will be needed to identify any recurrence or metastasis.
Author contributions
Data curation: Guiping Chen. Yibiao Zhou
Funding acquisition: Bin Zhang, Min Dai.
Methodology: Hucheng Liu. Yibiao Zhou
Project administration: Min Dai, Xuqiang Liu, Bin Zhang.
Resources: Min Dai, Hucheng Liu.
Supervision: Hucheng Liu, Min Dai, Bin Zhang.
Writing – original draft: Qiang Xu, Houyun Gu.
Writing – review & editing: Qiang Xu.
Qiang Xu orcid: 0000-0001-8043-2855.
Footnotes
Abbreviation: CT = computed tomography.
QX and HYG contributed equally to this work and should be considered co-first authors.
This project was supported by The Foundation of Health Department of Jiangxi Province (2016A073) and the Education Department Project of Jiangxi Province (GJJ160127).
The consent for publication of the manuscript and the related images from the patients and their relatives has been obtained by the First Affiliated Hospital of Nanchang University.
Publication of this report and the accompanying images were approved by the ethics committee of The First Affiliated Hospital of Nanchang University.
The authors have no conflicts of interest to disclose.
References
- [1].Smoll NR, Gautschi OP, Radovanovic I, et al. Incidence and relative survival of chordomas: The standardized mortality ratio and the impact of chordomas on a population. Cancer 2013;119:2029–37. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- [3].Chugh R, Tawbi H, Lucas DR, et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist 2007;12:1344–50. [DOI] [PubMed] [Google Scholar]
- [4].George B, Bresson D, Herman P, et al. Chordomas: a review. Neurosurg Clin N Am 2015;26:437–52. [DOI] [PubMed] [Google Scholar]
- [5].Jeys L, Gibbins R, Evans G, et al. Sacral chordoma: a diagnosis not to be sat on? Int Orthop 2008;32:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clarke MJ, Dasenbrock H, Bydon A, et al. Posterior-only approach for en bloc sacrectomy: clinical outcomes in 36 consecutive patients. J Neurosurg 2012;71:357–64. [DOI] [PubMed] [Google Scholar]
- [7].Ozger H, Eralp L, Sungur M, et al. Surgical management of sacral chordoma. Acta Orthop Belg 2010;76:243–53. [PubMed] [Google Scholar]
- [8].Samson IR, Springfield DS, Suit HD, et al. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Jt Surg Am 1993;75:1476–84. [DOI] [PubMed] [Google Scholar]
- [9].Stacchiotti Silvia. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol 2010;17:211–9. [DOI] [PubMed] [Google Scholar]
- [10].McMaster Mary L. Chordoma: incidence and survival patterns in the United States, 1973 ± 1995. Cancer Causes Control 2001;12:1–1. [DOI] [PubMed] [Google Scholar]
- [11].Ferraresi V, Nuzzo C, Zoccali C, et al. Chordoma: clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mirra J, Nelson S, Della Rocca C. Fletcher CD, Unni K, Mertens F. Chordoma. Pathology and genetics of tumours of soft tissue and bone. Lyon, France: IARC Press; 2002. 316–7. [Google Scholar]
- [13].Choy HY. Immunohistochemical comparison of chordoma with chondrosarcoma, myxopapillary ependymoma, and chordoid meningioma. Appl Immunohistochem Mol Morphol 2009;17:131–8. [DOI] [PubMed] [Google Scholar]
- [14].Chandaw arkar RY. Sacrococcygeal chordomas: review of 50 consecutive patients. World J Surg 1996;20:717–9. [DOI] [PubMed] [Google Scholar]
- [15].Hanna SA, Aston WJS, Briggs TWR, et al. Sacral ch-ordoma: can local recurrence after sacrectomy be predicted. Clin Orthop Relat Res 2008;466:2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ozger A, Eralp L, Sungur M, et al. Surgical management of sacral chordoma. Acta Orthop Belg 2010;76:243–53. [PubMed] [Google Scholar]
- [17].York JE, Kaczaraj A, Abi-Said D, et al. Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 1999;44:74–80. [DOI] [PubMed] [Google Scholar]
- [18].Atalar H. Management of sacrococcygeal chordomas. Int Orthopaedics (SICO-T) 2006;30:514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rao BSS. Sacral chordoma—a report of two cases. Indian J Surg 2005;67:207–9. [Google Scholar]
- [20].Mavrogens AF. Embolization of bone tumors. Orthopedics 2011;34:303–10. [DOI] [PubMed] [Google Scholar]
- [21].Biagini R, Ruggieri P, Mercuri M, et al. Neurologic deficit after resection of the sacrum. Chir Organi Mov 1997;82:357–72. [PubMed] [Google Scholar]
- [22].Nakai S, Yoshizawa H, Kobayashi S, et al. Anorectal and bladder function after sacrifice of the sacral nerves. Spine 2000;25:2234–9. [DOI] [PubMed] [Google Scholar]
- [23].Todd LT, Jr, Yaszemski MJ, Currier BL, et al. Bowel and bladder function after major sacral resection. Clin Orthop 2002;397:36–9. [DOI] [PubMed] [Google Scholar]


