Abstract
Background:
The primary aim of this study was to evaluate the effects of a once-a-day 5 mg dose of tadalafil, prescribed for 8 weeks, on the quality of life (QoL) of South Korean men with andropause symptoms, including erectile dysfunction (ED), using a single group, open-labeled, before-and-after preliminary trial. The secondary objective was to evaluate the effectiveness and safety of tadalafil for ED.
Methods:
Forty South Korean men (>35 years of age) with andropause symptoms including ED were enrolled into our trial. Andropause syndrome was defined using the androgen deficiency in aging males (ADAM) questionnaire and other screening tests, including testosterone levels. The following outcome measures were obtained at baseline and at 4 and 8 weeks of tadalafil treatment: physical examination, adverse effects, Short Form 12 Health Survey (SF-12) score, International Index of Erectile Function (IIEF-5) score, bioelectrical impedance analysis (BIA), and free radical testing.
Results:
Treatment increased the SF-12 Mental component score, used as a proxy measure of quality of life, from baseline to at 4 and 8 weeks (P < .05). In addition, the mean IIEF-5 score, which assesses sexual function, increased from baseline at 4 and 8 weeks (P < .05), with this increase being significant at both time points. No adverse effects were noted.
Conclusion:
Tadalafil (5 mg dose, once daily) is a safe and effective treatment to improve ED, and overall QoL, among Korean men with andropause symptoms, including ED.
Keywords: andropause, erectile dysfunction, quality of life, tadalafil
1. Introduction
With aging of the general population, there has been an increasing interest in the care of men with andropause symptoms and their quality of life (QoL). A study of late-onset hypogonadism, conducted in Korea, reported that 64.6% of men between the ages of 40 and 70 years may experience andropause syndrome, with erectile dysfunction (ED) being the most frequent symptom, reported in 71% of cases.[1]
Andropause syndrome is an age-related clinical and biochemical syndrome that is characterized by symptoms of ED and testosterone deficiency, with a resultant significant decrease in QoL; it also adversely affects the functions of multiple tissues and organs, including bone, fat tissue, muscle, hematopoiesis, brain, and skin.[2] The typical clinical symptoms of andropause syndrome can be summarized into 3 categories: changes in physical characteristics (abdominal obesity, decreased muscle mass and strength, decreased bone mineral density, decrease in body hair, and skin alterations), changes in mood (depression, decreased concentration and cognitive function, and increased fatigue), and decreased sexual function (diminished libido and erectile quality).[3]
Penile erection is mainly mediated by the neurotransmitter nitric oxide (NO), via cyclic guanosine monophosphate (cGMP). Phosphodiesterase type 5 inhibitors (PDE-5I) inhibit the hydrolysis of cGMP into GMP, causing an increase in NO to relax smooth muscle and increase blood flow, thereby facilitating the erectile response.[4]
Tadalafil, which is currently used for the treatment of ED, inhibits the PDE-5 enzyme to maintain an erection. A previous study evaluated the effects of different doses of tadalafil on erectile function, compared to those of a placebo.[5] This study found that a pro re nata tadalafil dose of 2.5 or 5 mg, taken once daily (OaD) for 12 weeks, improved andropause symptom including erectile function.
Giuliano et al[6] conducted a study based on the assumption that treatment using sildenafil (PDE-5I), another inhibitor of PDE-5, would improve ED symptoms and, consequently, improve the QoL of men with ED. These authors reported an improvement in QoL parameters related to sexual function, as well as improvement in the medical outcomes study and Short Form 12 Health Survey (SF-12), indicating that andropause symptoms, including ED, influence overall QoL, rather than merely causing discomfort. Novák et al[7] indicated that andropause syndrome affects all areas of QoL, including vitality, physical, emotional, mental, social, and sexual function. In particular, ED has been associated with a loss of meaning and value of life, as well as leading to psychological frustration, feelings of helplessness and shame, family disagreement, social conflict, and lower self-confidence, as well as having an adverse effect on couple intimacy and sex life harmony.[7–9]
Considering that the ultimate goal of treatment for men with andropause symptoms, including ED, is not only to improve ED symptoms but also to improve overall QoL, our primary aim in this study was to evaluate the effects of a 5 mg OaD dose of tadalafil, prescribed for 8 weeks, on QoL of Korean men with andropausal symptoms, including ED, using a single group, open-labeled, before-and-after preliminary trial. The secondary objective was to evaluate the effectiveness and safety of the 5 mg OaD dose of tadalafil on ED in order to confirm previously reported findings on the safety of this drug for the treatment of ED.
2. Materials and methods
2.1. Study design and participants
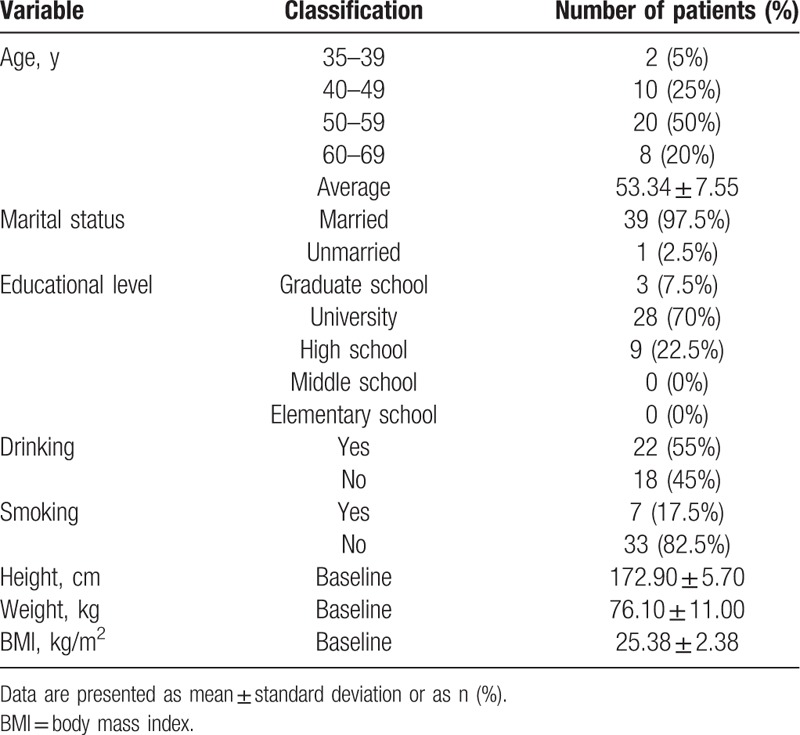
Men > 35 years of age, who sought consultation and treatment for ED at the University Hospital in Northern Gyeonggi, South Korea, between March and May 2016, were recruited for participation. After providing informed consent, participants underwent screening with those meeting our eligibility criteria (Table 1) being enrolled. The relevant general characteristics of our study group, such as age, marital status, education, and smoking and drinking habits, are summarized in Table 2.
Table 1.
Inclusion and exclusion criteria.

Table 2.
General characteristics of the study group (n = 40).
The study was approved by the Institutional Review Board of the Catholic Medical Center's Research Ethical Committee (UC16MISV0046), and the trial was registered (ClinicalTrial.gov Identifier: NCT02943356).
2.2. Protocol and evaluation
Participants were given an enrollment number and received a 5 mg OaD dose of tadalafil for 8 weeks. The following outcome measures were collected for analysis: SF-12 score, IIEF-5 score, serum levels of testosterone, bioelectrical impedance analysis (BIA) of body composition, and free radical testing. All outcome measures were obtained at baseline, at the mid-point of the treatment period (4 weeks) and at the end of the treatment period (8 weeks). In addition, physical examination, including vital signs, and adverse effect monitoring were performed to evaluate drug safety, with measures also obtained at baseline and at 4 and 8 weeks (Fig. 1). Evaluation at the mid-point of the treatment period (4 weeks) was included to monitor for adverse effects of tadalafil, as well as to measure the time-dependent improvement effect on QoL. The severity of adverse effects was classified based on the following 3 grades: grade 1, mild; grade 2, moderate; and grade 3, severe. The degree of causality between tadalafil and adverse effects was evaluated on a 5-point scale: 1, definitely related; 2, probably related; 3, possibly related; 4, unlikely related; and 5, not related.
Figure 1.
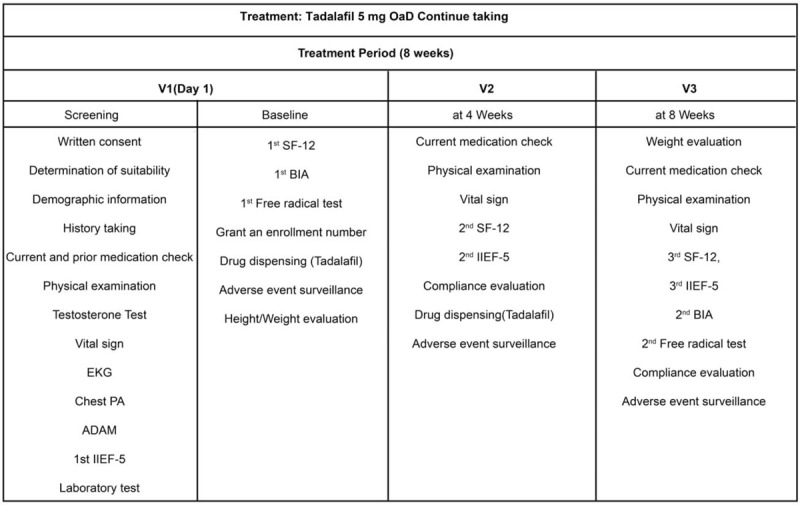
Study design. ADAM = androgen deficiency in aging males, BIA = bioelectrical impedance analysis, EKG = electrocardiography, IIEF-5 = International Index of Erectile Function, OaD = once daily, PA = posteroanterior, SF-12 = Short Form 12 Health Survey.
2.3. Screening measures
2.3.1. Androgen deficiency in aging males (ADAM) questionnaire
Andropause symptoms were measured using the Korean version of the ADAM questionnaire, originally developed by Morley et al[10] and translated into Korean by Kim et al.[11] The questionnaire consists of 10 items evaluating libido, energy, strength and endurance, height, enjoyment of life, feelings of sadness and moodiness, erections, and the ability to play sports, to sleep, and to work. The questionnaire uses a dichotomous “yes” or “no” answer scale. Positive responses to questions regarding libido or erection, as well as a positive answer on ≥3 of the remaining items were used as criteria for andropause syndrome.[1,12]
2.4. Outcome measures
2.4.1. Observational and clinical tests
Demographic and anthropometric measurements were obtained at baseline (Table 2), with social factors obtained through history-taking during the screening visit. The medical records of individuals enrolled onto the study were reviewed to obtain the medical history within the last 6 months, as well as medication history. Results of the physical examination, including vital signs, and laboratory tests performed, are reported in Table 3.
Table 3.
Laboratory tests.

2.4.2. SF-12 questionnaire
Health-related QoL was measured using the Korean version of SF-12, which is adapted from the original developed by Ware et al,[13] and used in this study with permission from the copyright owner, QualityMetric Incorporated. The instrument consists of 12 items measuring 2 component scores, the Physical Component Score (PCS) and the Mental Component Score (MCS), with the response scales for each item varying between 2, 4, and 6 points. The PCS includes the evaluation of physical functioning, role—physical, bodily pain (BP), and general health (GH), while the MCS evaluates vitality, social functioning, role—emotional (RE), and mental health (MH). The overall health score is quantified as the weighted average of all 8 domains. The QoL score on the SF-12 was calculated using the method provided by QualityMetric Incorporated, with a higher score indicative of a higher QoL. The reliability of the SF-12 has previously been established (Cronbach's α, 0.81–0.84).[14]
2.4.3. The IIEF-5 questionnaire
The IIEF-5 questionnaire is an abridged version of IIEF designed to the severity of ED. The questionnaire consists of 5 items evaluating the satisfaction with erectile function and sexual intercourse. Each item is scored on a 5-point scale, from “0” to “5,” and the scores for each item are summed to yield a total score.[15]
2.4.4. BIA and free radical test
BIA (Biodynamics BIA 450, Biodynamics Corporation, Seattle, Washington, DC) measures body composition by passing a low-level electrical current through the body to determine the bioelectrical impedance to. The BIA method has been widely used to assess body composition across a variety of diseases and health statuses, including obesity, diabetes mellitus, heart failure, and hemodialysis.[16] From this perspective, BIA could be useful to evaluate possible associations between body composition and QoL. All BIA analyses were performed with participants in the supine position, on a nonconductive surface, with electrodes placed on the right hand and foot. Six components of the BIA were measured, and all assessments were performed by the same evaluator.
Free radicals are by-products of the metabolic processes of the body and are “harmful oxygen” molecules associated with cell apoptosis and the development of several diseases, including different types of cancers, chronic diseases, and aging. The FORT free radical test measures the level of free radicals in the body, with levels referenced to the standard level 600,[17] classified as follows: >600, very severe oxidative stress; 401 to 600, high oxidative stress; 341 to 400, moderate oxidative stress; 311 to 340, low oxidative stress; 231 to 300, cautionary levels of oxidative stress; 160 to 230, normal level; and <160, requiring consultation. The free radical test was measured using a spectrophotometer, at a wavelength of 505 nm (Form CR 2000, Callegari, Parma, Italy), with intra- and interassays coefficients of variation (CV) of <5%, and a sensitivity of 4.0528. When measuring a known concentration of control serum (control substance), the specificity is within ±10% of the known concentration.
2.4.5. Total testosterone level
Testosterone is a steroid male hormone (androgen) produced by male testicular endocrine tissue (Leydig cells). Testosterone determines secondary sex characteristics, sexual desire, and erection, as well as increases metabolic processes in muscles, bones, bone marrow, the immune system, and the brain.[18]
Total testosterone level was measured from venous blood samples in the fasting state, in the morning (7–11 am), with measured levels normalized for age and classified into normal (≥3.5 ng/mL) and low (<3.5 ng/mL) levels. Analyses were performed using GAMMACOUNTER (Cobra5010, Canberra Packard, Pangebourne, UK), with a CV of <10% (range, 3.3–6.2%) for intra- and interassay precision.
2.5. Sample size and statistical analysis
2.5.1. Sample size
The primary outcome of our study was the change in the SF-12 QoL score, with the changes in BIA, free radical level, and IIEF-5 score being evaluated as secondary outcomes. Quantitative variables were reported as the mean ± standard deviation (SD) or median, as appropriate, with qualitative variables reported as count and proportion (n, %). Using the methods of Tong et al,[19] the sample size was calculated assuming a change of 8.78 points on the SF-12 QoL score, with a SD of 17 points. Sample size calculation was performed using PASS 12 (NCSS, LLC., Kaysville, UT), with 32 participants required to detect a change in QoL at a level of significance of 0.05 and power of 80%. Considering a dropout rate of 20%, 40 participants were enrolled into the study.
2.5.2. Statistical analysis
As all 40 participants completed the study protocol, the full analysis set and the per-protocol analysis set included the 40 participants in the assessment of QoL, changes in IIEF-5 score, SF-12 score, BIA, and free radical testing before and after tadalafil administration. For further safety evaluation, adverse effects, vital signs (blood pressure, pulse, and body temperature), and physical examination were used as input variables. Statistical analysis was performed using SPSS (version 17, IBM Corp, Armonk, NY). The paired t test was used to evaluate the change in score from baseline, with the Wilcoxon signed rank test being used when normality was not verified, as determined using the Shapiro–Wilk test. A P value < .05 was considered statistically significant.
3. Results
3.1. Study participants
Of the 102 men who sought treatment in our clinic during the study period (8 weeks), 40 met our inclusion and exclusion criteria and were enrolled into the trial. The other 82 were excluded due to not meeting the inclusion criteria (42), declining to participate (32), and 8 due to other reasons. All 40 participants enrolled completed all components of the study protocol.
3.2. General characteristics of the study participants
Relevant characteristics of our study group are reported in Table 2, and summarized as follows: age, 53.34 ± 7.55 years (range, 40–59 years); height 172.90 ± 5.70 cm; weight, 76.10 ± 11.00 kg; body mass index (BMI), 25.38 ± 2.38 kg/m2; all participants, with the exception of one, were married; 77.5% had a college education or above; 22 participants (55.00%) consumed alcohol regularly; and 7 (17.50%) were smokers.
3.3. Testosterone level and ADAM questionnaire
With regards to testosterone level, 30 participants (75.0%) had normal levels and 10 (25%) had low levels (Table 4). On the ADAM questionnaire, 30 participants (75.0%) reported a decrease in libido, with 5 (12.5%) reporting a decrease in the rigidity of penile erection. In addition, 24 participants (60.0%) reported a lack of energy, 30 (75.0%) reported a decrease in strength and/or endurance, 11 (27.5%) reported decreased height, 11 (27.5%) reported a decreased enjoyment of life, 10 (25.0%) reported being sad and/or moody, 10 (25.0%) reported a recent deterioration in their ability to play sports, 15 (37.5%) required sleep after dinner, and 17 (42.40%) reported a recent deterioration in their work performance (Table 5).
Table 4.
The range of testosterone level according to age.

Table 5.
Androgen deficiency in aging males questionnaire (n = 40).

3.4. Vital signs and physical examination
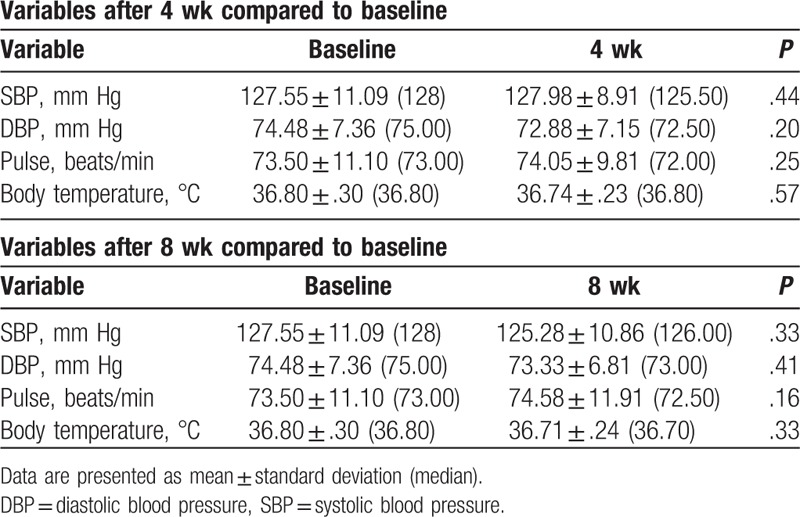
There was an overall decrease in systolic blood pressure, from baseline, of 1.58 ± 12.70 mm Hg at 4 weeks and 2.28 ± 13.35 mm Hg at 8 weeks of treatment, with a decrease in diastolic blood pressure of 1.60 ± 7.85 mm Hg at 4 weeks and 1.15 ± 8.82 mm Hg at 8 weeks. There was an increase in pulse rate, from baseline, of 0.55 ± 3.77 beats/min at 4 weeks and 1.08 ± 4.72 beats/min at 8 weeks, whereas the body temperature decreased by 0.03 ± .30°C at 4 weeks and 0.05 ± 0.38°C at 8 weeks. The change in vital signs from week 4 to week 8 was nonsignificant (Table 6). The physical examination of all participants was within normal limits at all measurement time points.
Table 6.
Vital sign and physical measurement scores at 4 and 8 weeks.
3.5. Change in the SF-12 scores
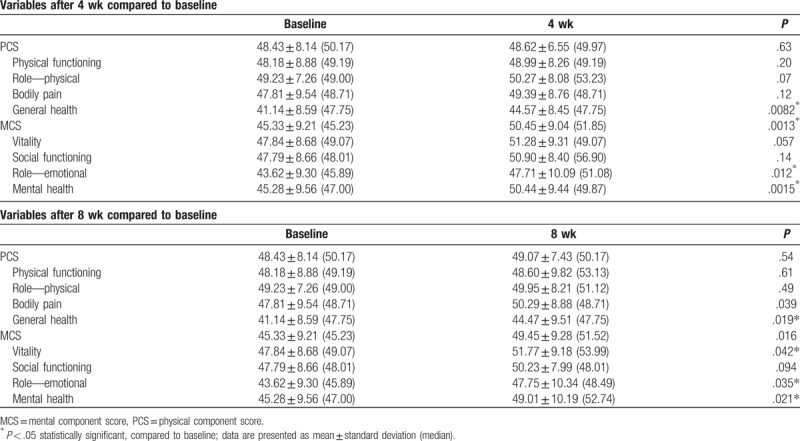
There was an overall nonsignificant increase in PCS, from baseline, of 0.47 ± 5.63 at 4 weeks and 0.73 ± 7.07 at 8 weeks. By item, there was a significant increase, from baseline, in the GH score of 3.43 ± 7.41 at 4 weeks and 3.33 ± 7.50 at 8 weeks. Other increases in specific item scores, reported in Table 7, included: MCS, 4.85 ± 8.15 at 4 weeks and 4.39 ± 8.48 at 8 weeks (both significant); vitality, 3.44 ± 10.58 at 4 weeks (nonsignificant) and 3.93 ± 9.91 at 8 weeks (significant); RE, 3.86 ± 9.14 at 4 weeks and 3.60 ± 8.94 at 8 weeks (both significant); and MH, 5.16 ± 9.07 at 4 weeks and 3.73 ± 7.64 at 8 weeks (both significant).
Table 7.
Short Form-12 questionnaire scores after 4 and 8 weeks.
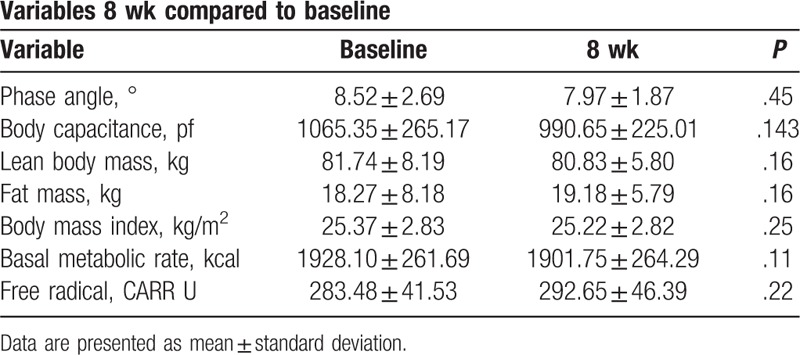
3.6. Change in BIA and free radical test
There was no change in BIA variables at either 4 or 8 weeks. The mean free oxygen radical level at baseline was 283.48 ± 41.53 (CARR U), classified as a cautionary level, with a nonsignificant increase, from baseline, of 9.18 ± 36.33 (CARR U) at 8 weeks (Table 8).
Table 8.
Bioelectrical impedance analysis and free radical test scores at 8 weeks.
3.7. Change in IIEF-5 scores
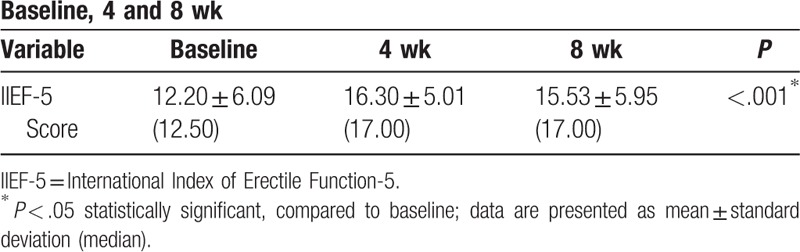
The mean IIEF-5 score, which assesses sexual function, increased significantly from baseline, by 4.10 ± 5.52 points at 4 weeks and 3.33 ± 4.77 points at 8 weeks (Table 9).
Table 9.
International Index of Erectile Function-5 score at the 3 measurement time points.
3.8. Safety outcomes
With regard to adverse effects, the following were observed: 1 case (2.5%) of stomatitis at baseline; 5 cases (12.50%) of dyspnea, fatigue, hand tingling, swelling around the eye, and muscle tension at 4 weeks; and 2 cases (5.0%) of tingling of the feet and nausea at 8 weeks. All adverse effects at 4 and 8 weeks were considered to be mild. On evaluation of the causal relationship between the drug and the adverse effects, all cases were deemed “unlikely” or “not” related to the drug. Twenty-four participants (60.0%) reported taking other medications before the study, with 26 participants (65.0%) taking a combination of medications at 4 weeks and 25 (62.5%) at 8 weeks. The use of combined medications was not a contraindication in our protocol.
4. Discussion
We conducted this pilot study to evaluate the possible clinical utility of tadalafil (5 mg, OaD) to improve the QoL of Korean men over 35 years old with andropause symptoms, including ED. The 5 mg daily dose of tadalafil produced a significant improvement in the MCS component of QoL at 4 and 8 weeks. Specifically, we identified an increase in MH and RE (5.16 ± 9.07 and 3.86 ± 9.14, respectively) at 4 weeks, with a further increase in MH, RE, and vitality (3.73 ± 7.64, 3.60 ± 8.94, and 3.93 ± 9.91, respectively) at 8 weeks. On the other hand, although the mean change in PCS subitems increased from baseline at 4 and 8 weeks, only the change in GH was significant. Based on our results, we propose that tadalafil (at a dose of 5 mg, OaD) produced a significant improvement in the overall QoL in our study group. This improvement in QoL appears to be related to an increase in self-confidence associated with improved sexual function and sex life, and improved partner relationship, findings which are consistent with those of a previous study in which sildenafil (PDE-5I) was used.[7] The absence of an improvement in PCS likely reflects our exclusion of physical disease in our selection criteria. A previous study evaluating the clinical utility of tadalafil on QoL in patients with benign prostatic hypertrophy (BPH) reported a meaningful increase in the International Prostate Symptom Score (IPSS) within 1 week in 65% of patients, with a meaningful increase achieved in 80% of patients by 4 weeks. Therefore, our 8-week period of treatment was sufficient to evaluate meaningful effects.[20] In that study, however, it was not possible to ascertain that increases in the IPSS specifically reflected increases in QoL, as most of the IPSS indices were related to urinary symptoms, with only one index related to QoL. Despite this limitation, the study is significant in that it demonstrated the association between the use of tadalafil and QoL improvement. Another previous study also reported an improvement in erectile function among men with ED using tadalafil, with this increase improving the relationship between men and their wives and, therefore, their overall QoL.[21] As expected in our study, we identified a significant increase in the IIEF-5 score, which assesses specific improvement in sexual function, at 4 and 8 weeks (4.10 ± 5.52 and 3.33 ± 4.77, respectively; P < .01). These findings are consistent with the aforementioned previous studies on the effects of tadalafil on ED. Although we included BIA measures in our analysis, the effects of tadalafil on body composition have not yet been well-studied and clarified. Therefore, we are not surprised by the lack of effect of tadalafil on BIA measures. However, we had not anticipated the lack of effect of tadalafil on free oxygen radicals at 8 weeks. We had postulated that as a PDE-5I, tadalafil would increase the concentration of NO and, therefore, reduce the level of free radicals. We had based our hypothesis on previous studies that had indicated and lowering of free radicals with NO release from endothelial cells, except under ischemic conditions.[22–24] Our findings may reflect the multiple health and environmental factors that influence free radical levels, including exercise, vitamins, healthy diet, smoking, obesity, and aging which modify the antioxidant capacity and, hence, levels of free radicals.[25] Consequently, it was difficult to evaluate the direct association between the use of tadalafil and free radical test results in our study.
The mean total testosterone level among our participants was 5.92 ± 3.74 ng/mL, with normal levels ranging between 3.5 and 9 ng/mL, among study populations and countries. Only 25% of our participants were classified as having a low testosterone level (<3.5 ng/mL), with the other participants having a normal level of testosterone, despite the presence of andropause symptoms, including ED. Possible explanations for these cases, which are common in clinical practice, include changes in the sensitivity of tissues and receptors to hormones, differences in hormone concentrations in tissues, degeneration of the hormone itself, and increases in the level of sexual hormone-binding globulin (SHBG). According to previous studies, the risk of ED in andropausal men is associated with the decrease in testosterone levels. As such, traditionally, the testosterone level is used as a biochemical indicator of andropause syndrome, with the prevalence of ED being higher among those with a lower testosterone level.[26] However, a relatively recent study reported that the blood concentration of male hormones may not be representative of the amount of hormonal activity, which is influenced by the function of the hormone itself.[27] Moreover, as SHBG increases with aging, blood levels of testosterone might not accurately represent the hormone status in middle-aged individuals. Therefore, the portion of biologically active testosterone should be measured.[27] It is also important to note that there are age-related decreases in other hormones as well in men, including dehydroepiandrosterone, growth hormone, insulin-like growth factor-I, and melatonin.[28] However, according to the guidelines of the International Men's Society and Korean Andropausal Society, testosterone replacement therapy (TRT) should be initiated when the serum level of testosterone reduces to <250 ng/dL[12] and/or in the presence of andropause symptoms, with these criteria being consistent across different countries. Therefore, the efficacy of TRT may be limited for men with andropause symptoms, including ED, who have a total testosterone level > 250 ng/dL. In our study, the use of 5 mg OaD of tadalafil improved the QoL of men with andropause symptoms, including ED, regardless of whether their total testosterone level was below <250 ng/dL or not.
In terms of compliance with using tadalafil and its safety, all participants fully adhered to the study protocol over the 8 weeks of the study period, with no drop-outs and good drug tolerability. This might reflect the convenience of the OaD dose and the relative short duration of prescription (8 weeks). The recommendation provided by physicians regarding the appropriateness of tadalafil as an optimal treatment suited to each patient's needs and the provision of a full explanation of the advantages and disadvantages of the drug might have helped to increase compliance. These results are consistent with those of a previous observational study that reported that physicians can influence patients’ decision regarding treatment for ED, with >86% of patients in that study either starting to use tadalafil, or switching to a 5 mg OaD dose, and continuing to treatment for 6 months.[29] A randomized, double-blind, placebo-controlled trial evaluating the effects of a 12-week prescription of a 5 mg OaD dose of tadalafil among 354 Korean men with BPH reported beneficial effects of the treatment, with the drug being well-tolerated.[30] With regards to safety, we identified only transient or mild adverse effects in our study group, which did not hinder participants’ daily function. Moreover, the 5 mg tadalafil dose had no significant effect on vital signs, despite the vasodilation effects of tadalafil. Our results confirm those of Kang et al who reported on the safety and efficacy of a 5 mg OaD dose of tadalafil among 162 Korean men. Improved blood pressure and heart rate were found after an 8-week treatment.[31] Adverse effects identified included facial flushing in 6% of patients at 4 weeks, and headaches in 4% of patients at 8 weeks.
The limitations of our study need to be acknowledged. First, this is a single group, open-labeled, before-and-after preliminary trial, without a control group, and included a relatively small number of participants (n = 40). However, we do acknowledge that even for a preliminary study, a randomized placebo-controlled trial would be more appropriate. Moreover, both QoL and ED were evaluated using self-reported questionnaires. Therefore, the objectivity of our findings is insufficient for the generalization of our results. Second, several variables, such as educational level, religion, quality of the partner relationship, monthly income, exercise, hobbies, marital status, and frequency of sexual intercourse may affect QoL, and could have been included as confounding variables. Lastly, the 8-week period of observation, from the initial screening to the end point of the study, was relatively short, which may not be sufficiently long to fully evaluate QoL and safety of the drug.
Our study demonstrated that a daily 5 mg dose of tadalafil for 8 weeks was effective in improving overall QoL in Korean men with andropause symptoms, including ED, and was safe to use. We present an alternative treatment to improve ED and overall QoL in this clinical population. Future trials, with a larger sample size, longer duration of follow-up, a control group, and more objective assessments are needed to confirm our results.
Author contributions
Conceptualization: Keun-Sang Yum, Kang Kon Lee.
Formal analysis: Kang Kon Lee.
Investigation: Keun-Sang Yum.
Writing – original draft: Joong Gyo Lee, Byung Duk Kim, Chang Hee Han.
Writing – review & editing: Keun-Sang Yum, Joong Gyo Lee, Byung Duk Kim, Chang Hee Han, Kang Kon Lee.
Footnotes
Abbreviations: ADAM = androgen deficiency in aging males, BIA = bioelectrical impedance analysis, BMI = body mass index, BPH = benign prostatic hypertrophy, cGMP = cyclic guanosine monophosphate, CV = coefficient of variation, ED = erectile dysfunction, GH = general health, IIEF-5 = International Index of Erectile Function-5, IPSS = International Prostate Symptom score, MCS = mental component score, MH = mental health, NO = nitric oxide, OaD = once daily, PCS = physical component score, PDE-5I = phosphodiesterase type 5 inhibitors, QoL = quality of life, RE = role—emotional, SD = standard deviation, SF-12 = Short Form 12 Health Survey, SHBG = sexual hormone-binding globulin, TRT = testosterone-replacement therapy.
Funding: The present study was supported by Hanmi Pharm© (14, Wiryeseong-daero, Songpa-gu, Seoul, Korea sujin.park82@hanmi.co.kr). We thank medical-excellence (Crop.) Team Bio Statistician (www.medicalexellenc.co.kr) for help in the Statistical analysis.
The authors have no conflicts of interest to disclose.
References
- [1].Lee MW, Park H. A study on late-onset of hypogonadism, erectile dysfunction, depression, and quality of life among middle-aged male worker [in Korean]. Korean J Adult Nurs 2013;25:483–93. [Google Scholar]
- [2].Morales A, Lunenfeld B. Investigation, treatment and monitoring of late-onset hypogonadism in males. Official recommendations of ISSAM. International Society for the Study of the Aging Male Aging Male. Aging Male 2002;5:74–86. [PubMed] [Google Scholar]
- [3].Kim KM. Latest knowledge of treatment in andropause [in Korean]. Korean J Health Promot 2012;1:117–21. [Google Scholar]
- [4].Scaglione F, Donde S, Hassan TA, et al. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin Ther 2017;39:370–7. [DOI] [PubMed] [Google Scholar]
- [5].Kim ED, Seftel AD, Goldfischer ER, et al. A return to normal erectile function with tadalafil once daily after an incomplete response to as-needed PDE5 inhibitor therapy. J Sex Med 2014;11:820–30. [DOI] [PubMed] [Google Scholar]
- [6].Giuliano F, Peña BM, Mishra A, et al. Efficacy results and quality-of-life measures in men receiving sildenafil citrate for the treatment of erectile dysfunction. Qual Life Res 2001;10:359–69. [DOI] [PubMed] [Google Scholar]
- [7].Novák A, Brod M, Elbers J. Andropause and quality of life: findings from patient focus groups and clinical experts. Maturitas 2002;43:231–7. [DOI] [PubMed] [Google Scholar]
- [8].Attia AA, Hassan FA, Kamel MI, et al. Quality of life in erectile dysfunction patients and their partners responding to tadalafil versus sildenafil citrate. Egypt J Dermatol Venerol 2013;33:32–6. [Google Scholar]
- [9].Bai WJ, Li HJ, Jin JJ, et al. A randomized clinical trial investigating treatment choice in Chinese men receiving sildenafil citrate and tadalafil for treating erectile dysfunction. Asian J Androl 2017;19:500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000;49:1239–42. [DOI] [PubMed] [Google Scholar]
- [11].Kim SW, Oh SJ, Paick JS, et al. Development of the Korean-translation of androgen deficiency in aging males (ADAM) questionnaire [in Korean]. Korean J Urol 2004;45:674–9. [Google Scholar]
- [12].Moon KH. Chang JY. Guidelines of Andropause. Andropause. [in Korean]. Seoul, Korea: Koon Ja Publishers; 2009. 343–7. [Google Scholar]
- [13].Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. [DOI] [PubMed] [Google Scholar]
- [14].Lee SH, Yang SO. The effects of chronic musculoskeletal pain and depression on health-related quality of life by gender in community-dwelling older adults [in Korean]. J Korean Acad Community Health Nurs 2010;21:21–30. [Google Scholar]
- [15].Ahn TY, Lee DS, Hong JH, et al. Validation of an abridged Korean version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction [in Korean]. Korean J Urol 2001;42:535–40. [Google Scholar]
- [16].Bae JH, Kim YM, Kim KH, et al. Review on bioelectrical impedance analysis in traditional East Asian medicine [in Korean]. J Physiol Pathol Korean Med 2013;27:717–29. [Google Scholar]
- [17].Dal Negro RW, Visconti MC, Micheletto C, et al. Normal values and reproducibility of the major oxidative stress obtained thanks to FORM system. G Ital Mal Torace 2003;57:199–209. [Google Scholar]
- [18].Bagatell CJ, Bremner WJ. Androgens in men—uses and abuses. N Engl J Med 1996;334:707–15. [DOI] [PubMed] [Google Scholar]
- [19].Tong SF, Ng CJ, Lee BC, et al. Effect of long-acting testosterone undecanoate treatment on quality of life in men with testosterone deficiency syndrome: a double blind randomized controlled trial. Asian J Androl 2012;14:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oelke M, Shinghal R, Sontag A, et al. Time to onset of clinically meaningful improvement with tadalafil 5 mg once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: analysis of data pooled from 4 pivotal, double-blind, placebo controlled studies. J Urol 2015;193:1581–9. [DOI] [PubMed] [Google Scholar]
- [21].Rubio-Aurioles E, Kim ED, Rosen RC, et al. Impact on erectile function and sexual quality of life of couples: a double-blind, randomized, placebo-controlled trial of tadalafil taken once daily. J Sex Med 2009;6:1314–23. [DOI] [PubMed] [Google Scholar]
- [22].Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, et al. Ischemia/reperfusion injury. J Surg Res 2002;105:248–58. [DOI] [PubMed] [Google Scholar]
- [23].Lefer AM, Lefer DJ. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc Res 1996;32:743–51. [PubMed] [Google Scholar]
- [24].McVary KT, Monnig W, Camps JL, et al. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 2007;177:1071–7. [DOI] [PubMed] [Google Scholar]
- [25].Kang YJ, Lee KJ, Min KB, et al. The Correlation between the smoking and oxygen free radicals in men [in Korean]. Korean J Health Promot 2012;12:129–36. [Google Scholar]
- [26].Makhlouf AA, Mohamed MA, Seftel AD, et al. Hypogonadism is associated with overt depression symptoms in men with erectile dysfunction. Int J Impot Res 2008;20:157–61. [DOI] [PubMed] [Google Scholar]
- [27].Kim KM. Late-onset hypogonadism [in Korean]. Korean J Fam Med 2013;3:245–54. [Google Scholar]
- [28].Jones R, Lopez KH. Reproductive Aging. Human Reproductive Biology, 4th ed. San Diego, United States: Science Direct; 2014:119–131. [Google Scholar]
- [29].Buvat J, Hatzichristou D, Boess FG, et al. Continuation and effectiveness of tadalafil once daily during a 6-month observational study in erectile dysfunction: the EDATE study. Int J Clin Pract 2004;68:1087–99. [DOI] [PubMed] [Google Scholar]
- [30].Kang DH, Lee JY, Park SY, et al. Efficacy and safety of tadalafil 5 mg administered once daily in Korean men with erectile dysfunction: a prospective, multicenter study [in Korean]. Korean J Urol 2010;51:647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee SW, Paick JS, Park HJ, et al. The efficacy and safety of tadalafil 5 mg once daily in Korean men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: an integrated analysis. World J Mens Health 2014;32:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


