Abstract
We aimed to investigate the clinical significance and prognostic value of miR-145 in hepatocellular carcinoma (HCC).
HCC tissue samples and adjacent normal liver tissues were obtained from 139 patients diagnosed with HCC. The relationships between the expression level of miR-145 and clinicopathologic factors were evaluated by Chi square test. Kaplan–Meier survival analysis with the log-rank test was used to evaluate the association between miR-145 expression and HCC prognosis.
miR-145 was significantly down-regulated in HCC tissues compared with the adjacent noncancerous tissues (P < .001). Its expression level was significantly correlated with tumor size (P = .010), tumor number (P = .033), lymph node metastasis (P < .000), TNM stage (P < .001) and tumor differentiation (P < .001). Kaplan–Meier curves with log rank test showed that the overall survival of the patients with low miR-145 expression was significantly shorter in comparison with the high miR-145 expression patients (P = .043). Furthermore, multivariate analysis using the Cox proportional hazards model for all variables showed that miR-145 expression was an independent prognostic factor for overall survival (P = .033).
Our results indicate that low expression of miR-145 is an independent poor prognostic factor for patients with HCC. Further investigations are needed to confirm our findings.
Keywords: biomarker, HCC, hepatocellular carcinoma, microRNA-145, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men and the seventh in women.[1,2] It is the third leading causes of cancer-related death in the world. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) has a major role in the development of the disease, therefore, the diagnosis of hepatic fibrosis and inflammation in patients with chronic hepatitis is essential for therapeutic and prognostic implications[3–6,7] Recently, new markers, including specific gene signatures, have been explored to stratify the risk of recurrence and to improve management of HCC, however, predictive biomarkers are still urgently needed.
MicroRNAs (miRNAs) are a class of endogenous, small non-coding RNAs (19–22 nucleotides in length) that regulate gene expression at post-transcriptional level. MiRNAs are found to be involved in many important functions in development, cell differentiation, and regulation of cell cycle and apoptosis, and their expression is abnormally regulated in cancer by a variety of mechanisms, including amplification, deletion, mutation, and epigenetic silencing.[8,9]
MicroRNA-145 (miR-145) is commonly down-regulated in various malignancies, and it is considered as a tumor suppressor miRNA.[10–13] In addition, data from clinical studies indicate that low miR-145 expression correlates with poor prognostic outcomes.[14] Previously, Ding et al investigated the role of miR-145 in the progression of HCC. Their results showed that miR-145 was frequently down-regulated in HCC tissues and cell lines. Over-expression of miR-145 in HCC cell lines significantly inhibited cell proliferation, migration, and invasion in vitro, suggesting miR-145 as a potential new diagnostic and therapeutic target for the treatment of HCC.[15] However, the clinical significance and prognostic value of miR-145 in HCC have not been investigated. In the present study, we investigate the clinical significance of miR-145 in HCC.
2. Materials and methods
2.1. Patients and tissue samples
HCC tissue samples and adjacent normal liver tissues were obtained from 139 patients diagnosed with HCC who had undergone a routine hepatic resection between May 2009 and February 2016. None of the patients had received preoperative radiotherapy or chemotherapy prior to surgical resection. In this study, the duration of overall survival was defined as from the date of the initial treatment completion to the date of death or last known date alive. HCC tissues and tissues from the surrounding non-cancerous liver were immediately transferred to Allprotect tissue reagent (Qiagen, Hilden, Germany) and stored at −80 °C until further examination. The study was approved by the Ethics Committee of Beijing Chaoyang Hospital affiliated to Capital Medical University. And all the patients provided written informed consent.
2.2. RNA Isolation and qRT-PCR
Total RNA was extracted from tissues with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer instructions. miRNAs were reverse transcribed to complementary DNA (cDNA). Quantitative mRNA analysis was performed using real-time PCR analysis (TaqMan, PE Applied Biosystems, Foster City, CA, USA). Reaction conditions included: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 57 °C for 20 s, and 72 °C for 15 s. Relative expression was calculated using the comparative Ct. Each sample was carried out in triplicate. U6 small nuclear RNA was used as an endogenous control. The following primers were used: miR-145, forward 5’-GTC CTC ACG GTC CAG TTT-3’ and reverse 5’-TTT GGC ACT AGC ACATT-3’; U6, forward 5’-CTC GCT TCG GCA GCACA-3’ and reverse 5’-AAC GCT TCA CGA ATT TGCGT-3’.
2.3. Statistical analysis
Statistical difference between two groups was analyzed using a student t test. The relationships between the expression of miR-145 and clinicopathologic factors were evaluated by Chi square test. Kaplan–Meier survival analysis with the log-rank test was used to evaluate the association between miR-145 expression and HCC prognosis. Hazard ratios (HRs) and 95% CIs for miR-145 expression were calculated from univariate and multivariate Cox regression model. In this study, a P-value of less than .05 was considered statistically significant. All analyses were performed with the statistical package for social science (SPSS) version 18 (SPSS Institute, Chicago, IL, USA).
3. Results
3.1. miR-145 is down-regulated in HCC
To analyze the expression of miR-145 in HCC, we collected 139 pairs of human HCC samples and their adjacent non-tumorous liver tissues. The expression level of miR-145 was detected by qRT-PCR. As shown in Figure 1, miR-145 was significantly down-regulated in HCC tissues compared with the adjacent noncancerous tissues (P < .001), suggesting that miR-145 may play antitumor suppressor role in HCC.
Figure 1.
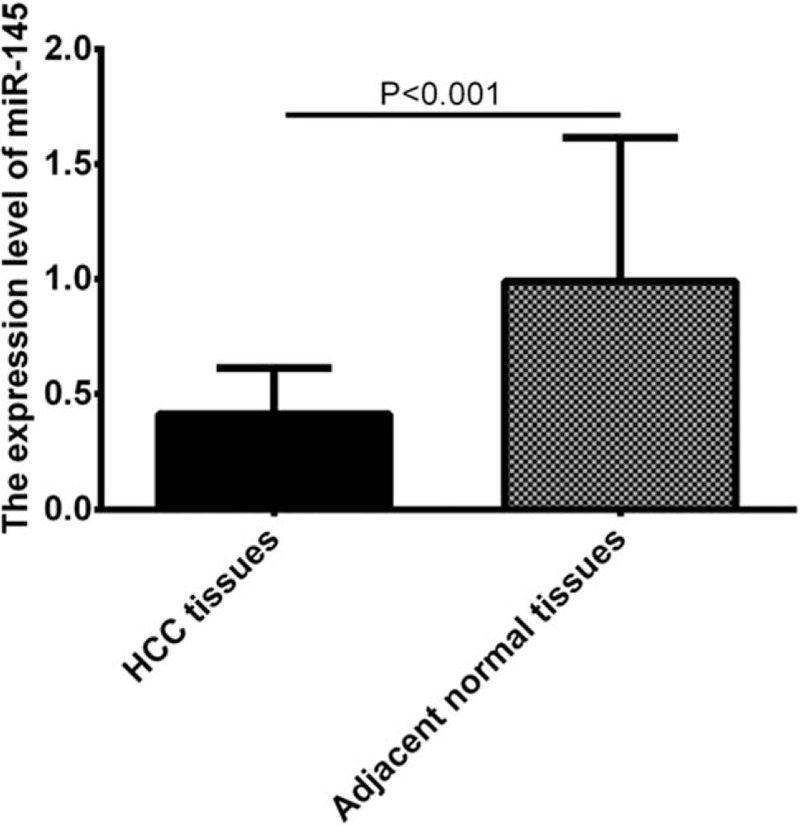
miR-145 was significantly down-regulated in HCC tissues compared with the adjacent noncancerous tissues. HCC = hepatocellular carcinoma.
3.2. Correlation of miR-145 expression with clinicopathological characteristics of HCC
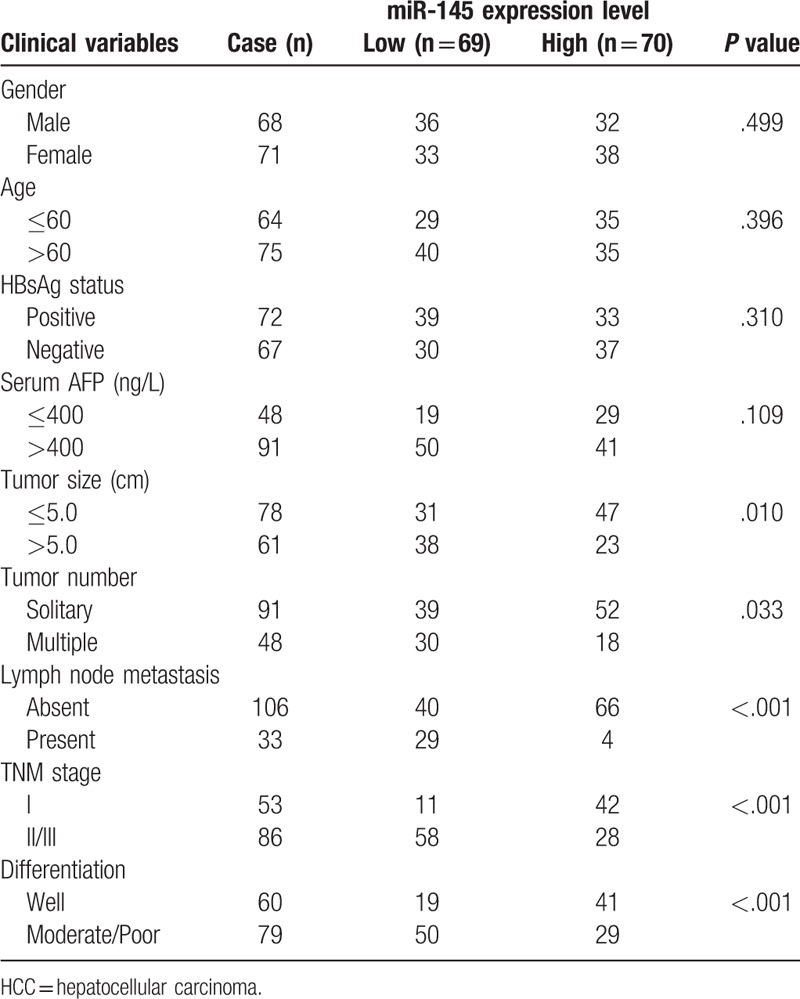
The associations of miR-145 expression with various clinicopathological parameters of HCC are summarized in Table 1. The median expression level of miR-145 was used to classify the patients with HCC into two groups. The expression level of miR-145 was significantly correlated with tumor size (P = .010), tumor number (P = .033), lymph node metastasis (P < .000), TNM stage (P < .001) and tumor differentiation (P < .001). However, no significant correlation was observed between miR-145 expression and other clinicopathologic characteristics (all P > .05).
Table 1.
The associations between miR-145 expression and clinicopathological parameters in 139 patients with HCC.
3.3. Low expression level of miR-145 was associated with shorter overall survival in HCC
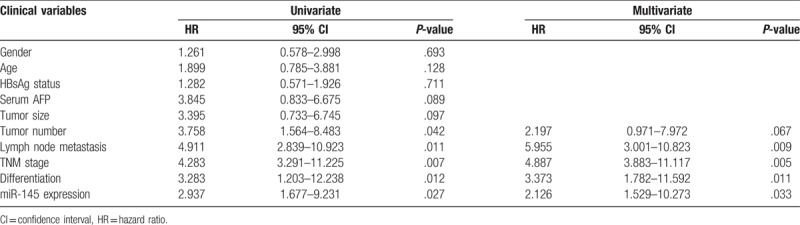
To further investigate the correlation between miR-145 expression level and prognosis of HCC, we followed up the overall survival of the HCC patients. Kaplan–Meier curves with log rank test showed that the overall survival of the patients with low miR-145 expression was significantly shorter in comparison with the high miR-145 expression patients (P = .043, shown in Fig. 2). Furthermore, multivariate analysis using the Cox proportional hazards model for all variables showed that miR-145 expression was an independent prognostic factor for overall survival (P = .033, shown in Table 2).
Figure 2.
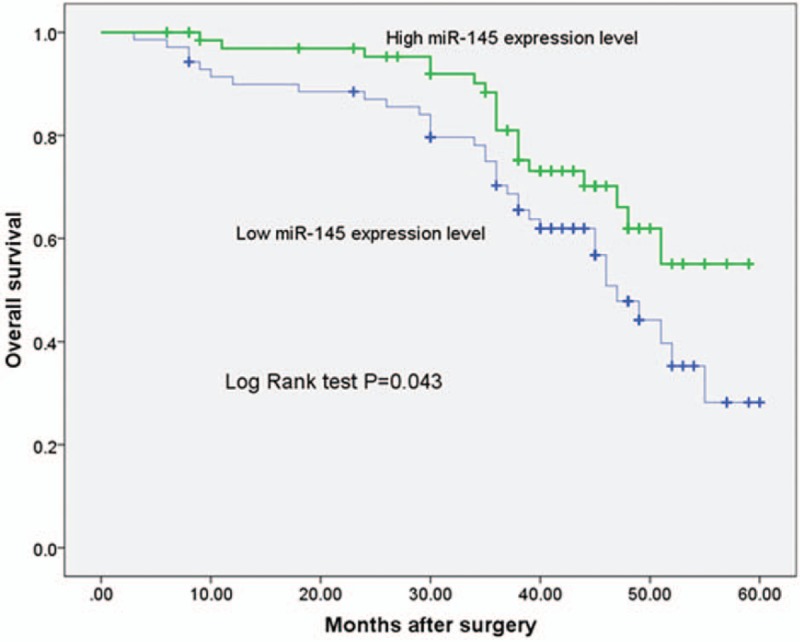
Kaplan–Meier survival curve in relation to miR-145 expression level in patients with HCC. HCC = hepatocellular carcinoma.
Table 2.
COX regeression regression analysis on the relationship of clinicopathologic characteristics and prognosis.
4. Discussion
Despite the tremendous efforts by physicians and researchers to develop early diagnostic methods or innovative treatment strategies to improve the clinical outcomes in patients with HCC, the majority of the HCC patients, especially those in the developing countries such as China, still experience very poor prognosis.[16,17] Thus, it is critical to elucidate the underlying molecular mechanisms of human HCC, search novel biomarker, and develop efficient treatment plans to suppress tumor growth, recurrence or metastasis in patients with HCC.
miRNAs are small non-coding RNAs involved in several physiological and pathological processes, such as cell growth and differentiation, inflammation, and carcinogenesis by regulating gene expression on a post-transcriptional level.[18] Recently, miRNAs are found to be promising biomarkers for cancer diagnosis and prognosis, and increasing studies on miRNAs as biomarkers have been reported.[19,20]
miR-145 is commonly down-regulated in various malignancies, and it is considered as a tumor suppressor miRNA. Zheng et al found that miR-145 acted as a tumor suppressor in breast cancer and its down-regulation in tumor tissues might contribute to the progression of breast cancer through a mechanism involving ROCK1, suggesting miR-145 as a potential new diagnostic and therapeutic target for the treatment of breast cancer.[21] Li et al found that miR-145 down-regulation was remarkably related with the occurrence of a phenotype of osteosarcoma with more aggressiveness. The univariate analysis showed that a lower miR-145 level was associated with unfavorable prognosis in osteosarcoma patients. In osteosarcoma cells, cell proliferation, motility, and invasion were inhibited by ectopic over-expression of miR-145 in vitro. These results indicate miR-145 plays a crucial role in inhibiting invasive and metastatic of osteosarcoma.[22]
Several studies have identified a significant relationship between miR-145 expression levels and the prognosis of various malignant cancers. For example, Zhang et al found that miR-145 expression was significantly associated with lymph node metastasis, metastasis stage, and distant metastasis. Furthermore, patients with low miR-145 expression had poorer overall survival time than those with high miR-145 expression. Moreover, univariate and multivariate Cox analysis showed that miR-145 was an independent prognostic indicator for gastric cancer, indicating that miR-145 can be used as a marker of poor prognosis in gastric cancer patients.[23] Shen et al found that miR-145 was down-regulated in NSCLC tissues, and down-regulation of miR-145 was correlated with late clinical stage and poorly differentiated carcinoma. Furthermore, low expression level of miR-145 could also predict chemotherapy resistance and shorter disease-free survival. These findings indicated that miR-145 expression might be a useful prognostic marker that could be used for predicting poor differentiation, chemo-resistance, and shore disease-free survival.[24] Wang et al found that miR-145 expression was significantly down-regulated in cervical cancer tissues when compared with corresponding adjacent normal tissues. Furthermore, Kaplan–Meier analysis showed that cervical cancer patients with low miR-145 expression had shorter overall survival time than those with high miR-145 expression. When analyzed with a multivariate Cox regression model, miR-145 was identified as an independent prognostic factor for overall survival.[25]
Previously, Ding et al investigated the role of miR-145 in the progression of HCC. Their results showed that miR-145 was frequently down-regulated in HCC tumors and cell lines. Over-expression of miR-145 in HCC cell lines significantly inhibited cell proliferation, migration, and invasion in vitro. ROCK1 was identified as a target of miR-145, and ectopic expression of miR-145 down-regulated ROCK1. Together, these findings indicate that miR-145 acts as a tumor suppressor and its down-regulation in tumor tissues may contribute to the progression and metastasis of HCC through a mechanism involving ROCK1, suggesting miR-145 as a potential new diagnostic and therapeutic target for the treatment of HCC.[15] Wang et al found that miR-145 expression was down-regulated in HCC tissues. Furthermore, miR-145 could inhibit HCC through targeting insulin receptor substrate 1 (IRS1), and its downstream signaling, implicating the loss of miR-145 regulation might be a potential molecular mechanism causing aberrant oncogenic signaling in HCC.[26] However, the clinical significance and prognostic value of miR-145 in HCC have not been investigated. Here we investigate the clinical significance of miR-145 in HCC. In the present study, we collected 139 pairs of human HCC samples and their adjacent non-tumorous liver tissues. We found that miR-145 was significantly down-regulated in HCC tissues compared with the adjacent noncancerous tissues, consisted with the results of previous studies.[15,26,27] The associations of miR-145 expression with various clinicopathological parameters of HCC are analyzed. The expression level of miR-145 was significantly correlated with tumor size, tumor number, lymph node metastasis, TNM stage, and tumor differentiation, suggesting that miR-145 was associated with the progression of HCC. To further investigate the correlation between miR-145 expression level and prognosis of HCC, we followed up the overall survival of the HCC patients. Kaplan–Meier curves with log rank test showed that the overall survival of the patients with low miR-145 expression was significantly shorter in comparison with the high miR-145 expression patients. Furthermore, multivariate analysis using the Cox proportional hazards model for all variables showed that miR-145 expression was an independent prognostic factor for overall survival. Our research has the following limitations: Firstly, the number of enrolled patients was not enough. Secondly, the follow-up time was not long enough. Therefore, more studies with longer follow-up are needed in the future to confirm our conclusions.
5. Conclusions
In conclusion, our findings indicated that miR-145 may be a potential novel prognostic marker for HCC, and further studies are still need to confirm our findings.
Author contributions
Conceptualization: Ping Li.
Data curation: Ping Li.
Formal analysis: Ping Li.
Investigation: Qiang He.
Methodology: Qiang He.
Project administration: Qiang He.
Resources: Qiang He.
Software: Hua Fan.
Supervision: Hua Fan.
Validation: Hua Fan.
Visualization: Hua Fan.
Writing – original draft: Ping Li.
Writing – review and editing: Hua Fan.
Footnotes
Abbreviations: cDNA = complementary DNA, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, IRS1 = insulin receptor substrate 1.
The authors declare that they have no conflicts of interest.
References
- [1].Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1632–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Besheer T, Arafa M, El-Maksoud MA, et al. Diagnosis of cirrhosis in patients with chronic hepatitis C genotype 4: role of ABCB11 genotype polymorphism and plasma bile acid levels. Turk J Gastroenterol 2018;29:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Razek A, Khashaba M, Abdalla A, et al. Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol Med 2014;119:903–9. [DOI] [PubMed] [Google Scholar]
- [5].Razek AA, Abdalla A, Omran E, et al. Diagnosis and quantification of hepatic fibrosis in children with diffusion weighted MR imaging. Eur J Radiol 2011;78:129–34. [DOI] [PubMed] [Google Scholar]
- [6].Besheer T, El-Bendary M, Elalfy H, et al. Prediction of fibrosis progression rate in patients with chronic hepatitis C genotype 4: role of Cirrhosis risk score and host factors. J Interferon Cytokine Res 2017;37:97–102. [DOI] [PubMed] [Google Scholar]
- [7].Pang RW, Joh JW, Johnson PJ, et al. Biology of hepatocellular carcinoma. Ann Surg Oncol 2008;15:962–71. [DOI] [PubMed] [Google Scholar]
- [8].Lages E, Ipas H, Guttin A, et al. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed) 2012;17:2508–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Milazzo M, Fornari F, Gramantieri L. MicroRNA and hepatocellular carcinoma: biology and prognostic significance. Minerva Gastroenterol Dietol 2011;57:257–71. [PubMed] [Google Scholar]
- [10].Dong R, Liu X, Zhang Q, et al. miR-145 inhibits tumor growth and metastasis by targeting metadherin in high-grade serous ovarian carcinoma. Oncotarget 2014;5:10816–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khan S, Ebeling MC, Zaman MS, et al. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget 2014;5:7599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang J, Zhang JY, Chen J, et al. Prognostic role of microRNA-145 in various human malignant neoplasms: a meta-analysis of 18 related studies. World J Surg Oncol 2014;12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Naito Y, Yasuno K, Tagawa H, et al. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol Rep 2014;32:1720–6. [DOI] [PubMed] [Google Scholar]
- [14].Dai SL, Zhou J, Pan C, et al. Prognostic value of microRNA-145 in patients with various cancers: a meta-analysis. Cancer Biomark 2015;15:507–13. [DOI] [PubMed] [Google Scholar]
- [15].Ding W, Tan H, Zhao C, et al. MiR-145 suppresses cell proliferation and motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumour Biol 2016;37:6255–60. [DOI] [PubMed] [Google Scholar]
- [16].Dhir M, Melin AA, Douaiher J, et al. A Review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg 2016;263:1112–25. [DOI] [PubMed] [Google Scholar]
- [17].Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J 2012;18:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roderburg C, Luedde T. Circulating microRNAs as markers of liver inflammation, fibrosis and cancer. J Hepatol 2014;61:1434–7. [DOI] [PubMed] [Google Scholar]
- [20].D’Anzeo M, Faloppi L, Scartozzi M, et al. The role of micro-RNAs in hepatocellular carcinoma: from molecular biology to treatment. Molecules 2014;19:6393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zheng M, Sun X, Li Y, et al. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol 2016;37:8189–96. [DOI] [PubMed] [Google Scholar]
- [22].Li Y, Liu J, Liu ZZ, et al. MicroRNA-145 inhibits tumour growth and metastasis in osteosarcoma by targeting cyclin-dependent kinase, CDK6. Eur Rev Med Pharmacol Sci 2016;20:5117–25. [PubMed] [Google Scholar]
- [23].Zhang Y, Wen X, Hu XL, et al. Downregulation of miR-145-5p correlates with poor prognosis in gastric cancer. Eur Rev Med Pharmacol Sci 2016;20:3026–30. [PubMed] [Google Scholar]
- [24].Shen H, Shen J, Wang L, et al. Low miR-145 expression level is associated with poor pathological differentiation and poor prognosis in non-small cell lung cancer. Biomed Pharmacother 2015;69:301–5. [DOI] [PubMed] [Google Scholar]
- [25].Wang Q, Qin J, Chen A, et al. Downregulation of microRNA-145 is associated with aggressive progression and poor prognosis in human cervical cancer. Tumour Biol 2015;36:3703–8. [DOI] [PubMed] [Google Scholar]
- [26].Wang Y, Hu C, Cheng J, et al. MicroRNA-145 suppresses hepatocellular carcinoma by targeting IRS1 and its downstream Akt signaling. Biochem Biophys Res Commun 2014;446:1255–60. [DOI] [PubMed] [Google Scholar]
- [27].Wang G, Zhu S, Gu Y, et al. MicroRNA-145 and MicroRNA-133a inhibited proliferation, migration, and invasion, while promoted apoptosis in hepatocellular carcinoma cells via targeting FSCN1. Dig Dis Sci 2015;60:3044–52. [DOI] [PubMed] [Google Scholar]


