Abstract
Rationale:
Fecal microbiota transplantation (FMT) has been used in a wide variety of diseases. In this article, we reported a 46-year-old female with diabetic neuropathy (DN) achieved remission by the treatment of FMT.
Patient concerns:
The patient with an 8-year history of diabetes and hypertension was admitted to hospital due to sensitive pain of her right thigh and poor blood glucose control. The traditional hypoglycemic and analgesic treatment were useless to her symptoms.
Diagnosis:
Diabetic-induced neuropathy was considered.
Interventions:
This patient received twice FMTs for 3 months.
Outcomes:
After twice FMTs, the clinical response of patient was pleasant. The glycemic control was improved, with a remarkable relief of the symptoms of painful DN in particular. No obvious adverse effects were observed during the FMTs and follow-up observation-testing.
Lessons:
We proposed that FMT could be a promising treatment in patients with diabetes or diabetes-related complications like DN. FMT also appeared to be definitely safer and more tolerable than the pharmacologic treatment in patients with DN.
Keywords: diabetic neuropathy, fecal microbiota transplantation, gut microbiota, type 2 diabetes mellitus
1. Introduction
Diabetes mellitus (DM) is a chronic, multifactorial disease and poor diabetic control will lead to the development of painful diabetic neuropathy (DN). DN is the most frequent complication of DM and finally contributes to the increase of morbidity and mortality.[1] It includes peripheral neuropathy, single neuropathy, and autonomic neuropathy, which mainly affects the distal end of the lower extremities, often characterized by loss of sensation, pain, numbness, gait disorder even amputation. Despite a high prevalence rate, the pathological mechanism of DN remains unknown.[2,3] Early detection of DN can only be achieved by assessing the nerve fibers, and current treatment of DN is limited to certain non-specific drugs with side effects and possibility of abuse.[1,4] DN has a significant impact on the health-related quality of life.
Fecal microbiota transplantation (FMT), also known as microbiota treatment, is given from the donor's fecal microbiota suspension to the recipient's intestine.[5] The accumulation of data now indicates that the gut microbiota correlates with the central nervous system (CNS) and participates in the regulation of cognition and pain.[6] However, only few studies have reported the clinical application of microbes in neurogenic disorders. FMT can reconstruct the composition of gut microbiota and may be a useful tool in the treatment of various diseases,[7] including inflammatory bowel disease,[8] metabolic syndrome,[9] certain neuropsychiatric symptoms,[10] etc. To our knowledge, we reported the first case of FMT to reach palliation of diabetic neuropathic pain and glycemic homeostasis.
2. Case report
A 46-year-old woman, with a history of 8-year DM and arterial hypertension, was admitted to the Second Affiliated Hospital of Nanjing Medical University in April 2017 due to poor blood glucose control and limb numbness. The onset of initial symptoms was at the age of 39, with no obvious symptom of thirsty, polydipsia (drink 4–5L/day), polyuria, and unexplained weight loss. After oral glucose tolerance test (OGTT) and determination of blood pressure, she was diagnosed with type two diabetes mellitus (T2DM) and hypertension. Then the patient started to be treated with insulin glargine injection (36 units per night), Mitiglinide Calcium Hydrate tablets (1.5 g/day) and Metformin Hydrochloride Tablets (30 mg/day) to maintain glucose homeostasis, as well as amlodipine besylate left circumflex (2.5 mg/day) to decrease the blood pressure. However, the blood glucose was still not effectively controlled, with a fasting plasma glucose of 9.0 to 13 mmol/L, a 2-h glucose of 14 to 18 mol/L. With the development of the T2DM, the complications gradually appeared, including diabetic retinopathy, diabetic peripheral neuropathy, especially the unusual sensitive pain and perception abnormality of the right thigh, which responded poorly after the treatment of lipoic acid and common analgesics. Painful diabetic peripheral neuropathy also caused sleep interference and anxiety, which greatly influenced the quality of the patient's life.
In April 2017, the patient underwent the first FMT. The fresh fecal microbial suspension was prepared under an automatic purification system (GenFMTer; FMT Medical, Nanjing, China) in our fecal microbiota bank system. Then it was delivered to her terminal ileum through endoscopy (Trial: ChiCTR-ONC-17011792) under anesthesia. The laboratory protocol and clinical work flow were noted in a recent report.[11] The complete study protocol was approved by institutional ethics committees of The Second Affiliated Hospital of Nanjing Medical University ([2015]ky044) and written informed consent was obtained from the participant. A week after the first FMT, the symptom of hyperalgesia was markedly reduced within the right thigh of the patient. Additionally, according to the effective control of blood glucose, her daily insulin injection dosage was adjusted to 24 units per night during the follow-up period. However, the patient also experienced some mild side effects, such as nausea, vomiting and diarrhea. Fortunately, a few days later, the side effects were slowly disappearing. Three months later, the patient received a second FMT. In view of the fact the stable glucose allowed us to make the participant stop taking Mitiglinide Calcium Hydrate Tablets. Since the first FMT, limb pain and paresthesia were significantly reduced during the treatment and follow-up period, until the date of this submission, the patient did not use any painkillers or drugs for alleviating the pain of DN.
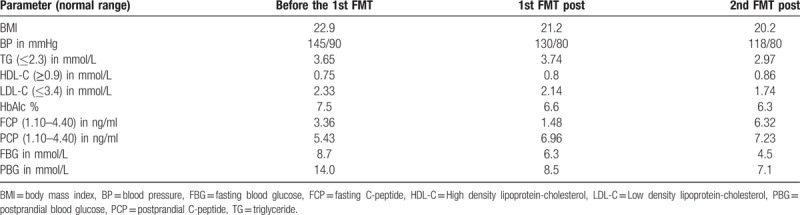
On the second day after admission, magnetic resonance imaging (MRI) of the lumbar spine showed lumbar disc degeneration. Before and after the treatment, the electromyogram (EMG) manifested improvement of motor conduction velocity in tibial nerve (Fig. 1), while the sensory dysfunction of the nerve was irreversible. Visual analogue scale (VAS) pain score was significantly decreased. The pain level dropped from 7.2 (Severe pain) to 2.5 (Mild pain). We found that her blood glucose gradually became stable under the current dose of drugs and the declining trend of blood glucose was obvious (Fig. 2). Before and after treatment, the blood pressure and blood lipid of this patient were significantly declined, and the weight was also reduced. More clinical parameters are shown in Table 1.
Figure 1.
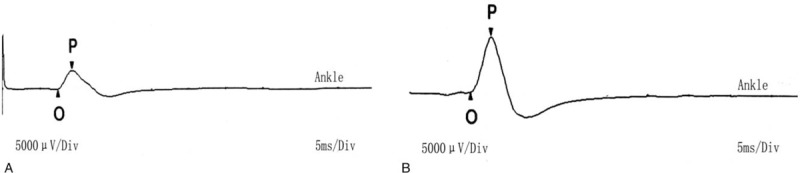
Nerve conduction velocity [Right tibial nerve sport].
Figure 2.
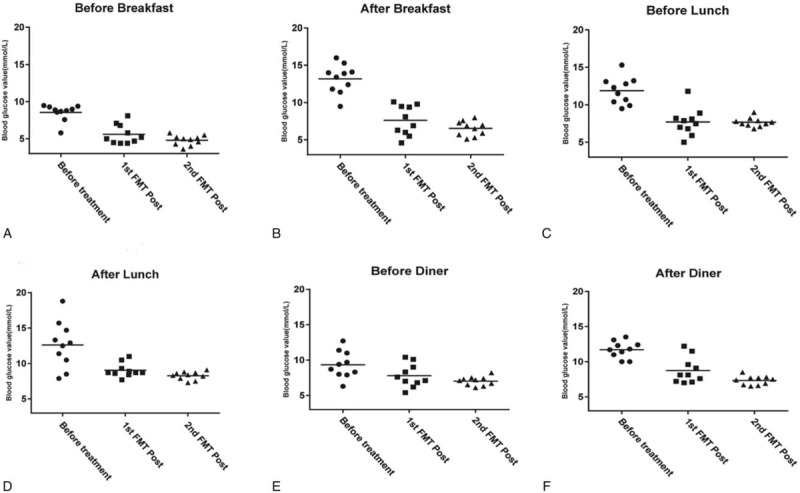
The changes of blood glucose during ten days after 2 fecal microbiota transplantations (FMT). (Before breakfast: 6 am; After breakfast: 9 am; Before lunch: 11 am; After lunch: 14 pm; Before diner 17 pm; After diner: 20 pm).
Table 1.
Clinical parameter changes of the patient during follow-up.
3. Discussion
Currently, the pain relief drugs are the main choice for treatment with DN.[12] The available treatments remained unsatisfying. To the best of our knowledge, this is the first case reported in an adult female on the therapy of FMT for painful diabetic neuropathy. The amelioration of patient's symptoms is encouraging. After the treatment, the participant has never taken any analgesic to mitigate the pain of DN. Since the first FMT, the symptoms have never appeared till now, our follow-up work plans to last for 2 years.
In this case, unfortunately, we did not carry out a thorough study of the mechanism and also did not conduct a specific analysis of gut microbes, but further follow-up study would be conducted in the near future. Although it has been mentioned that FMT may contribute to some neurological disorders,[10] there is no published literature on the use of FMT in diabetic neuropathic pain. It is noteworthy that the blood glucose of this patient fluctuated significantly after FMT, and the dosage of hypoglycemic drugs also have been reduced obviously. So far, the participant still has a good glycemic control. Numerous reports have demonstrated that gut microbiota had an important impact on insulin sensitivity and metabolic diseases[13,14] through several pathways, including endotoxin, inflammation, gut-brain axis. Gut-brain axis is a biaxial signaling axis between gastrointestinal tract and nervous system. Gut-brain axis can regulate pain, behavior and energy metabolism. Intestinal microbiota may affect the nervous system dysfunction through several mechanisms:
-
(1)
stimulation of host immune responses with cytokines as signaling mediators;
-
(2)
synthesis of neuroactive metabolites;
-
(3)
alteration of neuronal circuits by bacterial metabolites.[15,16]
We speculated that the role of FMT in improvement of diabetic neuralgia was associated with the regulation of gut-brain axis, while no article has described its specific mechanism. These evidences suggest that FMT treatment might provide a possibility to explore potential therapeutic approaches in diabetes and its complications. However, the optimal glycemic control is essential to avoid the chronic complication and the follow-up is very important after the treatment of the second FMT. FMT procedure also needs to be standardized about donor selection, stool preparation, delivery route, and dosing. Further studies to assess long-term outcomes are needed to provide the better guidance on clinical decision-making, patient selection, and clinical practice.
Author contributions
Conceptualization: Faming Zhang.
Data curation: Xiaolong Ye, Dafa Ding.
Funding acquisition: Dafa Ding.
Methodology: Huijuan Yong.
Resources: Bin Song, Xiaoling Zheng, Bota Cui, Yibing Lu, Heng Miao, and Dafa Ding.
Supervision: Dafa Ding.
Writing – original draft: Tingting Cai.
Footnotes
Abbreviations: CNS = central nervous system, DM = diabetes mellitus, DN = diabetic neuropathy, EMG = electromyogram, FMT = fecal Microbiota Transplantation, MRI = magnetic resonance imaging, OGTT = oral glucose tolerance test, T2DM = type two diabetes mellitus.
Written Informed consent was obtained from the individual participant included in the study.
This study was reviewed and approved by the Second Affiliated Hospital of Nanjing Medical University, Nanjing Medical University Institutional Review Board.
None of the authors have any potential conflicts of interest associated with this research.
This work was supported by the grants from Major Research and Development Project of Jiangsu (BE2016800) and Nanjing Science and Technology Plan Project (BE201715015).
The authors have no conflicts of interest to disclose.
References
- [1].Todorovic SM. Painful diabetic neuropathy: prevention or suppression. Int Rev Neurobiol 2016;127:211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig 2017;8:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dosenovic S, Jelicic KA, Miljanovic M, et al. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg 2017;125:643–52. [DOI] [PubMed] [Google Scholar]
- [4].Papanas N, Ziegler D. Emerging drugs for diabetic peripheral neuropathy and neuropathic pain. Expert Opin Emerg Drugs 2016;21:393–407. [DOI] [PubMed] [Google Scholar]
- [5].Zhang F, Cui B, He X, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell 2018;9:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- [7].Millan B, Laffin M, Madsen K, et al. Fecal microbiota transplantation: beyond clostridium difficile. Curr Infect Dis Rep 2017;19:31doi:10.1007/s11908-017-0586-5. [DOI] [PubMed] [Google Scholar]
- [8].Laffin M, Madsen KL. Fecal microbial transplantation in inflammatory bowel disease: a movement too big to be ignored. Clin Pharmacol Ther 2017;102:588–90. [DOI] [PubMed] [Google Scholar]
- [9].Zhou D, Pan Q, Shen F, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 2017;7:1529doi:10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013;155:1451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015;13:298doi:10.1186/s12967-015-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deli G, Bosnyak E, Pusch G, et al. Diabetic neuropathies: diagnosis and management. Neuroendocrinology 2013;98:267–80. [DOI] [PubMed] [Google Scholar]
- [13].Carvalho BM, Saad MJ. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm 2013;2013:986734doi:10.1155/2013/986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hwang I, Park YJ, Kim YR, et al. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J 2015;29:2397–411. [DOI] [PubMed] [Google Scholar]
- [15].Sherwin E, Sandhu KV, Dinan TG, et al. May the force be with you: the light and dark sides of the microbiota-gut-brain axis in neuropsychiatry. CNS Drugs 2016;30:1019–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Russo R, Cristiano C, Avagliano C, et al. Gut-brain axis: Role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 2018;25:3930–52. [DOI] [PubMed] [Google Scholar]


