Abstract
Background:
A lumbar herniated intervertebral disc (LHIVD) is a common problem that usually causes low back pain and radiating pain. The effectiveness of Bosinji, one of the herbal medicines used for low back pain and radiating pain in patient with LHIVD, has been reported in several studies; however, little clinical evidence is available owing to the methodological limitations in previous studies. Hence, the present study aims to establish the clinical evidence regarding the efficacy and safety of Bosinji in improving pain, function, and quality of life in LHIVD patients.
Method/design:
This is a multicenter, open-label, randomized, controlled, and equivalence trial with 2 parallel arms. A total of 74 patients who have low back pain and radiating pain due to LHIVD will be recruited and randomly allocated to the experimental group and control group. The patients in the experimental group and control group will take 2.5 g of Bosinji granule (1.523 g of Bosinji extract) or Loxonin tablet (60 mg of loxoprofen) 3 times a day for 6 weeks. Additionally, both groups will receive the same acupuncture treatment once a week for 6 weeks as a concurrent treatment. Changes in the 100-mm visual analogue scale (VAS) for low back pain after 6 weeks from baseline will be assessed as the primary outcome. Furthermore, the 100-mm VAS for radiating pain, Oswestry disability index (ODI), Roland–Morris disability questionnaire (RMDQ), EuroQol 5 Dimensions 5 Levels (EQ-5D-5L), global perceived effect (GPE), and deficiency syndrome of kidney index (DSKI) will be used to evaluate secondary outcomes. Outcomes will be assessed at baseline and at 3, 6, and 10 weeks after screening. For the safety evaluation, laboratory examinations including complete blood count, liver function test, renal function test, blood coagulation test, inflammation test, and urine analysis will be conducted before and after taking the medications.
Discussion:
The results of this trial will be used to establish clinical evidence regarding the use of Bosinji with acupuncture treatment in the treatment of patients with LHIVD.
Trial registration number:
NCT03386149 (clinicaltrials.gov) and KCT0002848 (Clinical Research Information Service of the Republic of Korea).
Keywords: bosinji, herbal medicine, low back pain, lumbar disc herniation, lumbar herniated intervertebral disc, radiculopathy
1. Introduction
A lumbar herniated intervertebral disc (LHIVD) is defined as a medical condition that causes compression and irritation of the dural sac and nerve roots due to displacement of the intervertebral disc in the lumbar region.[1] As low back pain and radiating pain, which are the most common symptoms of LHIVD, greatly affect the daily life and working status of patients, pain relief is a major concern in the conservative treatment of patients with LHIVD.[2,3]
Herbal medicines have been used clinically for the treatment of LHIVD mainly in Asian countries, and there have been a number of reports of evidence of their effectiveness.[4,5] Bosinji, the commercial name of Ucha-Shinki-hwan (Goshajinkigan in Japanese), which consists of 10 crude drugs, is one of the herbal formulations traditionally used for low back pain with radiating pain.[6]
Each component of Bosinji has shown several pharmacological effects in experimental studies; these effects may explain the mechanism of its effect on LHIVD. Aconitum carmichaelii exerts an anti-nociceptive effect by stimulating spinal kappa-opioid receptors via release of dynorphin,[7] as well as a blood flow-increasing effect by promoting the production of nitric oxide.[8] Catalpol, an iridoid glucoside found in Rehmannia glutinosa, also has an anti-nociceptive effect associated with modulation of neuroinflammation in the spinal cord in rats.[9] Other components of Bosinji such as Corni officinalis,[10]Alisma orientale,[11]Poria cocos,[12]Paeonia suffruticosa,[13] and Cinnamomum cassia,[14] show anti-inflammatory effects.
Experimental research has shown Bosinji to be possessing properties similar to those of its individual components. Similar to Aconitum carmichaelii, Bosinji has an anti-nociceptive effect that involves the activation of kappa-opioid receptors, thereby reducing paresthesia,[15] as well as vasodilating effect via increase in nitric oxide production.[16] Nakanishi et al have reported that Bosinji suppresses the expression of tumor necrosis factor-α,[17] which is a critical molecular mediator in the pathogenesis of radiating pain.[1]
Based on the mechanisms revealed in experimental studies, Bosinji have also been shown to be clinically effective in the treatment of LHIVD in observational studies.[18,19] One randomized controlled trial (RCT) reported that the effectiveness of Bosinji was similar to that of nonsteroidal anti-inflammatory drugs (NSAIDs) for low back pain and paresthesia in the lower extremities; however, this trial carried many unclear risks of bias and methodological limitations, and hence, a definite conclusion could not be arrived at.[20]
For the clinical application of Bosinji in treating low back pain with radiating pain caused by LHIVD, reliable evidence from pragmatic RCT reflecting the actual clinical situation in Korean medical clinics where herbal medicine treatment is conducted with acupuncture treatment concurrently[21] is required. Therefore, we will evaluate the equivalence of Bosinji to NSAIDs in concurrent with acupuncture using a rigorously designed, full-scale RCT protocol that includes assessments on pain, function, quality of life (QoL), and safety.
2. Methods
2.1. Trial design
This study is a multicenter, open-label, randomized, controlled, and equivalence trial with 2 parallel arms (1:1 ratio). The trial will be conducted at the Kyung Hee University Hospital at Gangdong (KHUHGD), Kyung Hee University Medical Center (KHUMC), Dongguk University Bundang Oriental Hospital (DUBOH), and Daegu Korean Medicine Hospital of Daegu Haany University (DKMHDHU). The efficacy and safety of Bosinji in patients with low back pain with radiculopathy due to LHIVD will be evaluated by comparison with loxoprofen, one of the NSAIDs.
This protocol has been approved by the Institutional Review Boards (IRBs) of the institutions (KHUHGD: KHNMCOH 2017-08-002, KHUMC: 170918-HR-039, DUBOH: 2017-0007, and DKMHDHU: DHUMC-D-17019). The trial has also been registered at clinicaltrials.gov, which is a website of the United States National Institutes of Health (registration number: NCT03386149), as well as in the Clinical Research Information Service of the Republic of Korea (registration number: KCT0002848). All research procedures comply with Korean Good Clinical Practice (KGCP) and the Declaration of Helsinki. The methodology was established in accordance with the Standard Protocol Items: Recommendation for Interventional Trials (SPIRIT)[22] and with the revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA).[23]
2.2. Participants
The inclusion criteria for this study are as follows: age over 19 years; radiating pain consistent with abnormalities in the lumbar spine that are more severe than bulging, as shown on computerized tomography (CT) or magnetic resonance imaging (MRI)[24,25]; low back pain score between 40 and 80 points on the 100-mm pain visual analogue scale (VAS); and voluntary participation and provision of a signed informed consent form after a detailed explanation of the clinical trial has been provided.
Participants who have the following characteristics will be excluded:
-
1.
congenital abnormalities or surgical history in the lumbar region;
-
2.
red flag signs that may indicate cauda equina syndrome, such as bladder and bowel dysfunction or saddle anesthesia;
-
3.
tumor, fracture, or infection in the lumbar region;
-
4.
injection in the lumbar region within 1 week of the screening;
-
5.
psychiatric disorder currently being treated, such as depression or schizophrenia;
-
6.
liver function abnormality (aspartate transaminase [AST] or alanine transaminase [ALT] >100 U/L for men/70 U/L for women);
-
7.
renal function abnormality (serum creatinine >2.0 mg/dL);
-
8.
other diseases that could affect or interfere with therapeutic outcomes, including severe gastrointestinal disease, cardiovascular disease, hypertension, diabetes, renal disease, liver disease, or thyroid disorder;
-
9.
contraindications for NSAIDs, including concurrent disease, hypersensitivity reaction, or other medication;
-
10.
conditions for which acupuncture is contraindicated, for example, skin disease or hemostatic disorder (international normalized ratio >2.0 or taking anticoagulant);
-
11.
pregnancy, breastfeeding or having pregnancy plans;
-
12.
other condition for which herbal medicine treatment is contraindicated; and
-
13.
participation in other clinical trials within 1 month of the screening.
2.3. Procedure
A total of 74 participants with LHIVD will be recruited at four institutions, each of which will recruit an appointed number of patients (KHUHGD: 20, KHUMC: 18, DUBOH: 18, and DKMHDHU: 18). All participants who can read and write in Korean will be informed that they may voluntarily participate and that they can withdraw their consent at any stage. They will also be given essential information regarding the study protocol, including purpose, selection of participants, interventions by random allocation, schedule, expected benefits and risks, alternative treatment options, and confidentiality. Those who agree and sign the informed consent form will be screened through the assessment of demographics, medical history, present illness, vital sign, pregnancy test, and laboratory test. If they meet the eligibility criteria, the participants will be randomly allocated to the experimental group or control group. After random allocation, the assigned group will undergo a 6-week treatment, and assessment of outcome measure will be performed at 3, 6, and 10 weeks later according to schedule (Fig. 1).
Figure 1.
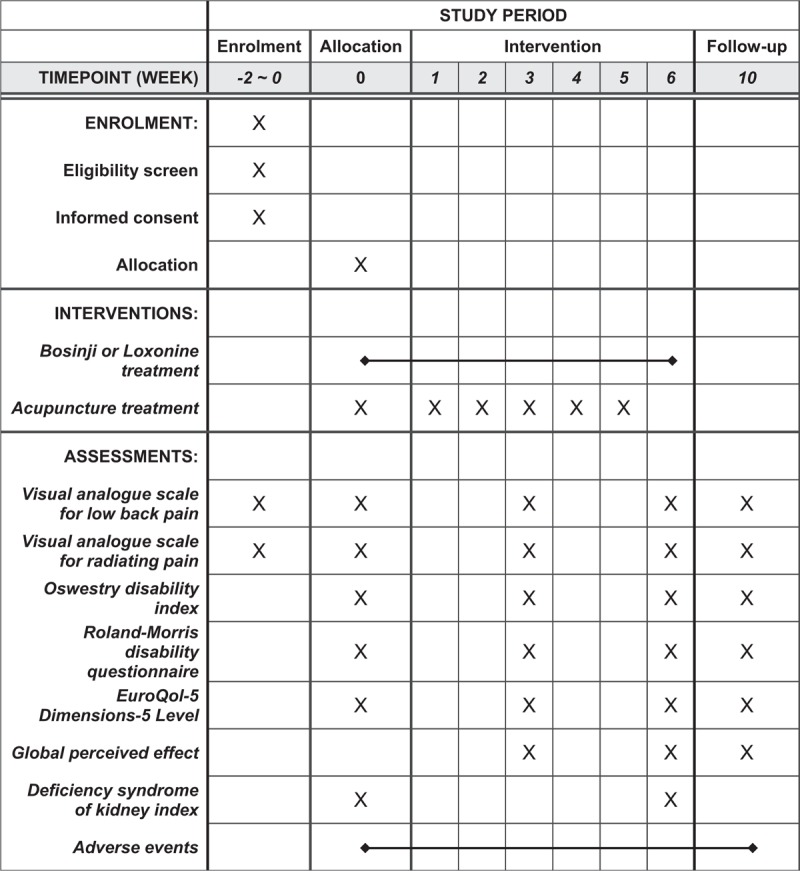
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) figure.
2.4. Interventions
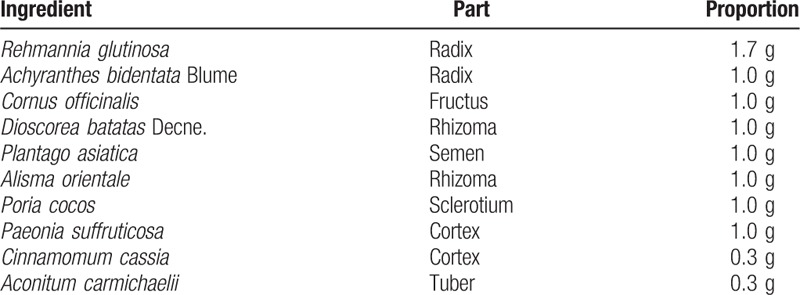
In the experimental group, 2.5 g of Bosinji granule (1.523 g of Bonsinji extract, Tsumura Co., Tokyo, Japan) will be orally administered 3 times a day at 30 minutes after every meal for 6 weeks. Bonsinji extract comprises a mixture of extract from the following 10 crude drugs in fixed proportions (Table 1). Bosinji is manufactured by Tsumura Co. in compliance with Good Manufacturing Practice standards. In the preparation phase, each crude drug undergoes macroscopic and microscopic examination. The extract and its components are also subjected to physicochemical tests. Bosinji granule is produced by a process involving decoction, separation, concentration, drying, and granulation. To ensure consistency and safety, multiple quality tests, including quantitative analysis of the components, microbial limits test, and residual pesticides test, are performed.
Table 1.
Composition of Bosinji extract.
In the control group, Loxonin tablet (60 mg of loxoprofen, Dong Wha Pharm Co., Ltd, Seoul, Korea) will be orally administered 3 times a day at 30 minutes after each meal for 6 weeks. Three weeks of medication will be provided at week 0 and week 3, and the remaining medication will be withdrawn to confirm the medication compliance.
2.5. Concurrent treatment
As a concurrent treatment, an identical acupuncture treatment will be conducted once a week for all participants regardless of group during the medication period. Acupuncture treatment will be performed on 20 predefined acupoints using 40 mm x 0.25 mm acupuncture needles according to STRICTA (Table 2). The selection of acupoints and details of the procedure have been modified from similar studies[26,27] by a committee of Korean medical doctors (KMDs) experienced in LHIVD and acupuncture. Acupuncture treatment will be performed by KMDs who have completed or have been taking a specialist acupuncture and moxibustion course for at least 3 years. To maintain homogeneity of treatment among the participating centers, all practitioners will undertake a training course together in advance.
Table 2.
Details of acupuncture treatment using the STRICTA 2010 checklist.

2.6. Outcomes
All assessments will be performed according to schedule by blinded independent researchers who are not involved in the intervention. The primary outcome will be a change in VAS for low back pain. The secondary outcomes will be evaluated using the VAS for radiating pain, Oswestry disability index (ODI), Roland-Morris disability questionnaire (RMDQ), EuroQol 5 Dimensions 5 Levels (EQ-5D-5L), global perceived effect (GPE), and deficiency syndrome of kidney index (DSKI). Additionally, all adverse events will be recorded at each visit.
2.6.1. Primary outcome measure
2.6.1.1. 100-mm VAS for low back pain
The intensity of low back pain will be evaluated using the 100-mm VAS at pre-trial screening and at weeks 0 (baseline), 3, 6 (primary endpoint), and 10 (follow-up sessions).[28] Participants will be asked to record their pain intensity within the past week on a 100-mm linear scale (0, absence of pain; 100, worst pain imaginable). Changes from baseline after 6 weeks of treatment will be compared between the groups as the primary outcome.
2.6.2. Secondary outcome measures
2.6.2.1. 100-mm VAS for radiating pain
The intensity of radiating pain in the lower extremities will also be assessed using a 100-mm VAS at pre-trial screening and at weeks 0, 3, 6, and 10. Participants will be asked to record their pain intensity within the past week on a 100-mm linear scale (0, absence of pain; 100, worst pain imaginable).
2.6.2.2. Oswestry disability index (ODI)
Inability to function in daily life due to low back pain will be assessed using the ODI at weeks 0, 3, 6, and 10. The ODI questionnaire consists of 10 sections: pain, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. Each question is scored from 0 to 5, and the total score is calculated as a percentage disability.[29]
2.6.2.3. Roland-Morris disability questionnaire (RMDQ)
Physical disability due to low back pain will be assessed using the RMDQ at weeks 0, 3, 6, and 10. The RMDQ contains 24 sentences describing the discomfort that may occur with low back pain. Participants will need to check the sentences that would best describe their life on that day, and the score is based on the total number of sentences checked (from 0 to 24).[30]
2.6.2.4. EuroQol 5 Dimensions 5 Levels (EQ-5D-5L)
The QoL and general health status will be assessed using the EQ-5D-5L at weeks 0, 3, 6, and 10. The EQ-5D-5L consists of the EQ-5D descriptive system and EQ-VAS. The EQ-5D descriptive system assesses the following five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension is rated from 1 to 5 (1, no problem; 2, slight problems; 3, moderate problems; 4, severe problems; and 5, extreme problems). The EQ-VAS scale can be used to assess a patient's current health status. It is a 20-cm scale numbered from 0 to 100 (0, the worst health they can imagine; 100, the best health they can imagine).[31]
2.6.2.5. Global perceived effect (GPE)
The subjective change of symptom will be assessed using the GPE at weeks 3, 6, and 10. The participants will score their perceived change after treatment using a 7-point scale (1, worst ever; 2, much worse; 3, worse; 4, not improved and not worse; 5, slightly improved; 6, much improved; and 7, best ever).[32]
2.6.2.6. Deficiency syndrome of kidney index (DSKI)
The deficiency syndrome of kidney (DSK), a concept of the Korean medical diagnosis system, will be assessed using DSKI at weeks 0 and 6. The DSKI shows the severity of the DSK by scoring the 12 DSK-related symptoms from 0 to 2 points (1, no symptom; 2, moderate; and 3, severe).[33]
2.7. Safety
To confirm the safety of the medications, the following laboratory tests will be carried out at the pre-screening point and at week 6 when the medication treatment is completed; complete blood count (white blood cells, red blood cells, hemoglobin, hematocrit, and platelet), liver function test (total protein, albumin, AST, ALT, alkaline phosphatase, total bilirubin, and γ-glutamyl transferase), renal function test (uric acid, blood urea nitrogen [BUN], and creatinine), fasting plasma glucose, prothrombin time, activated partial thromboplastin time, erythrocyte sedimentation rate, C-reactive protein, and urine analysis (color, specific gravity, pH, protein, glucose, ketone, urobilinogen, bilirubin, nitrite, blood, and white blood cell). Additionally, AST, ALT, BUN, and creatinine will be examined at week 3. For women of childbearing potential, a urine human chorionic gonadotropin test will be performed using a stick-type pregnancy tester, and they will be educated about the need for medically acceptable contraception during the study period.
At each visit, the researchers will measure the vital signs, such as blood pressure, pulse rate, and body temperature and collect information about the occurrence of adverse event (AE) and changes in the drug being taken. Information about expected AEs, as well as a contact number, will be given to the participants along with the informed consent form before the pre-trial screening. If AEs do occur, the principal investigator will evaluate the severity of the incident, as well as its relation to the interventions, and provide proper examination and treatment in accordance with the compensation rules. The progress of all AEs will be recorded in the case report form (CRF) and handled in accordance with the standard operation protocol (SOP) of the IRB and the regulation of KGCP.
2.8. Sample size
The sample size was calculated based on a previous similar study (assuming σ = 9.18,  = 10),[20] and the equivalence margin for the primary outcome was set at 18.[34] With a 0.05 significance level, 80% power, and 1:1 ratio, we calculated the adequate sample size using the following formula:
= 10),[20] and the equivalence margin for the primary outcome was set at 18.[34] With a 0.05 significance level, 80% power, and 1:1 ratio, we calculated the adequate sample size using the following formula:
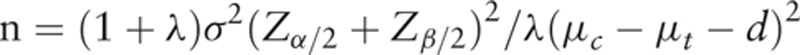 |
Finally, we determined that the sample size required in each group should be 37 participants by considering the dropout rate (20%) and medication compliance rate (95%) on the calculated value. The 74 participants will be recruited separately at the four research sites; each site will recruit the following numbers of participants: KHUHGD: 20, KHUMC: 18, DUBOH: 18, and DKMHDHU: 18.
2.9. Randomization and allocation concealment
A total of 74 participants will be randomly allocated to the experimental group or control group according to a block randomization procedure with a 1:1 ratio after stratifying them by institutions. The randomization sequence will be generated by an independent statistician using the package “blockrand” 1.3 of R (The R Foundation for Statistical Computing, Vienna, Austria). For allocation concealment, sealed, opaque envelopes containing random code will be sent to each institution. The clinical research coordinator will open the envelope and allocate participants to their groups after screening.
2.10. Blinding
The medications used in this study are in the form of tablets and granules and can easily be distinguished visually. Therefore, because it is impossible to achieve patient blinding, this research was designed as an open-label study. However, for objective analysis, evaluators and statisticians do not provide information on group assignments.
2.11. Statistical methods
The data will be corrected using the “last observation carried forward” method and then analyzed using the “intention-to-treat” principle. The independent t test and Chi-square test will be used to compare the differences in general characteristics between groups. As a primary outcome, changes in the 100-mm VAS for low back pain from baseline to the end of the end of the medication treatment will be compared between groups using an independent t test. Equivalence will be evaluated based on the equivalence margin of 18 mm and 95% confidence interval (CI). To compare the outcomes of each session with the baseline values, an analysis of covariance will be used. Trends over time and time-by-treatment interactions will be analyzed using a repeated measures analysis of variance. All statistical analyses will be performed using PASW statistics 18 for Windows, and the statistical significance level will be set at .05.
2.12. Data collection and management
All research data will be collected and double-checked by two independent researchers based on source documents including the CRF. All sensitive information obtained from the trial will be confidentially preserved, and personally identifiable information will be discarded after a certain period, in accordance with the SOP. All researchers will be given training in protecting the privacy of participants.
2.13. Quality control
To maintain the quality of the trial, the study procedure and documents will be periodically monitored by the Korean Medicine Clinical Trial Center.
3. Discussion
Bosinji is a granule-shaped herbal medicine derived from Ucha-Shinki-hwan, which is described in a medical book called “Je-Saeng-Bang” written in the 13th century.[6] Due to the characteristic of this herbal formulation composed of 10 kinds of herbs, Ucha-Shinki-hwan has been reported to have beneficial effects on various diseases including chemotherapy-induced neuropathy,[35–37] diabetic neuropathy,[38] and overactive bladder.[39,40] In addition, its effect on low back pain with radiating pain was reported in an RCT; however, there were many unclear risks of bias in random sequence generation, allocation concealment, outcome assessment blinding, and incomplete outcome data. In addition, since the outcome measurement was only limited to pain, it is not enough to confirm the definite evidence due to the low quality of the report.[20]
To confirm the efficacy of Bosinji compared with NSAIDs for low back pain and radiculopathy caused by LHIVD, this RCT was designed in a methodologically rigorous, full-scale setting. The indicators for pain, function, QoL, and safety will be comprehensively evaluated, and DSKI will be assessed to reflect the diagnostic conception of traditional Korean medicine. Because Ucha-Shinki-hwan was originally designed to treat a group of complex symptoms called DSK,[6] not a single specific symptom, it is necessary to verify the relation between the therapeutic effect and traditional diagnostic system.
Loxoprofen, used as an active control intervention in this study, is one of the NSAIDs in the propionic acid group.[41] The clinical benefit of NSAIDs for LHIVD remains controversial; however, they are most commonly used for conservative treatment of LHIVD because inflammatory signaling plays an important role in the production of nerve pain.[1,2] Therefore, NSAIDs have been used as a control intervention in studies attempting to identify a new therapeutic agent for LHIVD.[42] Although patient blinding is not possible due to the differences in the shape of medications, bias can be minimized through blinding of assessors and statisticians.
As a concurrent treatment, acupuncture will be practiced to reflect the actual clinical situation in Korea. While about 90% of patients who visit Korean medical institutions due to low back pain choose acupuncture as a primary treatment, about 30% of patients opt for herbal medicine treatment.[21] Therefore, evaluating the effects of herbal medicine along with acupuncture may help determine the practical usefulness.
The results of this study will suggest clinical evidence comparing Bosinji with NSAIDs by providing data about the changes in various measurements from the rigorously conducted trial. These findings will help clinicians to utilize Bosinji with acupuncture as a therapeutic option for patients with LHIVD.
Author contributions
Conceptualization: Byung-Kwan Seo.
Funding acquisition: Byung-Kwan Seo.
Methodology: Eun-Jung Kim, Dongwoo Nam, Hyun-Jong Lee, Jae-Soo Kim, Yeon-Cheol Park, Yong-Hyeon Baek, Sang-Soo Nam, Byung-Kwan Seo.
Project administration: Bonhyuk Goo, Sung-Jin Kim, Eun-Jung Kim, Dongwoo Nam, Hyun-Jong Lee, Jae-Soo Kim, Byung-Kwan Seo.
Supervision: Byung-Kwan Seo.
Writing – original draft: Bonhyuk Goo, Sung-Jin Kim.
Writing – review & editing: Eun-Jung Kim, Dongwoo Nam, Hyun-Jong Lee, Jae-Soo Kim, Yeon-Cheol Park, Yong-Hyeon Baek, Sang-Soo Nam, Byung-Kwan Seo.
Footnotes
Abbreviations: AE = adverse event, ALT = alanine transaminase, AST = aspartate transaminase, BUN = blood urea nitrogen, CRF = case report form, DKMHDHU = Daegu Korean Medicine Hospital of Daegu Haany University, DSK = deficiency syndrome of kidney, DSKI = deficiency syndrome of kidney index, DUBOH = Dongguk University Bundang Oriental Hospital, EQ-5D-5L = EuroQol 5 Dimensions 5 Levels, GPE = global perceived effect, IRB = Institutional Review Board, KGCP = Korean Good Clinical Practice, KHUHGD = Kyung Hee University Hospital at Gangdong, KHUMC = Kyung Hee University Medical Center, KMD = Korean medical doctor, LHIVD = lumbar herniated intervertebral disc, NSAIDs = nonsteroidal anti-inflammatory drugs, ODI = Oswestry disability index, QoL = quality of life, RCT = randomized clinical trial, RMDQ = Roland–Morris disability questionnaire, SOP = Standard Operation Protocol, SPIRIT = Standard Protocol Items: Recommendation for Interventional Trials, STRICTA = Standards for Reporting Interventions in Clinical Trials of Acupuncture, VAS = visual analogue scale.
Ethics approval and consent to participate: The protocol of this study has been approved by the IRB of 4 respective institutions (reference number: KHUHGD, KHNMCOH 2017-08-002; KHUMC, 170918-HR-039; DUBOH, 2017-0007; and DKMHDHU, DHUMC-D-17019), and the informed consent form is to be completed voluntarily before screening.
This study was supported by the Traditional Korean Medicine R&D program, which is funded by the Ministry of Health and Welfare through the Korea Health Industry Development Institute (KHIDI) (HB16C0061), and by a grant from Kyung Hee University in 2014 (KHU-20140689). As this study was an investigator-initiated trial, the funding bodies did not play any role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Amin RM, Andrade NS, Neuman BJ. Lumbar Disc Herniation. Curr Rev Musculoskelet Med 2017;10:507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vialle LR, Vialle EN, Suarez Henao JE, et al. Lumber Disc Herniation. Rev Bras Ortop 2010;45:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jordan J, Konstantinou K, O’Dowd J. Herniated lumbar disc. BMJ Clin Evid 2011;2011. [PMC free article] [PubMed] [Google Scholar]
- [4].Luo Y, Huang J, Xu L, et al. Efficacy of Chinese herbal medicine for lumbar disc herniation: a systematic review of randomized controlled trials. J Tradit Chin Med = Chung i tsa chih ying wen pan 2013;33:721–6. [DOI] [PubMed] [Google Scholar]
- [5].Zhang B, Xu H, Wang J, et al. A narrative review of non-operative treatment, especially traditional Chinese medicine therapy, for lumbar intervertebral disc herniation. Biosci Trends 2017;11:406–17. [DOI] [PubMed] [Google Scholar]
- [6].Kim MK, Kim BR, Kim YH, et al. A case study of a patient diagnosed as diabetic cystopathy with dysuria. J Inter Korean Med 2008;29:1123–9. [Google Scholar]
- [7].Suzuki Y, Goto K, Ishige A, et al. Antinociceptive effect of Gosha-jinki-gan, a Kampo medicine, in streptozotocin-induced diabetic mice. Jpn J Pharmacol 1999;79:169–75. [DOI] [PubMed] [Google Scholar]
- [8].Yamada K, Suzuki E, Nakaki T, et al. Aconiti tuber increases plasma nitrite and nitrate levels in humans. J Ethnopharmacol 2005;96:165–9. [DOI] [PubMed] [Google Scholar]
- [9].Wang Y, Zhang R, Xie J, et al. Analgesic activity of catalpol in rodent models of neuropathic pain, and its spinal mechanism. Cell Biochem Biophys 2014;70:1565–71. [DOI] [PubMed] [Google Scholar]
- [10].Park CH, Tanaka T, Yokozawa T. Anti-diabetic action of 7-O-galloyl-D-sedoheptulose, a polyphenol from Corni Fructus, through ameliorating inflammation and inflammation-related oxidative stress in the pancreas of type 2 diabetics. Biol Pharm Bull 2013;36:723–32. [DOI] [PubMed] [Google Scholar]
- [11].Tian T, Chen H, Zhao YY. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: a review. J Ethnopharmacol 2014;158(Pt A):373–87. [DOI] [PubMed] [Google Scholar]
- [12].Yasukawa K, Kaminaga T, Kitanaka S, et al. 3 beta-p-hydroxybenzoyldehydrotumulosic acid from Poria cocos, and its anti-inflammatory effect. Phytochemistry 1998;48:1357–60. [DOI] [PubMed] [Google Scholar]
- [13].Fu PK, Yang CY, Tsai TH, et al. Moutan cortex radicis improves lipopolysaccharide-induced acute lung injury in rats through anti-inflammation. Phytomedicine 2012;19:1206–15. [DOI] [PubMed] [Google Scholar]
- [14].Liao JC, Deng JS, Chiu CS, et al. Anti-inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med 2012;2012:429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higuchi H, Yamamoto S, Ushio S, et al. Goshajinkigan reduces bortezomib-induced mechanical allodynia in rats: possible involvement of kappa opioid receptor. J Pharmacol Sci 2015;129:196–9. [DOI] [PubMed] [Google Scholar]
- [16].Suzuki Y, Goto K, Ishige A, et al. Effects of gosha-jinki-gan, a kampo medicine, on peripheral tissue blood flow in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol 1998;20:321–8. [DOI] [PubMed] [Google Scholar]
- [17].Nakanishi M, Nakae A, Kishida Y, et al. Go-sha-jinki-Gan (GJG) ameliorates allodynia in chronic constriction injury-model mice via suppression of TNF-alpha expression in the spinal cord. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakagawa H, Yamaoka T. Experience using Gosha-jinki-gan for lumbar and lower extremity pain in the elderly. Ortho Traumatol 1995;44:219–21. [Google Scholar]
- [19].Hamaguchi S, Komatsuzaki M, Kitajima T, et al. A retrospective study assessing Kampo medicine for the treatment of lower extremity symptoms caused by lumbar spinal diseases. Kampo Med 2017;68:366–71. [Google Scholar]
- [20].Maejima S, Katayama Y. Traditional Chinese Medicines for the Spinal Disorders. Kampo & the newest therapy 2004;13:232–6. [Google Scholar]
- [21].Park JE, Jung HJ, Kim A, et al. Current state of pain treatment in oriental medicine. J Korean Ori Med 2011;32:23–41. [Google Scholar]
- [22].Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].MacPherson H, Altman DG, Hammerschlag R, et al. Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010;7:e1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA 2006;296:2441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Carragee EJ, Spinnickie AO, Alamin TF, et al. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine 2006;31:653–7. [DOI] [PubMed] [Google Scholar]
- [26].He Q, Xu L, Wu W, et al. Clinical observation on acupuncture combined with massage for treatment of lumbar intervertebral disc protrusion. J Guangzhou Uni Trad Chin Med 2010;27:242–5. [Google Scholar]
- [27].Fu X. Comparative study on the efficacy of 240 cases of lumbar disc herniation treated with acupuncture and Chinese massage. World J Integr Trad Western Med 2011;6:1058–60. [Google Scholar]
- [28].Revill SI, Robinson JO, Rosen M, et al. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976;31:1191–8. [DOI] [PubMed] [Google Scholar]
- [29].Jeon CH, Kim DJ, Kim SK, et al. Validation in the cross-cultural adaptation of the Korean version of the Oswestry Disability Index. J Korean Med Sci 2006;21:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee JS, Lee DH, Suh KT, et al. Validation of the Korean version of the Roland-Morris Disability Questionnaire. Eur Spine J 2011;20:2115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim SH, Ahn J, Ock M, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res 2016;25:1845–52. [DOI] [PubMed] [Google Scholar]
- [32].Kamper SJ, Ostelo RW, Knol DL, et al. Global perceived effect scales provided reliable assessments of health transition in people with musculoskeletal disorders, but ratings are strongly influenced by current status. J Clin Epidemiol 2010;63: 760-766.e761. [DOI] [PubMed] [Google Scholar]
- [33].Kim YJ, Kang JH, Kwak KI, et al. Observation of correlation between deficiency syndrome of kidney and bone mineral density in osteoporosis patients. Korean J Acupun 2014;31:99–107. [Google Scholar]
- [34].Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003;12:12–20. [DOI] [PubMed] [Google Scholar]
- [35].Kono T, Hata T, Morita S, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, doubleblind, placebocontrolled trial of goshajinkigan to prevent oxaliplatininduced neuropathy. Cancer Chemother Pharmacol 2013;72:1283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Abe H, Kawai Y, Mori T, et al. The Kampo medicine Goshajinkigan prevents neuropathy in breast cancer patients treated with docetaxel. Asian Pac J Cancer Prev 2013;14:6351–6. [DOI] [PubMed] [Google Scholar]
- [37].Nishioka M, Shimada M, Kurita N, et al. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol 2011;16:322–7. [DOI] [PubMed] [Google Scholar]
- [38].Tawata M, Kurihara A, Nitta K, et al. The effects of Goshajinkigan, a herbal medicine, on subjective symptoms and vibratory threshold in patients with diabetic neuropathy. Diabetes Res Clin Pract 1994;26:121–8. [DOI] [PubMed] [Google Scholar]
- [39].Kajiwara M, Mutaguchi K. Clinical efficacy and tolerability of Gosha-jinki-gan, Japanese traditional herbal medicine, in females with overactive bladder. Hinyokika Kiyo 2008;54:95–9. [PubMed] [Google Scholar]
- [40].Chughtai B, Kavaler E, Lee R, et al. Use of herbal supplements for overactive bladder. Rev Urol 2013;15:93–6. [PMC free article] [PubMed] [Google Scholar]
- [41].Sekiguchi H, Inoue G, Nakazawa T, et al. Loxoprofen sodium and celecoxib for postoperative pain in patients after spinal surgery: a randomized comparative study. J Ortho Sci 2015;20:617–23. [DOI] [PubMed] [Google Scholar]
- [42].Kanayama M, Hashimoto T, Shigenobu K, et al. New treatment of lumbar disc herniation involving 5-hydroxytryptamine2A receptor inhibitor: a randomized controlled trial. J Neurosurg Spine 2005;2:441–6. [DOI] [PubMed] [Google Scholar]


