Abstract
Background:
The Free and Cued Selective Reminding Test (FCSRT) is the most accurate test for the diagnosis of prodromal Alzheimer’s disease (AD). Recently, a novel cognitive test, the Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L), has been developed in order to provide an early diagnosis.
Objective:
To compare the diagnostic accuracy of the FCSRT and the LASSI-L for the diagnosis of AD in its preclinical and prodromal stages using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) as a reference.
Methods:
Fifty patients consulting for subjective memory complaints without functional impairment and at risk for AD were enrolled and evaluated using FCSRT, LASSI-L, and FDG-PET. Participants were evaluated using a comprehensive neurological and neuropsychological protocol and were assessed with the FCSRT and LASSI-L. FDG-PET was acquired concomitantly and used for classification of patients as AD or non-AD according to brain metabolism using both visual and semi-quantitative methods.
Results:
LASSI-L scores allowed a better classification of patients as AD/non-AD in comparison to FCSRT. Logistic regression analysis showed delayed recall and failure to recovery from proactive semantic interference from LASSI-L as independent statistically significant predictors, obtaining an area under the curve of 0.894. This area under the curve provided a better discrimination than the best FCSRT score (total delayed recall, area under the curve 0.708, p = 0.029).
Conclusions:
The LASSI-L, a cognitive stress test, was superior to FCSRT in the prediction of AD features on FDG-PET. This emphasizes the possibility to advance toward an earlier diagnosis of AD from a clinical perspective.
Keywords: Alzheimer’s disease, early detection, memory, mild cognitive impairment, neuropsychological assessment, positron emission tomography
INTRODUCTION
Alzheimer’s disease (AD) is the most frequent neurodegenerative disease, and its early diagnosis is a major aim in a clinical and research setting [1]. The typical form of AD is characterized by a memory impairment associated with neurodegeneration of the hippocampus and entorhinal cortex [2]. Indeed, neuropsychological assessment is a main tool to detect patients with cognitive impairment secondary to AD and to guide the use of biomarkers to confirm the diagnosis. In this regard, the Free and Cued Selective Reminding Test (FCSRT) has been recommended by the International Working Group for the assessment of episodic memory and diagnosis of prodromal AD [3, 4]. This test has been largely validated for the diagnosis of amnestic mild cognitive impairment (MCI) and mild AD [2], even showing better discrimination capacity than MRI volumes [5]. However, an earlier diagnosis of AD becomes more necessary, as it is thought that dis ease modifying treatments may be most effective in prodromal and preclinical stages of the disease. In this setting, accuracy of FCSRT and other traditional memory tests may be probably lower than more novel neuropsychological tests that stress the cognitive system [6, 7].
The incomplete accuracy of FCSRT and other memory tests is evident in the concept of amnestic MCI (aMCI), in which the boundaries between nor mal aging and dementia are often difficult to establish, and aMCI is not invariably linked to clinical deterio ration. Accordingly, the outcome of aMCI is variable, with an estimated risk of conversion to AD dementia between 5 and 20% [8]. This risk is heterogeneous and depends on several factors, including the cognitive tests used in each study. For this reason, use of biomarkers has been emphasized and it has shown to improve the outcome prediction. In this regard, amyloid biomarkers are considered the most sensitive biomarkers, but are also associated with apparent false positives with aging in individuals who may never have clinical symptoms in their lifetimes [9]. In contrast, biomarkers of neurodegeneration and, specifically 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET), has shown to be a very specific tool to predict the progression from MCI to AD in the following months with a high positive predictive value. Although the sensitivity of FDG PET is inferior to amyloid biomarkers, it is estimated around 83–89% [10], and the sensitivity and specificity has been considered similar in prediction of longitudinal changes and clinical diagnosis over 3–4 years [11]. For this reason, this technique could con tribute to the classification of patients with preclinical and prodromal AD in two groups: first, patients with hypometabolism in brain regions linked to AD (with high risk of progression to Alzheimer’s dementia); and second, those patients with normal metabolism (with lower risk of progression).
Although the available biomarkers can help us to better classify patients consulting for memory loss, these biomarkers are expensive, some invasive, and not completely available in all clinical scenarios. In this regard, over the last several years some novel cognitive tests have been developed specifically to contribute to the diagnosis of AD in the earliest stages [6, 7]. These tests could hypothetically detect AD earlier than traditional measures because they were specifically designed for the detection of prodromal and preclinical AD [7]. Among them, the Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) is characterized by a new paradigm in the assessment of memory evaluating semantic interference and, particularly, the ability to recovery from semantic proactive interference [12, 13]. The LASSI-L studies memory processes, including encoding, storage and learning, free and cued recall, proactive and retroactive semantic interference, recovery from the proactive interference, and delayed recall. Previous research has clinically validated the test for the diagnosis of aMCI and AD, and main scores have been correlated to amyloid load and hippocampal volumes [13–16]. However, to our knowledge, there are no studies comparing the diagnostic accuracy of LASSI-L and the current recommended test for prodromal AD (i.e., the FCSRT).
In this context, we aimed to compare the diagnostic accuracy of the FCSRT and the LASSI-L in the setting of preclinical and prodromal AD, using FDG-PET results as a reference.
METHODS
Design and participants
This cross-sectional study enrolled 50 individuals without dementia who consulted to a Department of Neurology of a tertiary hospital. The patients were recruited consecutively over a period of 6 months, with the following inclusion criteria: 1) patients consulting for memory loss, but with no significant impairment in daily living activities (Functional Activities Questionnaire <2) [17]; 2) one or more of the following features suggestive of increased risk of AD: a) family history of AD; b) age of onset of memory complaints; c) the characteristics of the described memory loss (for instance, progressive memory or other cognitive decline according to informants, repetitive comments, etc.); and 3) absence of depression, other psychiatric diseases, or neurological or medical comorbidities with a potential cognitive repercussion.
We studied all patients using a common proto col comprising of general cognitive assessments, the FCSRT, and the LASSI-L. FCSRT and LASSI-L were not used for diagnosis. In addition, 18F-FDG-PET scans were obtained. Two neurologists evaluated all participants, recruited the patients, and collected clinical information, including demographic data, and medical and neurological history. Two neuropsychologists carried out neuropsychological assessment, and two nuclear medicine physicians read FDG PET images. Neurologists, neuropsychologists, and nuclear medicine physicians were blinded to the information gathered by the other assessments.
The local ethics committee approved the research protocol and all patients signed a written informed consent prior to enrolling in the study.
Clinical and neuropsychological protocol
A comprehensive neuropsychological assessment was administered, inclusive of the following tests: Mini-Mental State Examination (MMSE), verbal span of attention (forward and backward digit span), the Corsi block-tapping test, Trail Making Test, Symbol Digit Modalities Test, Boston Naming Test, Visual Object and Space Perception Battery (subtests for object decision, progressive silhouettes, position discrimination, and number location), Judgement of Line Orientation, Tower of London-Drexel version, Rey-Osterrieth Complex Figure Test (copy and incidental memory at 3 and 30 min), and Stroop Color-Word Interference Test. These tests belong to the Neuronorma battery and were used to study the potential impairment of other cognitive domains beyond verbal memory, using the normative data from the Neuronorma study [18–24]. In this regard, the following domains were considered: visual memory, attention/executive functioning, language, and visuospatial/visuoperceptive function.
FCSRT and LASSI-L
The FCSRT and LASSI-L were administered to all participants during distinct assessment sessions. The FCSRT includes a learning list of 16 written words presented with a semantic cue to control for memory encoding. Patients are asked to retrieve the words spontaneously, then with the help of the semantic cue during three trials and, finally, free and cued delayed recall is examined 30 min later [19]. The FCSRT included the following scores: trial 1 free recall (FR-1), total free recall (TFR), total recall (free recall + cued recall) (TR), delayed free recall (DFR), delayed total recall (delayed free recall + delayed total recall) (DTR), intrusions (total number and different intrusions), and a sensitivity to cueing index (TR–TFR)/(48 – TFR). Regarding LASSI-L, two lists (lists A and B) of 15 common words, including fruits, musical instruments, and articles of clothing, are presented in printed words. List A is firstly presented and the patient is asked to remember the words free and with semantic cues. Then, List A is again presented, and cued recall is performed again. Then, the semantically related list B with 15 words is presented using the same procedure. After that, the patient is asked to recall the words from List A free and cued, and finally, a delayed free recall of all words is performed 20 min later [12, 16]. In LASSI-L, the following scores were registered: free recall for List A (FRA1), first cued recall trial for List A (CRA1), second cued recall trial for List A (CRA2; maximum storage), free recall for List B (FRB1), first cued recall trial for List B (CRB1), second cued recall trial for List B (CRB2; recovery from proactive semantic interference), short delay free recall of list A (SdFRA; retroactive semantic interference), short delay of cued recall for List A (SdCRA), and delayed recall (DR). Intrusions from competing lists and intrusions not related to the target LASSI-L words were also registered.
Raw scores were also adjusted by age and education according to the normative data of FCSRT and LASSI-L in our country [16, 19]. Thus, raw scores were transformed to scaled-scores (mean 10, standard deviation 3), considering 6/7 as the cutoff (6 = impaired, 7 = non impaired). An index to evaluate the recovery from proactive interference was calculated as the difference between CRA2 and CRB2 (scaled scores). A failure in the ability to recover from proactive semantic interference (frPSI) was considered when there was a difference of at least two points between CRA2 and CRB2 or when CRB2 was impaired (scaled score ≤6). Furthermore, a percentage of decline (PD) from CRA2 to CRB2 indicative of failure to recovery from proactive interference was calculated as (CRA2 – CRB2 / CRA2) × 100.
FDG-PET acquisition, preprocessing, and analysis
Images were acquired in a late generation PET CT following the current European guidelines for bran PET imaging [25], with the protocol described elsewhere [26]. Statistical Parametric Mapping version 8 (SPM 8, The Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College of London) was used for preprocessing and analysis. Images were realigned and normalized to the Montreal Neurological Institute space using a reference FDG template [27]. Automatic Anatomic Labeling (AAL) atlas and Marsbar software were used for region of interest analysis. Cerebellum was used as reference.
Patients were classified as AD/non-AD according to brain metabolism following two methods. Using the first method, brain images were evaluated by two independent nuclear medicine physician experts in brain imaging. Each rater completed a questionnaire addressing the regional brain metabolism, the diagnosis (AD versus normal metabolism), and the confidence in his/her own diagnosis. None of the participants had an alternative pattern of brain metabolism suggestive of other neurodegenerative disease or vascular cognitive impairment. Using the second method, brain metabolism was assessed semi-quantitatively using a Meta-ROI that comprises the most relevant brain regions associated to AD and has been previously validated for the diagnosis of AD. A cut-off value of 1.249 was used for the MetaROI [28, 29].
Statistical analysis
Statistical analysis was performed using IBM® SPSS Statistics 22.0 and R version 3.3.3 (pROC package) [30]. Results are shown as mean ± standard deviation or frequency (percentage). To compare qualitative and quantitative variables between groups, Fisher’s exact test and U-Mann Whitney tests were used, as appropriate. Cohen’s d was used to calculate effect size. We also estimated ROC curves and the area under the curve to evaluate the discrimination between groups by each score. The method proposed by DeLong et al. was used to compare the ROC curves [31]. Statistical significance was set as p < 0.05.
RESULTS
Sample characteristics
Fifty participants with a mean age of 71.74 ± 8.42 years were included. Mean education was11.70 ± 4.69 years, and 27 (54%) were men. Mean MMSE score was 27.62 ± 2.29 (Table 1) and it was 29–30 in 25 (50%) of cases. In 34 patients (68%) there was no impairment in general cognitive assessment, while in 2 cases there was an impairment in visual memory, in 5, executive dysfunction and in 9 cases (18%) two domains were altered. Twenty participants (40%) showed AD features according to the visual analysis, and 21 (42%) in the semi-quantitative analysis of FDG-PET images. The percentage of agreement between visual and semi-quantitative analysis was 74%.
Table 1.
Main sample characteristics. Raw and scaled scores (in brackets) are shown
| Age (years) | 71.74 ± 8.42 |
| Men | 27 (54%) |
| Years of education | 11.70 ± 4.69 |
| MMSE (0–30) | 27.62 ± 2.29 |
| TMT-A | 69.36 ± 40.27 (8.50 ± 3.25) |
| TMT-B | 195.83 ± 153.9 (7.52 ± 3.07) |
| SDMT | 29.69 ± 17.59 (9.29 ± 2.88) |
| Boston Naming Test (0–60) | 44.24 ± 8.85 (8.84 ± 2.72) |
| Semantic fluency (animals) | 16.70 ± 5.82 (8.88 ± 2.64) |
| Phonological fluency (words with “p”) | 12.94 ± 4.93 (9.92 ± 2.74) |
| Rey Complex Figure (copy accuracy) (0–36) | 26.70 ± 6.71 (8.52 ± 2.30) |
| Judgement Line Orientation (0–30) | 19.82 ± 6.29 (9.02 ± 3.71) |
| Tower of London (correct moves) (0–10) | 3.42 ± 2.54 (9.58 ± 4.37) |
| VOSP (object decision) (0–20) | 15.86 ± 3.15 (10.29 ± 3.49) |
| VOSP (discrimination of position) (0–20) | 18.86 ± 2.57 (15.86 ± 3.15) |
Accuracy of FCSRT and LASSI-L for diagnosis of AD metabolic features
Using visual analysis of FDG-PET images to classify the patients, 11 (55%) of patients with AD and 17 (56.7%) of cases with non-AD were impaired in DTR-FCSRT (p = 0.907). Regarding TR-FCSRT, this score was impaired in 12 (60%) of AD and 17 (56.7%) of non-AD patients (p = 0.815). Regarding LASSI-L, CRA2 (maximum storage), CRB2 (failure to recover from proactive semantic interference), and DR (delayed recall) were respectively impaired in 11 (55.0%), 13 (65.0%), and 16 (80%) cases with AD, and in 10 (33%), 8 (26.7%), and 13 (43%) of patients with non-AD (p = 0.110 for CRA2; p = 0.007 for CRB2, and p = 0.010 for DR). Furthermore, frPSI was found in 16 (80%) of cases with AD and 10 (33.3%) of patients with non-AD (p = 0.001).
Using semi-quantitative analysis, 13 (61.9%) of patients with AD and 15 (51.7%) of cases with non-AD were impaired in DTR-FCSRT (p = 0.474). Regarding TR-FCSRT, this score was impaired in 14 (66.7%) of AD and 15 (51.7%) of non-AD patients (p = 0.291). Regarding LASSI-L, CRA2, CRB2, and DR were respectively impaired in 11 (52.4%), 12(57.1%), and 16 (76.2%) cases with AD, and in 10(34.5%), 9 (31.0%), and 13 (44.8%) of patients with non-AD (p = 0.206 for CRA2; p = 0.065 for CRB2, and p = 0.027 for DR). Furthermore, a frPSI was found in 15 (71.4%) of cases with AD and 11 (37.9%) of patients with non-AD (p = 0.019).
The alteration of any cognitive domain beyond verbal memory was not associated to a pattern of AD in FDG-PET either in visual or semi-quantitative analysis (Supplementary Tables 1 and 2).
We also performed the same analysis restricted to those patients in which visual and semi-quantitative analysis of PET images were coincident (n = 37). In this case, there were non-statistically significant differences in the frequency of impairment in the main scores of the FCSRT in the groups with AD (11,78.6% in TR and 10, 71.4% in TDR) and non-AD (14,60.9% in TR and 14, 60.9% in TDR) (p = 0.228 and0.387), respectively. In contrast, the LASSI-L showed statistically significant differences in CRA2, CRB2, DR, and in the index of recovery from proactive interference (frPSI). These scores were respectively impaired in 9 (64.3%), 10 (71.4%), 13 (92.9%), and 12 (85.7%) cases with AD, and in 8 (34.8%), 6(26.1%), 10 (43.5%), and 7 (30.4%) cases with non-AD (p = 0.08 for CRA2, p = 0.009 for CRB2, 0.003 for DR, and 0.001 for the index of recovery from proactive interference.
Mean scores of each test were also compared, showing statistically significant differences in FRA1, FRB1, CRB2 and DR, as shown in Table 2 (see also Supplementary Tables 1 and 2 for the same comparison but using visual and semi-quantitative analysis as reference). Effect size was d = 0.73 for TDR-FCSRT and 1.12 for DR.
Table 2.
Comparison between groups with AD and non-AD in FDG-PET analysis. Only patients with agreement between visual and semi-quantitative analysis are included
| AD | Non-AD | U-Mann Whitney (p-value) | |
|---|---|---|---|
| Age | 72.57 ± 8.29 | 70.39 ± 9.13 | 139.0 (0.490) |
| Years of education | 11.64 ± 5.59 | 12.04 ± 4.39 | 156.0 (0.874) |
| FR1-FCSRT | |||
| Raw | 3.00 ± 2.18 | 4.43 ± 3.04 | 118.0 (0.175) |
| Scaled | 6.86 ± 3.13 | 8.83 ± 3.92 | 113 (0.131) |
| TR-FCSRT | |||
| Raw | 21.43 ± 11.84 | 28.04 ± 10.89 | 111.5 (0.121) |
| Scaled | 4.29 ± 2.46 | 6.00 ± 3.35 | 113.5 (0.131) |
| TDR-FCSRT | |||
| Raw | 5.57 ± 4.51 | 8.74 ± 4.09 | 94.0 (0.036) |
| Scaled | 4.29 ± 5.96 | 5.96 ± 4.54 | 134.0 (0.388) |
| Index of Sensitivity to Cueing (%) | 33.87 ± 21.13 | 44.42 ± 21.44 | 121.5 (0.216) |
| FRA1 | |||
| Raw | 4.86 ± 1.95 | 6.87 ± 2.75 | 87.4 (0.020) |
| Scaled | 5.57 ± 3.00 | 8.22 ± 4.11 | 92.0 (0.029) |
| CRA1 | |||
| Raw | 6.64 ± 2.34 | 7.65 ± 3.08 | 131.0 (0.343) |
| Scaled | 7.14 ± 2.59 | 8.00 ± 4.11 | 145.5 (0.623) |
| CRA2 | |||
| Raw | 8.71 ± 2.55 | 10.04 ± 2.45 | 5.09 ± 2.42 |
| Scaled | 5.71 ± 3.12 | 7.48 ± 3.80 | 115.5 (0.151) |
| FRB1 | |||
| Raw | 3.57 ± 1.86 | 5.09 ± 2.42 | 96.0 (0.038) |
| Scaled | 6.36 ± 1.94 | 8.70 ± 2.38 | 62.5 (0.002) |
| CRB1 | |||
| Raw | 3.57 ± 1.86 | 5.35 ± 2.79 | 150.0 (0.727) |
| Scaled | 7.64 ± 1.49 | 8.09 ± 3.35 | 142.5 (0.558) |
| CRB2 | |||
| Raw | 6.21 ± 3.06 | 8.83 ± 2.79 | 88.0 (0.021) |
| Scaled | 5.00 ± 2.77 | 8.09 ± 3.80 | 80.5 (0.011) |
| SdFRA | |||
| Raw | 2.71 ± 1.89 | 4.65 ± 2.72 | 91.5 (0.028) |
| Scaled | 7.29 ± 2.61 | 8.70 ± 2.99 | 110.5 (0.110) |
| SdCRA | |||
| Raw | 5.86 ± 1.91 | 7.04 ± 2.51 | 101.5 (0.060) |
| Scaled | 8.43 ± 2.37 | 9.43 ± 3.42 | 111.5 (0.117) |
| DR | |||
| Raw | 6.64 ± 5.42 | 12.87 ± 6.34 | 73.0 (0.006) |
| Scaled | 3.14 ± 2.21 | 7.17 ± 4.64 | 72.0 (0.005) |
| PD | 31.54 ± 24.40 | 10.53 ± 25.36 | 88.5 (0.022) |
ROC curves of scores
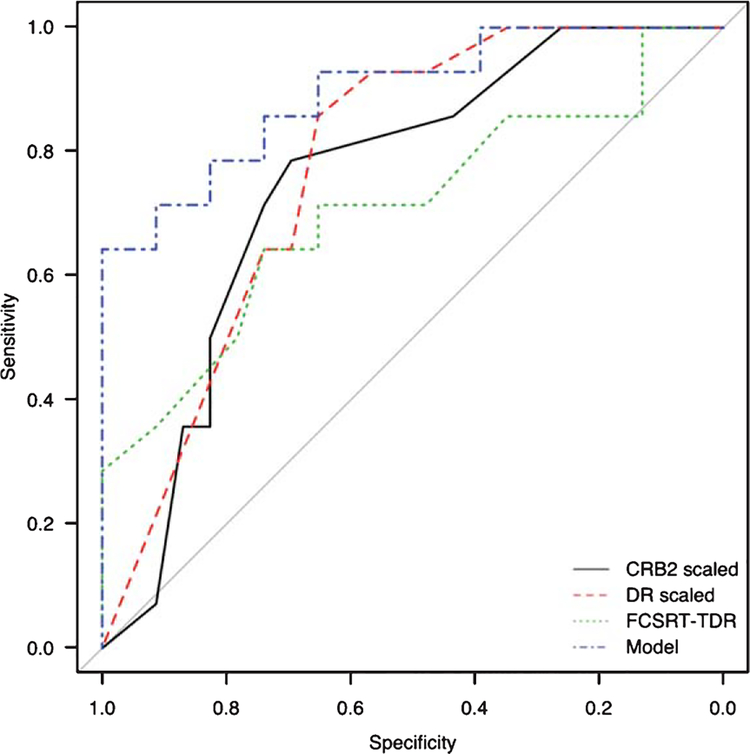
In the group of patients with agreement between visual and semi-quantitative analysis of FDG-PET images, ROC curves and areas under the curve (AUC) were estimated for the discrimination of patients with and without AD metabolic features (Fig. 1). The AUC was 0.776 for DR (scaled score), 0.773 for DR (raw score), 0.750 for CRB2 (scaled score), 0.727 for CRB2 (raw score), 0.660 for CRA2 (raw score), 0.641 for CRA2 (scaled score), 0.654 for TR-FCSRT (raw score), 0.648 for TR-FCSRT (scaled score), 0.708 for TDR-FCSRT (raw score), and 0.548 for TDR-FCSRT (scaled score). All ROC curves were statistically significant (p < 0.05) except CRA2 (raw and scaled score), TR-FCSRT (raw and scaled score), and TDR-FCSRT (scaled score).
Fig. 1.
ROC curves and cutoff points for the discrimination between AD and non-AD. CRB2 (scaled score) (black), DR (scaled score) (red), TDR-FCSRT (raw score) (green) and logistic regression model (blue).
PD from CRA2 to CRB2 obtained an AUC of 0.643 in visual analysis, 0.702 in semi-quantitative analysis, and 0.725 in cases with agreement of both methods. A cutoff point of 35% of PD achieved a sensitivity of 35–49% and a specificity of 86.7–91.3%. A more sensitive cutoff point of 11% provided a sensitivity of 75–81% and a specificity of 36.7–43.5% (other cutoff points are shown in Supplementary Table 3).
Logistic regression analysis
A logistic regression analysis (enter method) was estimated for the differential diagnosis between AD and non-AD using the group of patients with agreement between both methods of FDG-PET imaging analysis. The variables introduced to the model were: TDR-FCSRT, CRA2, CRB2, DR, and frPSI. Only frPSI and DR were independent statistically significant predictors (Table 3), yielding a 78.5% of correct classification, with a Nagelkerke’s R2 of 0.55. Hosmer-Lemeshow test was not significant (p = 0.214), indicating no evidence of poor fit of the model. This model obtained an AUC of0.894 (p < 0.001). This AUC was greater than the AUC of the best FCSRT score (0.894 vs. 0.708 in TDR-FCSRT, p = 0.029).
Table 3.
Results of the binary logistic regression analysis for predicting AD
| β0 | OR | 95% CI | p-value | |
|---|---|---|---|---|
| TDR-FCSRT | −0.146 | 0.864 | 0.650–1.149 | 0.315 |
| CRA2 | 0.244 | 1.276 | 0.708–2.300 | 0.417 |
| CRB2 | 0.201 | 1.222 | 0.694–2.152 | 0.486 |
| DR | −0.277 | 0.758 | 0.576–0.999 | 0.049 |
| frPSI | −3.072 | 0.019 | 0.004–0.605 | 0.019 |
| Constant | 0.463 | 1.588 | - | 0.804 |
β0: constant of the model (coefficient of the explicative variable); OR, odds ratio; CI, confidence interval.
DISCUSSION
This is the first investigation to compare the diagnostic accuracy of FCSRT and LASSI-L in a cohort of patients presenting for memory loss with an increased risk of prodromal AD. Patients enrolled in the study did not have functional impairment, had only mild deficits or were largely preserved in cognitive assessment. This represents a group of patients that could be categorized as subjective memory complaints, pre-MCI or early MCI, but potentially at risk of AD because of family history, age or clinical characteristics. In this setting, LASSI-L scores detected more accurately than FCSRT, those patients with AD features on FDG-PET and this superiority in the diagnostic accuracy remained across the different methods used for classification of FDG-PET imaging (visual, semi-quantitative analysis and agreement between both). Interestingly, LASSI-L scores that provided a better discrimination were CRB2 and DR. In contrast, CRA2 (as well as TR and TDR of the FCSRT) were not statistically significant in several of the parameters used to evaluate their diagnostic accuracy. CRB2 is the score directly associated to the recovery from the PSI. The failure to recover from proactive interference effects (frPSI) is a unique feature of the LASSI-L and has been associated with increased global and regional amyloid load among otherwise neuropsychologically normal community-dwelling elders [13] and decreased MRI volumes and cortical thickness in AD prone areas among those with aMCI [14, 15].
Likewise, DR results from the several processes that include forgetting originally recalled targets and adequate recovery from the PSI also is necessary to obtain an optimal score. In contrast, CRA2, TR-FCSRT, and TDR-FCSRT are mainly linked to encoding and storage of verbal information. This suggests that the recovery from PSI could potentially be a better marker of AD in preclinical and prodromal stages than those measures only associated to semantic encoding and cueing.
There are several similarities and differences between FCSRT and LASSI-L that likely explain our findings. Both are verbal memory tests that use a controlled memory encoding procedure with a semantic cue along several trials, assess free and cued recall, and include a delayed recall trial at 20–30 min. However, FCSRT evaluates the ability to learn a list of 16 non-prototypical words (e.g., crow) belonging to 16 semantic categories (e.g., bird) during 3 trials. In contrast, LASSI-L evaluates the learning of a first list of 15 words of three semantic categories (fruits, musical instruments, and articles of clothing) during 2 trials and, then, a second list of 15 different words belonging to the identical semantic categories. The second list must be learned under the effects of proactive semantic interference and a subsequent learning provides a unique opportunity to probe semantic interference effects. Retroactive interference is subsequently evaluated asking the patient to remember the items of the first list. However, LASSI L generates a “memory stress condition” [13] with PSI and evaluates its impact during CB2 and DR, which encompasses the several processes involved in episodic memory. This could explain a higher sensitivity than other traditional memory tasks [12].
Importantly, the present study employed logistic regression analysis that showed that DR and frPSI were predictors of AD features on FDG-PET, not requiring FCSRT or CRA2. The model achieved a good fit and obtained a higher AUC. This value was especially high if we consider the fact that it allowed discrimination between AD versus non-AD among patients consulting for memory loss to a Department of Neurology (i.e., not healthy controls). This has direct implications in clinical practice, because the high frequency of patients consulting for memory loss and the increasingly need of an early diagnosis of AD [32]. Furthermore, LASSI-L was evenly tolerated as FCSRT, although practicality of each test was not objectively measured.
Our study has some limitations. First, its cross-sectional design does not allow excluding totally the possibility that some patients classified as non-AD might progress to AD during the follow-up. Second, amyloid biomarkers (PET or CSF) were not available. Due to the mean age of our patients, we preferred FDG to amyloid PET because of the limited positive predictive value in older population [33]. However, this issue may limit our conclusions about the sensitivity, but not the specificity, of the tests evaluated. In this regard, LASSI-L scores were more specific, but follow-up would be interesting to find out if those patients with impairment in the test and/or the extent to which those with greater or lesser frPSI may progress to AD dementia. It is acknowledged that FDG-PET interpretation in preclinical and prodromal AD may be sometimes more challenging than in other clinical situations. To reduce this limitation, PET scans were assessed with two highly experienced nuclear medicine physicians with more than 10 years of experience as well as with visual and semi-quantitative methods, with consistent results within different methods. These methodologies have previously demonstrated high accuracy in the detection of AD brain glucose metabolism pattern in patients with amnestic MCI [34].
In conclusion, our study found that LASSI-L was superior to the FCSRT in the prediction of AD features on FDG-PET in patients with only memory complaints at risk of AD. This contributes to the increasing evidence showing recovery from PSI as a key neuropsychological marker of AD and emphasizes the possibility to advance toward an earlier diagnosis of AD from a clinical perspective. Further studies are necessary to evaluate the potential advantages of LASSI-L in other clinical settings, such as population-based studies, clinical follow-up or in the differential diagnosis with other neurodegenerative diseases, where FCSRT has demonstrated a major role in cognitive assessment [35–38].
Supplementary Material
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0604r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170604.
REFERENCES
- [1].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sarazin M, Berr C, De Rotrou J, Fabriguole C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B (2007) Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 69, 1859–1867. [DOI] [PubMed] [Google Scholar]
- [3].Dubois B, Feldman HH, Jacova C, DeKosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol 6, 734–746. [DOI] [PubMed] [Google Scholar]
- [4].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bate man R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert MO, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s dis ease: The IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [5].Sánchez-Benavides G, Peña-Casanova J, Casals-Coll M, Gramunt N, Molinuevo JL, Gómez-Ansón B, Aguilar M, C, Martínez-Parra C, Frank-García A, Robles A, Antunez Fernández-Martínez M, Blesa R, for the NEURONORMA Study Team (2014) Cognitive and neuroimaging profiles in mild cognitive impairment and Alzheimer’s disease: Data from the Spanish Multicenter Normative Studies (NEU RONORMA Project). J Alzheimers Dis 41, 887–891. [DOI] [PubMed] [Google Scholar]
- [6].Rentz DM, Parra Rodriguez MA, Amariglio R, Stern Y, Sperling R, Ferris S (2013) Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimers Res Ther 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Loewenstein DA, Curiel RE, Duara R, Buschke H (2017) Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer’s disease. Assessment, doi:10117/1073191117691608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ghosh S, Libon D, Lippa C (2014) Mild cognitive impairment: A brief review and suggested clinical algorithm. Am J Alzheimers Dis Other Dement 29, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jack CR Jr, Wiste HJ, Weigand SD, Rocca WA, Knop man DS, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Preboske GM, Pankratz VS, Vemuri P, Petersen RC (2014) Age-specific population frequencies of cerebral Beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: A cross-sectional study. Lancet Neurol 13, 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perani D, Schillaci O, Padovani A, Nobili FM, Iaccarino L, Della Rosa PA, Frisoni G, Caltagirone C (2014) A survey of FDG- and amyloid-PET imaging in dementia and GRADE analysis. Biomed Res Int 2014, 785039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jagust W, Reed B, Mungas D, Ellis W, Decarli C (2007) What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 69, 871–877. [DOI] [PubMed] [Google Scholar]
- [12].Crocco E, Curiel RE, Acevedo A, Czaja SJ, Loewenstein DA (2014) An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features in Alzheimer’s disease. Am J Geriatr Psychiatry 222, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, Wicklund M, Crocco E, Pontecorvo M, Joshi AD, Rodriguez R, Barker WW, Hidalgo J, Duara R (2016) A novel cognitive stress test for the detection of preclinical Alzheimer Disease: Discriminative properties and relation to amyloid load. Am J Geriatr Psychiatry 24, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loewenstein DA, Curiel RE, Wright C, Sun X, Alperin N, Crocco E, Czaja SJ, Raffo A, Penate A, Melo J, Capo K, Gamez M, Duara R (2017) Recovery from proactive semantic interference in mild cognitive impairment and normal aging: Relationship to atrophy in brain regions vulnerable to Alzheimer’s disease. J Alzheimers Dis 56, 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Loewenstein DA, Curiel RE, DeKosky S, Rosselli M, Bauer R, Grieg-Custo M, Penate A, Li C, Lizagarra G, Golde T, Adjouadi M, Duara R (2017) Recovery from proactive semantic interference and MRI volume: A replication and extension study. J Alzheimers Dis 59, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Matías-Guiu JA, Curiel RE, Rognoni T, Valles-Salgado M, Fernández-Matarrubia M, Hariramani R, Fernández-Castro A, Moreno-Ramos T, Loewenstein DA, Matías-Guiu J (2017) Validation of the Spanish version of the LASSI-L for diagnosing mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 56, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. J Gerontol 37, 323–329. [DOI] [PubMed] [Google Scholar]
- [18].Peña-Casanova J, Blesa R, Aguilar M, Gramunt-Fombuena N, Gómez-Ansón B, Oliva R, Molinuevo JL, Robles A, Bar-quero MS, Martínez-Parra C, Frank-García A, Fernández M, Antúnez C, Alfonso V, Sol JM; NEURONORMA Study team (2009) Spanish Multicenter Normative Studies (NEU RONORMA Project): Methods and sample characteristics. Arch Clin Neuropsychol 24, 307–319. [DOI] [PubMed] [Google Scholar]
- [19].Peña-Casanova J, Gramunt-Fombuena N, Quiñones-úbeda S, Sánchez-Benavides G, Aguilar M, Badenes D, Molinuevo JL, Robles A, Barquero MS, Payno M, Antunez C, Martínez-Parra C, Frank-García A, Fernández M, Alfonso V, Sol JM, Blesa R, for the NEURONORMA, Study Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Rey-Osterrieth Complex Figure (Copy and Memory), and Free and Cued Selective Reminding Test. Arch Clin Neuropsychol 24, 371–393. [DOI] [PubMed] [Google Scholar]
- [20].Peña-Casanova J, Quiñones-úbeda S, Gramunt-Fombuena N, Aguilar M, Casas L, Molinuevo JL, Robles A, Rodríguez D, Martínez-Parra C, Frank-García A, Barquero MS, Antunez A, Fernández M, Molano A, Alfonso V, Sol JM, Blesa R, Study NEURONORMA, Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Boston naming test and token test. Arch Clin Neuropsychol 24, 343–354. [DOI] [PubMed] [Google Scholar]
- [21].Pena-Casanova J, Quiñones-úbeda S, Gramunt-Fombuena N, Quintana-Aparicio M, Aguilar M, Badenes D, Cerulla N, Molinuevo JL, Ruiz E, Robles A, Barquero MS, Antúnez C, Martínez-Parra C, Frank-García A, Fernández M, Alfonso V, Sol JM, Blesa R, Study NEURONORMA, Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for verbal fluency tests. Arch Clin Neu ropsychol 24, 395–411. [DOI] [PubMed] [Google Scholar]
- [22].Peña-Casanova J, Quintana-Aparicio M, Quiñones-úbeda S, Aguilar M, Molinuevo JL, Serradell M, Robles A, Bar-quero MS, Martínez-Parra C, Villanueva C, Antunez Frank-García A, Aguilar MD, Fernández M, Alfonso V, Sol JM, Blesa R, Study NEURONORMA, Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the visual object and space perception battery-abbreviated, and judgement of line orientation. Arch Clin Neuropsychol 24, 355–370. [DOI] [PubMed] [Google Scholar]
- [23].Peña-Casanova J, Quiñones-úbeda S, Gramunt-Fombuena N, Quintana M, Aguilar M, Molinuevo JL, Serradell M, C, Martínez-Robles A, Barquero MS, Payno M, Antunez Parra C, Frank-García A, Fernández M, Alfonso V, Sol JM, Blesa R, Study NEURONORMA, Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for the Stroop color-word interference test and the Tower of London-Drexel. Arch Clin Neuropsychol 24, 413–429. [DOI] [PubMed] [Google Scholar]
- [24].Peña-Casanova J, Quiñones-úbeda S, Quintana-Aparicio M, Aguilar M, Badenes D, Molinuevo JL, Torner L, Robles C, Martínez-Parra A, Barquero MS, Villanueva C, Antunez C, Frank-García A, Sanz A, Fernández M, Alfonso V, Sol JM, Blesa R, Study NEURONORMA, Team (2009) Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch Clin Neuropsychol 24, 321–341. [DOI] [PubMed] [Google Scholar]
- [25].Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Nagren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K; European Association of Nuclear Medicine Neuroimaging Committee (2009) EANM Procedure guide lines for PET brain using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36, 2103–2110. [DOI] [PubMed] [Google Scholar]
- [26].Matías-Guiu JA, Cabrera-Martín MN, Valles-Salgado M, Pérez-Pérez A, Rognoni T, Moreno-Ramos T, Carreras JL, Matías-Guiu J (2017) Neural basis of cognitive assessment in Alzheimer’s disease, amnestic mild cognitive impairment, and subjective memory complaints. Am J Geriatr Psychiatry 25, 730–740. [DOI] [PubMed] [Google Scholar]
- [27].Della Rosa PA, Cerami C, Gallivanone F, Prestia A, Caroli A, Castiglioni I, Gilardi MC, Frisoni G, Friston K, Ash-burner J, Perani D (2014) A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12, 575–593. [DOI] [PubMed] [Google Scholar]
- [28].Landau SM, Harvey D, Madison CM, Reiman EM, Fos ter NL, Aisen PS, Petersen RC, Shaw LM, Trojanowski JQ, Jack CR Jr, Weiner MW, Jagust WJ.; Alzheimer’s Dis ease Neuroimaging Initiative (2010). Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ; Alzheimer’s Dis ease Neuroimaging Initiative (2011) Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 32, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez M (2011) pROC: An open-source package for JC, Muller R and S+to analyze and compare ROC curves. BMC Bioin formatics 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].DeLong ET, DeLong DM, Clarke-Pearson DL (1988) Com paring the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 44, 837–844. [PubMed] [Google Scholar]
- [32].Matias-Guiu JA, García-Azorín D, García-Ramos R, Basoco E, Elvira C, Matías-Guiu J (2015) Study of outpatient neurological care in the Region of Madrid: The impact of implementing free choice of hospital. Neurología 30, 479–487. [DOI] [PubMed] [Google Scholar]
- [33].Laforce R Jr, Rabinovici GD (2011) Amyloid imaging in the differential diagnosis of dementia: Review and potential clinical applications. Alzheimers Res Ther 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morbelli S, Brugnolo A, Bossert I, Buschiazzo A, Frisoni GB, Galluzzi S, van Berckel BN, Ossenkoppele R, Perneczky R, Drzezga A, Didic M, Guedj E, Sambuceti G, Bottoni G, Arnaldi D, Picco A, De Carli F, Pagani M, Nobili F (2015) Visual versus semi-quantitative analysis of 18F-FDG-PET in amnestic MCI: An European Alzheimer’s Disease Consortium (EADC) project. J Alzheimers Dis 44, 815–826. [DOI] [PubMed] [Google Scholar]
- [35].Derby CA, Burns LC, Wang C, Katz MJ, Zimmerman ME, L’italien G, Guo Z, Berman RM, Lipton RB (2013) Screening for predementia AD: Time-dependent operating characteristics of episodic memory tests. Neurology 80, 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lemos R, Duro D, Simoes MR, Santana I (2014) The free and cued selective reminding test distinguishes frontotemporal dementia from Alzheimer’s disease. Arch Clin Neuropsychol 29, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Teichmnan M, Epelbaum S, Samri D, Levy Nogueira M, Michon A, Hampel H, Lamari F, Dubois B (2017) Free and Cued Selective Reminding Test: Accuracy for Alzheimer’s and neurodegenerative disease differential diagnosis: A large-scale biomarker-characterized monocenter cohort study (ClinAD). Alzheimers Dement 13, 913–923. [DOI] [PubMed] [Google Scholar]
- [38].Fernández-Matarrubia M, Matías-Guiu JA, Cabrera-Martín MN, Moreno-Ramos T, Valles-Salgado M, Carreras JL, Matías-Guiu J (2017) Episodic memory dysfunction in behavioral variant frontotemporal dementia: A clinical and FDG-PET study. J Alzheimers Dis 57, 1251–1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.