Abstract
Polydopamine is one of the simplest and most versatile approaches to functionalizing material surfaces, having been inspired by the adhesive nature of catechols and amines in mussel adhesive proteins. Since its first report in 2007, a decade of studies on polydopamine molecular structure, deposition conditions, and physicochemical properties have ensued. During this time, potential uses of polydopamine coatings have expanded in many unforeseen directions, seemingly only limited by the creativity of researchers seeking simple solutions to manipulating surface chemistry. In this review, we describe the current state of the art in polydopamine coating methods, describe efforts underway to uncover and tailor the complex structure and chemical properties of polydopamine, and identify emerging trends and needs in polydopamine research, including the use of dopamine analogs, nitrogen-free polyphenolic precursors, and improvement of coating mechanical properties.
Keywords: pPolydopamine, mussel, material-independent, surface coating, catecholamine
Graphical abstract:
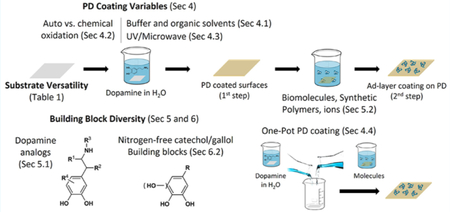
1. INTRODUCTION
Polydopamine (PD) is a uniquely adaptable and simple surface functionalization method, being the first single-step, material-independent surface chemistry when it was first reported in 2007.1 Since its introduction, PD has emerged as one of the most powerful tools available for modification of surfaces, achieving this status as a result of versatility, simplicity, and broad potential use in the biomedical, energy, consumer, industrial, military, and other sectors. In this review, we will summarize the evolution of the PD method since it was first reported ten years ago. We begin by describing the origin and basic features of PD, which arose as an outcome of mussel adhesive protein research. Next, we compare PD coatings to other commonly employed surface modification methods, followed by an account of how approaches to PD deposition have evolved from the original “recipe” to the current state-of-the-art PD preparation methods. We then provide a synopsis of the substrates and materials found to be amenable to modification with PD, and introduce selected emerging applications for these coatings. Finally, we conclude with a forward-looking statement on the opportunities and challenges in further development and implementation of PD and PD-like coatings.
2. INSPIRATION AND GENERAL FEATURES OF PD
The invention of PD originated from previous studies on one of Nature’s most celebrated families of wet adhesive biomolecules- the mussel adhesive proteins. These proteins, especially the mytilus foot proteins-3 and −5 (Mfp-3 and −5) located in the distal portion of the mussel byssus where the byssal foot engages the substrate surface,2–4 have two key features that inspired PD: (1) high catechol (3,4-dihydroxybenzene) content due to the presence of 3,4-dihydroxy-L-phenylalanine (DOPA); and (2) high primary and secondary amine content due to lysine (Lys) and histidine residues. The high concentration and intimate association of DOPA and Lys/ His was noted early on by Waite and co-workers as a remarkable feature of Mfp-5,3 leading to speculation that the combination of catechol and amine is a special one as it relates to interfacial adhesion. Early exploitation of catecholamines as building blocks for bioinspired materials include the synthesis of DOPA-Lys poly(amino acids),5 the tethering of catechol moieties to amine-rich polymers such as poly(ethylenimine)6,7 and chitosan8,9 and the use of short DOPA-Lys peptides as anchors for immobilization of antifouling polymers at solid–liquid interfaces.10 Although low molecular weight catecholamines such as dopamine were not considered in the context of adhesion prior to 2007, the coexistence of catechol and amine functional groups that is such a distinctive feature of mussel adhesive proteins is now understood to be a powerful combination (only recently was the physicochemical basis for the interfacial adhesive synergy between catechol and amine investigated in detail11).
The widespread adoption of PD originates from its simplicity, low cost and adaptability in a variety of science and applied engineering contexts. While variations of the coating method exist as discussed below, in its simplest manifestation coating an object with PD involves nothing more than simply immersing it in an aqueous alkaline solution of dopamine for an adjustable period of time (Figure 1). Spontaneous deposition of a conformal PD coating occurs during incubation, and this primary coating can be used without further modification or used as a “primer” onto which a subsequent secondary coating is applied. The composition and properties of the secondary coating is highly tailorable, therefore giving rise to the tremendous versatility and broad range of applications enjoyed by PD coatings. Dopamine·HCl is a commercially available and relatively inexpensive reagent (3.2 USD per gram when purchasing from Sigma-Aldrich). For little cost, one can make a one-liter solution of dopamine (1 mg/mL) that can be sprayed12 or used as an aqueous bath for dip-coating large surface areas.
Figure 1.
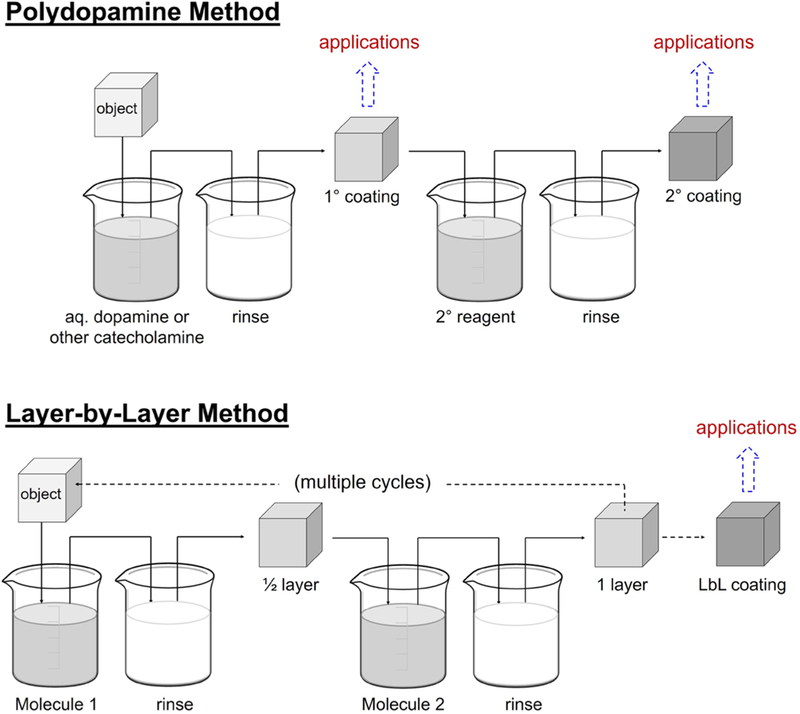
Schematic illustration of PD coating method with comparison to LbL coatings. Top: the traditional PD method takes place spontaneously in alkaline aqueous solutions, or with the addition of oxidants. A subsequent ad-layer (secondary coating) step can be undertaken. Secondary reagents are usually amine or sulfhydryl containing nucleophiles such as peptides, proteins, oligonucleotides, or nucleophilic natural or synthetic polymers. Bottom: the LbL method involves cyclic adsorption of typically polymeric components with intermediate rinsing steps. Often, dozens or even hundreds of cycles are used to build the coating.
The formation of PD coatings occurs by oxidative polymerization of dopamine, the details of which remain an active area of investigation. In fact, many features of PD formation and structure remain unknown. For this reason, and because other research and review papers with detailed mechanisms have been recently published,13–19 we will provide only a brief overview of existing theories of PD formation and structure here (Figure 2). There is little doubt that the initial driving force for PD formation is the oxidation of dopamine by dissolved oxygen at alkaline pH of the solution, as elimination of oxygen from the solution slows or eradicates PD formation. The oxidation product, dopamine-quinone, undergoes a nucleophilic intramolecular cyclization reaction leading eventually to the formation of 5,6-dihydroxyindole. In most existing theories of PD formation, these two compounds, dopamine-quinone and 5,6-dihydroxyindole (DHI), are key building blocks for PD, albeit through various proposed pathways. On one hand, it has been postulated that PD is composed entirely of noncovalent assemblies of dopamine, dopamine-quinone and DHI, whereas other hypotheses hold that these molecules polymerize to form a heteropolymer composed of catecholamine, quinone and indole repeat units. Alternatively, it has been suggested that PD is a eumelanin-like material composed of oligomeric building blocks generated spontaneously by further oxidation of DHI and coupling through 2–2′, 4–7′, 2–4′, and/or 2–7′ linkages. It should be mentioned that there is currently no consensus on the PD formation mechanism, and the proposed noncovalent and covalent pathways elaborated above should not be viewed as mutually exclusive, as it is possible and perhaps even probable that both covalent “polymerization” and “self-assembly” pathways contribute to PD formation.20 In addition, M. d’Ischia and co-worker found pyrrolecarboxylic acid (PCA), an oxidative degraded form of PD, with uncyclized dopamine/dopamine-quinone and cyclized DHI units when forming PD (Figure 3a).17 Ding et al. reported that (DHI) /PCA trimer complex can also be one of building blocks of PD18. This trimer is associated with others by noncovalent interactions to form the PD (Figure 3b). Additional uncertain aspects of PD formation that should be addressed by future studies, relate to how the aforementioned events occurring in solution lead to spontaneous deposition of a conformal coating on solid surfaces, and how the composition of the coating is the same as the product that can be isolated from the surrounding solution.
Figure 2.
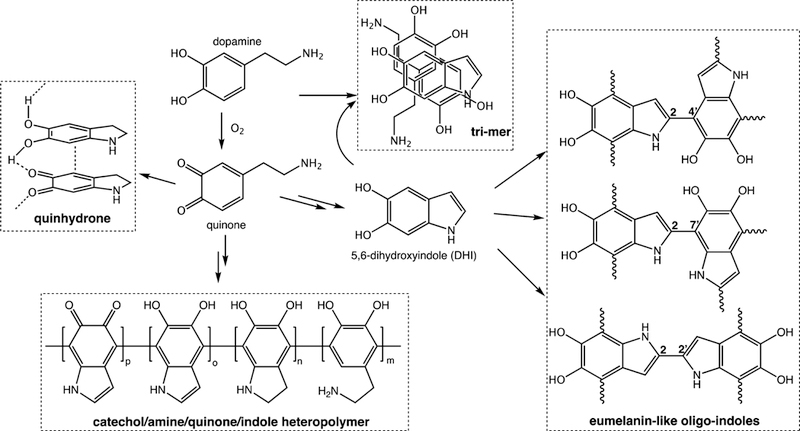
Current theories of polydopamine structure and formation. Auto-oxidation of dopamine leads to the formation of dopamine-quinone and 5,6-dihydroxyindole. Proposed mechanisms for polydopamine formation range from noncovalent self-assembly of subunits to form quinhydrone or trimer assemblies, and covalent coupling of subunits to yield a catecholamine/quinone/indole heteropolymer or eumelanin-like oligo-indoles. Adapted with permission from refs 13, 15, and 20. Copyright 2013 American Chemical Society, 2014 American Chemical Society, and 2012 Wiley-VCH.
Figure 3.
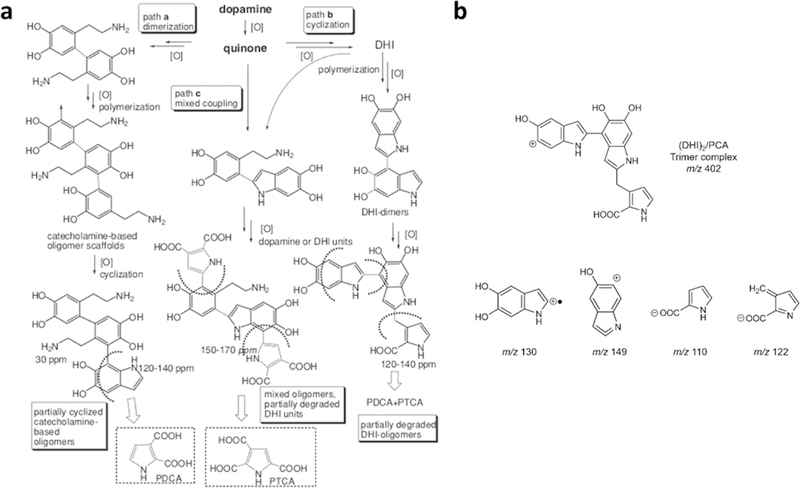
Pathway of pyrrolecarboxylic acid-involved PD formation. (a) Analysis of oxidative degradation products suggests PD formation pathways. Pyrrole-2,3-dicarboxylic acid (PDCA) is originated from partially cyclized catecholamine oligomers. Pyrrole-2,3,5-tricarboxylic acid (PTCA) is an outcome of DHI unit degradation. Reprinted with ref 17. Copyright 2013 Wiley-VCH. (b) DHI2/PCA trimer complex as a building block of PD. Reprinted with permission from ref 18. Copyright 2014 American Chemical Society.
3. PD IN COMPARISON TO OTHER COATING METHODS
The unique ability of PD to be deposited as a conformal thin film onto virtually all types, shapes and sizes of organic and inorganic surfaces through a simple dip coating process distinguishes it from other approaches to surface modification. Before the discovery of PD coatings, the three dominant methods in surface modification chemistry were self-assembled monolayer (SAM),21–23 layer-by-layer (LbL) assembly,24,25 and plasma treatment.26–28 In SAMs, end-functionalized alkanethiol molecules form ordered monolayers on noble metal surfaces through highly specific metal–thiolate bonds22 and therefore require matching surface–adsorbate chemistries. On the other hand, gas-phase plasma surface chemistry modifications are only transient because the modified surface properties change with time.
It is worthwhile to briefly compare PD and LbL coating methods (Figure 1), as both methods are arguably the most versatile and rely on adsorption of coating components at solid–liquid interfaces. Despite some similarities, there remain some important differences between PD and LbL coatings in terms of mode-of-action, properties, and substrate versatility. First, PD in its simplest form is a synthesis-free method in which the coating can be built in one step without the need to procure or synthesize sophisticated polymers or other coating components (ad-layer components notwithstanding). In contrast, polymers used in LbL methods are often synthesized specifically with a view toward LbL use or to provide a new function, and the deposition processes often involve many coating cycles. Second, formation of PD coatings involves in situ polymerization starting from its “monomer” dopamine, which is covalently/noncovalently polymerized at later stages (see above). Therefore, PD coatings are ideally suited for coating of 3D porous materials by infusion and in situ polymerization due to the low molecular weight of the dopamine building block. In contrast, polymers used in LbL assembly are typically high molecular weight and therefore will diffuse rather slowly into 3D substrates with small pores. Third, thickness of layers formed by LbL assembly can be easily controlled from a few nanometers to several micrometers by varying the number of deposition cycles, which in some cases can be hundreds of cycles. However, PD deposition is a kinetic process with a saturation limit of typically less than 50 nm due to dopamine depletion from the coating solution (it is possible to deposit additional PD layers onto a pre-existing PD layer). Fourth, the PD layer exhibits intrinsic chemical reactivity originating from the presence of catecholquinone moieties and radical species to which molecules with nucleophilic groups such as amine- (R-NH2) and thiolate- (R-S−) spontaneously react with PD (details are explained in section 5.2).29,30 Also, the PD coating is redox active, allowing electroless metallization and on surface synthesis of metal nanoparticles.31–33 In contrast, polymers specially prepared to provide such functions are needed for LbL coatings.
Although the range of substrates amenable to PD and LbL methods have substantial overlap, it is generally accepted that PD is a more suitable method to modify a broader array of surfaces, including materials that are normally difficult to coat (e.g., low surface energy solids). For example, without adaptation of the basic approach, PD can easily functionalize most metal oxides and noble metals as well as low energy surfaces such as polytetrafluoroethylene, polydimethylsiloxane, polystyrene, poly(lactic-co-glycolic) acid, polycarbonate, poly-(caprolactone), graphene, carbon nanotubes. A more compre-hensive list of materials functionalized by PD is summarized in Table 1. Notably, even extremely low energy surfaces including superhydrophobic/superomniphobic surfaces34 can be modi-fied with PD. Synergistic salt displacement at solid and liquid interfaces by catechol and amine groups11 is one of important mechanisms why PD exhibits coating capability to such a broad spectrum of materials surfaces listed in Table 1. Also, PD layers utilizes a variety of multiple binding mechanisms such as catechol-metal coordinations, electrostatic interactions, π−π interactions, hydrogen bonds, and covalent reactions (e.g., catechol-NH-R/catechol-S-R) depending upon chemistry of materials surfaces.16,35–40 However, influences of solid substrates on PD thickness and homogeneity of coatings require further studies. In contrast to the LbL method, the PD method requires no significant surface preparation and aggressive cleaning of substrates prior to coating deposition. Finally, it should be emphasized that the PD and LbL coating methods in some cases may be complementary toolkits. For example, catechol or catecholamine moieties which are the building blocks of PD, have been chemically tethered or end-functionalized to polymers for use in LbL. Stability and substrate versatility are enhanced when catechol-conjugated polymers are used in LbL depositions.6
Table 1.
List of Substrates Successfully Coated with PD
| materials (substrates) | substrate form | ref |
|---|---|---|
| polystyrene (PS) | 1 | |
| nanofiber | 41 | |
| polyethylene (PE) | 1 | |
| membrane | 42 | |
| polypropylene (PP) | 43, 44 | |
| nanofiber | 45 | |
| Polycarbonate (PC) | 1, 46 | |
| polyethylene terephthalate (PET) | 1, 47 | |
| PET/Ag hybrid fiber | 48 | |
| polyester | 44 | |
| poly(dimethylsiloxane) (PDMS) | 1, 47 | |
| PET/Ag hybrid fiber | 48 | |
| polyester | 44 | |
| poly(dimethylsiloxane) (PDMS) | 1, 49–53 | |
| polytetrafluoroethylene (PTFE, Teflon) |
54, 55 | |
| microtube | 56 | |
| poly(ether sulfone) | membrane | 57 |
| polyvinyl alcohol (PVA) | nanofiber | 58 |
| PVA/polyacrylic acid (PAA) | nanofiber | 59 |
| poly(vinyldiene fluoride) | nanofiber | 60 |
| poly(vinylidenefluoride) (PVDF) | membrane | 61 |
| polyether ether ketone (PEEK) | 1, 62 | |
| membrane | 63 | |
| polyurethane (PU) | 1 | |
| sponge/foam | 64, 65 | |
| poly(lactic-co-glycolic acid) (PLGA) | nanoparticle | 66 |
| poly(caprolactone) (PCL) | fiber | 67 |
| particle | 68 | |
| scaffold | 69, 70 | |
| polyimide (PI) | 55 | |
| cellulose | membrane | 71 |
| filter paper | 72 | |
| paper | 73 | |
| silk | fiber | 74 |
| nylon | membrane | 57 |
| graphene | 54 | |
| graphene oxide (GO) | 75, 76 | |
| carbon nanotube (CNT) | 77, 78 | |
| diamond | 79 | |
| diamond-like carbon | 80 | |
| SiO2 | 1, 81 | |
| nanoparticle | 82 | |
| membrane | 83 | |
| porous scaffold | 84 | |
| Si3N4 | 1 | |
| glass | 1, 85 | |
| bead | 86 | |
| tetraethyl orthosilicate | nanofiber (sol–gel) | 87 |
| clay | 88 | |
| quartz | 1, 89 | |
| fertilizer | 90 | |
| mica | 55 | |
| hydroxyapatite | 1 | |
| crystallization | 91, 92 | |
| calcium phosphate | cement | 93 |
| calcium carbonate | powder | 94 |
| TiO2 | 1, 95, 96 | |
| nanoparticle | 97 | |
| nanowire | 98, 99 | |
| nanotube | 100, 101 | |
| ZrO2 | nanocomposite | 102 |
| Nb2O3 | 1 | |
| Fe | 55 | |
| Fe3O4 | nanoparticle | 103 |
| Pd | 1 | |
| nanoparticle | 104, 105 | |
| Pt | 1, 106, 107 | |
| Cu | 1, 108, 109 | |
| Ag | 1 | |
| nanostructure | 110 | |
| Au | 1, 54, 111 | |
| ZnO2 | nanorod | 112 |
| Al | 44 | |
| Al2O3 | nanoparticle | 113 |
| Al(OH)3 | particle | 114 |
| GaAs | 1 | |
| In2O3/SnO2 (Indium Tin Oxide, ITO) | 115 | |
| stainless steel | 1, 116 | |
| porous | 117 | |
| CdS/CdSe | quantum dot | 118 |
| virus, E.coli | 119, 120 | |
| superhydrophobic surface | 121 | |
| water surface | air/water interface | 122–124 |
| PD capsule | 125–130 |
The two unique properties of a polydopamine coating, substrate flexibility combined with a variety of ad-layer properties by covalent/coordinate/noncovalent linkages with other molecules, allow virtually unlimited access to functional properties. The list of applications of PD is rapidly growing and seems to be limited only by the creativity of researchers using the method. A partial listing of PD applications demonstrated in just last 10 years include surfaces for stem and differentiated cell culture,85,131–134 cell patterning,135–137 microfluidics,138,139 antimicrobial surfaces,46,140,141 scaffold functionalization for tissue engineering,142–144 bioimaging,145,146 theragnostic,31,147,148 photothermal therapy,149,150 PLGA (nano)-particles66,151 and capsules for drug delivery,125,129,130 hydroxyapatite91,92,152 or calcium carbonate surface mineralization,94,153,154 artificial spores,155,156 immobilization of photocatalysts and/or interplay between PD and photocatalysts,157–159 Li-ion battery membranes,42,160–162 Li–air battery electrolytes,163,164 Li–sulfur battery cathode materials,165 Zn–air cathode materials,166 oil/water separation,167–169 atomic transfer radical polymerization,77,170,171 water detoxification,86,172 carbonization,173–175 membrane separation technologies,176–178 organocatalysts,179,180 and numerous others. It is certain that the scope of PD research and utilization will expand further in the years to come.
4. PD COATING METHODS
Many of the advancements that have occurred during the first decade of PD research relate to modifications of the coating recipe that was originally published in 2007. In this section we describe some of these key developments with an emphasis on choice of buffer and solvent, the use of chemical oxidants, utilization of external stimuli, and a description of a one-pot method that reduces the number of steps needed for functional coatings.
4.1. Choice of Buffer and Solvent.
The original method of PD coating employed 2 mg/mL of dopamine hydrochloride (synonyms: 3-hydroxytyramine hydrochloride; 2-(3,4-dihydroxyphenyl)ethylamine) dissolved in Tris buffer, pH 8.0–8.5.1 Dopamine concentration is an important tool in controlling deposition kinetics and roughness of surfaces. Recently, dopamine with a low concentration (<0.5 mg/mL) was used to functionalize nanostructures (i.e., particle,147,181 tube,182 and fiber 68) because low concentration of dopamine could effectively reduce PD particle formation by self-polymerization and interparticle aggregation, and such aggregates inevitably increase roughness of PD coatings.181 For instance, Au nanoparticles coated with dopamine (0.1 mg/ mL) are stable as monodisperse nanoparticles, but small aggregates of particles are observed at 0.4 mg/mL dopamine concentration.181 Likewise, a convenient method to minimize surface roughness is to decrease substrate immersion time to about 1–3 h in Tris buffer,183 and the coating process can be repeated twice or three times if desired to control thickness.155 Vincent Ball and co-workers clearly reveal concentration effects on the kinetics of PD deposition, thickness, roughness, and surface energy.184 The maximal film thickness is increased linearly with dopamine concentrations from 0.1 to 5 mg/mL (i.e., 20 nm for 0.5 mg/mL, 25 nm for 1 mg/mL, and 25–40 nm for 2 mg/mL). In contrast, the thickness of PD varies at high concentrations of dopamine (3 and 5 mg/mL). In general, the thick PD films are rough compared with roughness of thin ones, and the surface energy of PD is independent with dopamine concentrations. In addition to concentrations, the maximal film thickness increases from pH 5 to 8.5 of dopamine solutions. However, during PD formation Tris17 and unreacted dopamine20 are unavoidably incorporated into the coating, which may alter the physicochemical properties of the coating and alter further chemical reactions. To avoid Tris buffer incorporation,185–187 amine- free organic buffers (e.g., bicine) or inorganic (e.g., phosphate) buffers can be used instead, but the codeposition of PD nanoaggregates can be more problem-atic than when Tris buffer is used.
Choice of solvents is critical in some cases. While the vast majority of reports on PD describe the use of aqueous solvents, solvents with low surface tension such as methanol and ethanol can be used to modify hydrophobic and/or porous materials such as polyethylene (PE) membranes used in Li-ion batteries.42 As we discuss further below, the use of organic solvents may be advantageous in other ways, for example enhancing the drying rate of treated substrates through fast evaporation, preventing degradation of hydrolyzable substrates, coimmobilization of water insoluble molecules, etc.
4.2. Oxidation: Auto- Versus Chemical Oxidation.
Dissolved oxygen is essential for traditional PD formation via auto-oxidation at alkaline pH as was qualitatively shown in the original PD coating report.1 Later, direct evidence regarding the importance of dissolved oxygen in aqueous solutions was demonstrated for the study of microwave accelerated PD coating (see section 4.3 for details).188 Water-soluble, inorganic chemical oxidants such as sodium periodate (NaIO4), ammonium per(oxodi)sulfate ((NH4)2S2O8), potassium per-manganate (KMnO4), copper sulfate (CuSO4), and Fe(III) have been widely used.12,57,189 Vincent Ball and co-workers reported that superhydrophilic-superoleophobic PD coatings are achieved by the oxidants control.189 In the presence of (NH4)2S2O8 or CuSO4 (above 10 mM oxidant concentrations), the PD formation results in heterogeneous coating on surfaces. However, the PD coating generated by NaIO4 (the concentration lower than 30 mM) provides homogeneous surfaces. Furthermore, the thickness of the PD films in the presence of NaIO4 was 65 nm after 1 h incubation, which was far higher than that of CuSO4–catalyzed PD films (43 nm). When CuSO4 was used, copper ions (Cu2+) were also found in the PD coating, which is due to the chelation properties of the catechol moieties. Over the last several years, use of NaIO4 in PD coating has become more widespread. Through optimiza-tion of pH, concentration of dopamine, and stoichiometric ratio of [NaIO4]/[dopamine], ultrafast and thick (>50 nm) PD coatings were obtained at room temperature.12 Furthermore, hydrophilic coatings were obtained in the presence of large excess of NaIO4 due to the formation of carboxylic acid groups on surfaces.
4.3. Ultraviolet and Microwave Enhanced PD Coatings.
The generation of radical species by providing external energy such as ultraviolet (UV) light can also trigger PD formation as was shown by Levkin and co-workers.190,191 The main advantages of using light are to control onset and termination of PD coatings, and to deposit patterns of PD on substrates. Furthermore, this light-induced method is effective from slightly acidic to basic pH ranges. Thus, when one utilizes acidic conditions and UV light, initiation and termination of PD deposition can easily be controlled. The use of UV irradiation in conjunction with chemical derivatives of dopamine provides an additional level of control. A. del Campo and co-workers used nitro-dopamine derivatives, which exhibit photocleavable properties with a leaving group of ortho-nitrophenyl ethyl moiety.192 This chemistry provides new ways of controlling surface properties by detaching molecules that are tethered from surfaces.
Microwave irradiation of dopamine solution is another useful method to accelerate PD coating formation.188 To achieve a PD coating thickness of 18 nm by the conventional alkaline PD coating method requires several hours, whereas microwave PD coating method takes only 15 min. The fast PD coating kinetics in the microwave technique is claimed to be due to enhancement of oxygen tension in the coating solution because of vibration-involved heating mechanisms. Interestingly, dis-solved oxygen is ultimately removed in microwave heat-induced boiling, which was used to clearly demonstrate the importance of oxygen in PD formation.123
4.4. One-Pot PD Coatings.
For surface tethering of molecules containing amine (−NH2) and thiol (−SH) groups to PD, the “one pot” method offers a simplified approach to forming PD coatings.143 In the one-pot method, dopamine and polymer/biomolecule deposit simultaneously from solution, reducing the number of coating steps (Figure 4). An additional advantage of one-pot PD coating is that PD aggregation is largely suppressed because of dopamine/target molecule association. Representative one-pot coating studies include a comparative and quantitative analysis of protein immobilization for the conventional two-step approach vs the one-pot method,193 a tertiary amine coating for nanoscale silicifica-tion,160 and creation of special wettability properties such as superomniphilic and omniphobic surface.34 The initial study on one pot PD employed macromolecules included poly(vinyl alcohol), hyaluronic acid, dextran, and chitosan that have strong interactions with dopamine/PD,143 although future studies will likely reveal numerous other polymers that can be employed with this method.
Figure 4.

One-pot PD coatings. The one-pot method for preparing PD coatings utilizes a precursor solution containing a mixture of dopamine and molecules to be coimmobilized with PD. The method can use either the auto-oxidation approach at basic pH solution or chemical oxidants to produce functional substrates.
In general, we recommend use of a few milligrams of dopamine per mL of Tris pH 8.5 for 5–6 h in general purposes of solid substrate modifications. Overnight PD coating should be avoided for obtaining smooth surfaces because of generation of microsized PD aggregates. Also, a water/ethanol cosolvent recommends for PD coatings on hydrophobic surfaces or porous membranes because ethanol’s low surface tension. For ad-layer formations with general purposes of molecular immobilizations, one-pot PD coating should be considered with the conventional two-step PD coatings to obtain high density molecular immobilizations.
5. TAILORING PD FUNCTIONALITY THROUGH BUILDING BLOCK DIVERSITY
One of the defining features of PD is undoubtedly the rich array of possibilities for tailoring surface properties for various applications. Here we review the two main approaches to functional versatility of PD coatings: the use of dopamine chemical derivatives in the primary deposition, and secondary ad-layer formation on an underlying PD “primer”.
5.1. Chemical Derivatives of Dopamine.
As shown in Figure 5, dopamine exhibits four possible sites (amine, alkyl and aromatic) for chemical derivatization (leaving aside O-substituted dopamine derivatives because they eliminate the catechol).194 Perhaps the most obvious example of a dopamine derivative is 3,4-dihydroxy-L-phenylalanine (DOPA) (R1 = CO2H), a biologically important free amino acid that is an intermediate in melanin formation and is decarboxylated to form dopamine in vivo. Interestingly, oxidative polymerization of DOPA to form polyDOPA coatings using a PD-like method is generally less successful than PD, possibly due to electrostatic repulsive interactions between neighboring carboxylic acid groups that may disrupt polymerization and/or aggregation of oligomeric polyDOPA subunits during coating formation. Nevertheless, polyDOPA coatings were successfully applied to PE, PVDF, and PTFE membrane substrates and showed reduced static water-contact angles.195 Subsequently, it was shown that most limitations of polyDOPA coating formation on noble metals, polymers, and oxides could be overcome through the use of high ionic strength deposition conditions.196
Figure 5.
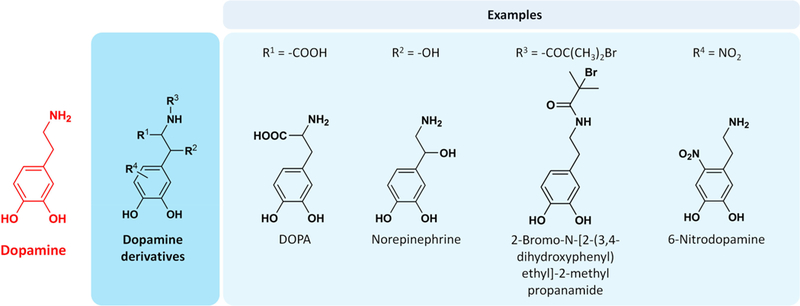
Chemical structures of dopamine derivatives. Opportunities exist for chemical substitutions of dopamine at alkyl (R1, R2), amino (R3), and aromatic (R4) sites, offering the potential for tailoring the formation and physicochemical properties of PD coatings.
Norepinephrine (R2 = OH) has been widely used for coating formation, with the unique aspect being that conformal poly(norepinephrine) coatings are ultrasmooth, with a uniform thickness ∼20 nm.197 In contrast, under similar deposition conditions, the thickness of a PD coating ranges from 30–50 nm with a number of PD nanoparticles present. Norepinephrine-derived coatings have been formed on various materials including Si/SiO2, glass, polystyrene, PDMS, and PTFE,198–201 and on substrates of various morphologies such as microchips,202 nanotubes,203 nanoparticles,204 nanofibers,205 and sponges.206 As an example of a benefit achieved through the use of chemical derivatives of dopamine, the additional hydroxyl group in norepinephrine allows for ring opening polymerization of lactone monomers from the surface in a two-step modification, resulting in grafted polyester.201
Substitutions at the primary amine (R3 position) have been the most widely used functional dopamine derivatives. Conjugations to the R3 primary amine intrinsically prevents indole formation, affecting the PD formation mechanism,194,207,208 but surface modification may occur by catechol-to-catechol conjugation pathway. One functional group for modification at the R3 position is 2-bromoisobutyryl bromide for initiating atom transfer radical polymerization (ATRP).209 In addition, pyrrole, pyridine, and methacrylate as R3 substituents have been used in surface functionalizations.52,53,81,96,100,210–212
A representative R4 substituted dopamine is 6-nitrodop-amine, which was mentioned previously as being photo-cleavable and used in the preparation of light-responsive smart surfaces.192 This compound is primarily used to functionalize surfaces of inorganic nanoparticles such as iron oxide and titania.213–217 6-Nitrodopamine is rather oxidation resistant compared to dopamine due to the presence of the electro-negative nitro groups. This potentially results in maintaining strong binding affinity to nanoparticles.215 Another R4-substituted dopamine is 5-hydroxydopamine218 (we describe the use of amine-free gallol-derived compounds below in Section 6.2).
5.2. Secondary (ad-layer) Functionality.
The second approach for tailoring functionality is to utilize the intrinsic chemical reactivity of the surface of PD to deposit an ad-layer. These secondary reactions may exploit noncovalent binding interactions, or covalent reactions with molecules containing nucleophilic or other reactive groups. Nearly all proteins, peptides, end-functionalized oligonucleotides, and a large population of small molecules are amenable to this approach. Other molecules, including synthetic polymers, can be modified or synthesized with functional groups enabling reactions with PD. Reaction conditions for ad-layer grafting are generally the same as PD formation (buffers with basic pH).
Early studies in this area involved thiol- or amine-terminated polymers grafted onto PD-coated surfaces through thiolcatechol or amine-catechol adducts by Michael-type addition reactions and/or Schiff-base formations (Figure 6),1,38–40,46,77,176,219–225 with subsequent expansion to bio-molecules (peptides, protein enzymes and oligonucleotides).226,227 Examples of proteins and peptides successfully immobilized onto PD are numerous and include albumin,228,229 lysozyme,230 bone morphogenic protein-2,142,231–233 trypsin,234 alkaline phosphatase,193 antifreeze proteins,235 collagen,236 collagenase,230 aquaporin,237 vitronectin (VN2)-derived peptides,238 Arg-Gly-Asp (RGD) peptides,142 epidermal growth factor,239 and many others. Ad-layer immobilization of (strept)avidin provides even more versatility through strong avidin–biotin reversible interactions.229
Figure 6.

Ad-layer functionalization with thiol- and amine-containing biomolecules on PD-coated substrates. Adapted with permission from refs 38, 39, and 40. Copyright 1999 American Chemical Society, 1987 American Society for Biochemistry and Molecular Biololgy, and 2006 American Chemical Society.
In the case of biomolecules, the promiscuous reactivity of catechols and quinones at the PD surface is normally thought to make these reactions poorly specific at best, however a recent report of a chemoselective reaction between catechol and the N-terminal amine of proteins and peptides suggests opportunities for chemospecific conjugations to PD.240 An interesting recent publication described the surprising orientation-specific immobilization of antifreeze proteins onto PD surfaces.235 Apparently the amino acid composition exposed on the ice binding and nonice binding faces of the protein allowed for selective immobilization in such a way as to orient the protein with the nonice binding oriented away from the surface, reducing ice formation at the modified surface.235
PD is also a good platform for surface tethering and release of small molecule drugs and therapeutic RNAs66,130,241,242 through electrostatic interactions, hydrogen bonds, π–π stacking, cation–π interactions.103,141,243–245 For instance, the amount of bound siRNA on PD substrates is larger than that of unmodified substrates and shows sustained release profiles over at least 7 days.242 In another study, siRNAs were successfully loaded onto surfaces of manganese oxide nanoparticles for delivery to target cells.246
6. FUTURE OPPORTUNITIES AND CHALLENGES IN PD COATINGS
With a view toward guiding further development of PD and related coating technologies in the coming years, in the section below we describe several areas that we feel are deserving of more attention by researchers.
6.1. Improving the Mechanical Properties of PD.
Considering its chemical resemblance to mussel adhesive proteins and its ability to form conformal coatings on a multitude of solid surfaces, one would predict that PD coatings should function well as mechanically robust coatings. An application of PD in which mechanical properties are important is in fiber and particulate composites, where dopamine has been used to enhance wetting and adhesion between phases (recently reviewed by Ball247). As an example of PD applications in composites, the mechanical properties of bioceramics composed of hydroxyapatite and gelatin modified with silane (HAp-Gemosil) was improved by incorporation of PD.248 The compressive strength of PD-incorporated HAp-Gemosil (HAp-Gemosilamine) at is approximately 100 MPa, higher than that of HAp-Gemosil (∼80 MPa). Several other recent reports have been published showing the ability of PD to enhance mechanical properties of composites.249,250
The application of PD to mechanical composites notwith-standing, the anecdotal experience of our laboratories, and that of several others, is that PD coatings in their current form do not perform particularly well as mechanical adhesives or in a context that requires resistance to delamination or abrasion. This is particularly true on flat surfaces and when PD is applied to low-surface-energy materials. Widespread incorporation of PD into products or components of devices may be further hindered unless improvements in PD mechanical properties can be realized.
Achieving these goals will likely require better under-standings of chemical composition and physical properties of PD coatings, which might lead to develop next-generation PD coating with improved mechanical properties. Surprisingly, few investigations of mechanical properties of PD have been performed in the past.123,245,251–254 The elastic modulus of PD has been measured to be in the GPa range in the dry state,123, 251 suggesting that PD is quite rigid. The importance of primary amines in PD adhesion was measured using the surface forces apparatus, suggesting that a role for surface salt displacement as well as π–π and cation–π interactions in adhesion.245 Moreover, chemical cross-linking of amines in the coating offers a mechanism for increasing PD modulus.251 You and co-workers performed an investigation of lap shear mechanical adhesion of PD films, characterizing the mechanical adhesion of PD while at the same time illustrating how chemical analogs affect adhesion strength.252 In addition to providing insight into PD polymerization mechanisms, an interesting outcome of this study was that extension of the alkyl chain linker between catechol and amine did not affect adhesion strength. New methods of measuring the adhesion strength of PD films to substrates may prove useful in screening new PD formulations for improved adhesion to substrates.254
6.2. Expanding the Chemical and Biological Diversity of PD-like Coatings.
A recent development in the field involves the use of nitrogen-free phenols and polyphenols as coating precursors. Catechol-containing molecules that form coatings include hydrocaffeic acid,255,256 alkylcatechol, 257–262 and thiol-terminated catecholic monomers.263 Gallol (2,3,4-triyhydroxyphenyl) based molecules such as pyrogallol and tannic acid are also emerging as useful precursors for coating formation. Surface coatings based on metal phenolic networks (MPN) are well-known examples.264,265 In the case of MPN coatings based on tannic acid, addition of transition metals such as Fe(III), Cr(III), V(III), Zn(II), or Cd(II)266 to tannic acid exhibits material-independent surface coatings by metal coordination network formation. Because of the fast metal–ligand coordination kinetics, the coating forms rapidly, which is an advantage of MPN. In contrast, PD coating requires several hours unless special fast coating processes are used. MPN coatings formed in this manner can provide support for surface PEGylation.267 Other important applications of MPN coatings formed in this manner can provide support for surface PEGylation.267 Other important applications of MPN coatings include protective coating for cell attachment,268,269 tooth desensitization,270 heavy metal removal,271 and protein immobilization.272,273
Nitrogen-free catechol and gallol-containing precursors are capable of forming coatings in the absence of metals via auto-oxidation in a manner that is similar to PD formation. The observation of spontaneous adsorption of phenolic compounds from beverages (e.g., tea, wine) rich in plant polyphenols onto surfaces led to a significant expansion of potential building blocks for spontaneous coating formation.274 This approach was first demonstrated with pyrogallol, tannic acid, epigalloca-techin gallate (EGCG), epicatechin gallate (ECG), and epigallocatechin (EGC), which form nanoscale coatings in a kinetically controlled auto-oxidation process that resembles that of PD formation, and then later expanded to at least 15 natural compounds that are known to form coatings by auto-oxidation.275 Many of the inherent advantages of PD, namely simplicity, substrate versatility and multifunctionality, are shared by plant polyphenol derived coatings. Some of these coatings have the advantage of being colorless, unlike PD coatings.274 Furthermore, some of the biological properties of plant polyphenols such as antibacterial activity, antioxidant activity, and other properties are conferred into the coatings. Already there is a rapidly growing body of reports employing nitrogen free polyphenol coatings for a variety of purpo-ses.267,273,276–304 An important consequence of the enormous biological diversity of plant polyphenols is that hundreds if not thousands of natural plant polyphenols can now be considered as coating precursors, with a broad range of biological and chemical properties. We anticipate that this large biological toolbox of coating precursors will be increasingly exploited in the coming years, leading to unforeseen coating properties and applications.
7. CONCLUSIONS
In its first ten years, PD has proven to be one of the most powerful and widespread surface coating methods due to its material-independent coating ability, the simplicity of the coating deposition process, and the unique and broad ranging capabilities for ad-layer formation leading to numerous practical uses. The thickness and PD coating properties can be tailored by parameters such as coating time, pH, solvent, dopamine concentration, and chemical additives including metal ions. In addition, target molecules such as natural/synthetic polymers, proteins, peptides, oligonucleotides, and numerous small molecules including drugs) can be readily immobilized by ad-layer formation or through one-pot coating methods. The use of chemical analogs of dopamine promises to further expand the properties and applications of PD coatings. Important challenges facing the field in the future include better understanding of PD formation mechanisms and elucidation of the chemical structure of PD, enhancement of mechanical robustness of PD, and extension of the general PD coating approach to more chemically diverse building blocks such as the nitrogen-free catechol and gallol compounds found in plant tissues. With advancements in these areas, it is likely that the family of PD and related coatings will be widely implemented in biomedical, energy, consumer products, agricultural, military, and other sectors.
ACKNOWLEDGMENTS
The authors acknowledge support by grants from the National Research Foundation of South Korea (Midcareer Scientist Grant, 2015021564; Nature-inspired Technology, NRF-2017M3C1B7014223, H.L.) and the US National Institutes of Health (R37 DE014193 and R01EB005772, P.B.M.).
Footnotes
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Lee H; Dellatore SM; Miller WM; Messersmith PB Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Waite JH; Tanzer ML Polyphenolic Substance of Mytilus edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Science 1981, 212, 1038–1040. [DOI] [PubMed] [Google Scholar]
- (3).Waite JH; Qin X Polyphosphoprotein from the Adhesive Pads of Mytilus Edulis. Biochemistry 2001, 40, 2887–2893. [DOI] [PubMed] [Google Scholar]
- (4).Silverman HG; Roberto FF Understanding Marine Mussel Adhesion. Mar. Biotechnol 2007, 9, 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Holowka EP; Deming TJ Synthesis and Crosslinking of L-DOPA Containing Polypeptide Vesicles. Macromol. Biosci 2010, 10, 496–502. [DOI] [PubMed] [Google Scholar]
- (6).Lee H; Lee Y; Statz AR; Rho J; Park TG; Messersmith PB Substrate-Independent Layer-by-Layer Assembly by Using Mussel-Adhesive-Inspired Polymers. Adv. Mater 2008, 20, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kim E; Song IT; Lee S; Kim JS; Lee H; Jang JH Drawing Sticky Adeno-Associated Viruses on Surfaces for Spatially Patterned Gene Expression. Angew. Chem., Int. Ed 2012, 51, 5598–5601. [DOI] [PubMed] [Google Scholar]
- (8).Ryu JH; Hong S; Lee H Bio-Inspired Adhesive Catechol-Conjugated Chitosan for Biomedical Applications: A Mini Review. Acta Biomater 2015, 27, 101–115. [DOI] [PubMed] [Google Scholar]
- (9).Shin M; Park SG; Oh BC; Kim K; Jo S; Lee MS; Oh SS; Hong SH; Shin EC; Kim KS; Kang SW; Lee H Complete Prevention of Blood Loss with Self-Sealing Haemostatic Needles. Nat. Mater 2017, 16, 147–152. [DOI] [PubMed] [Google Scholar]
- (10).Statz AR; Meagher RJ; Barron AE; Messersmith PB New Peptidomimetic Polymers for Antifouling Surfaces. J. Am. Chem. Soc 2005, 127, 7972–7973. [DOI] [PubMed] [Google Scholar]
- (11).Maier GP; Rapp MV; Waite JH; Israelachvili JN; Butler A Adaptive Synergy between Catechol and Lysine Promotes Wet Adhesion by Surface Salt Displacement. Science 2015, 349, 628–632. [DOI] [PubMed] [Google Scholar]
- (12).Hong SH; Hong S; Ryou M-H; Choi JW; Kang SM; Lee H Sprayable Ultrafast Polydopamine Surface Modifications. Adv. Mater. Interfaces 2016, 3, 1500857. [Google Scholar]
- (13).Liebscher J; Mrowczynski R; Scheidt HA; Filip C; Hadade ND; Turcu R; Bende A; Beck S Structure of Polydopamine: A Never-Ending Story? Langmuir 2013, 29, 10539–10548. [DOI] [PubMed] [Google Scholar]
- (14).Liu Y; Ai K; Lu L Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev 2014, 114, 5057–5115. [DOI] [PubMed] [Google Scholar]
- (15).d’Ischia M; Napolitano A; Ball V; Chen C-T; Buehler MJ Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res 2014, 47, 3541–3550. [DOI] [PubMed] [Google Scholar]
- (16).Yang J; Cohen Stuart MA; Kamperman M Jack of All Trades: Versatile Catechol Crosslinking Mechanisms. Chem. Soc. Rev 2014, 43, 8271–8298. [DOI] [PubMed] [Google Scholar]
- (17).Della Vecchia NF; Avolio R; Alfe M; Errico ME; Napolitano A; d’Ischia M Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater 2013, 23, 1331–1340. [Google Scholar]
- (18).Ding Y; Weng L-T; Yang M; Yang Z; Lu X; Huang N; Leng Y Insights into the Aggregation/Deposition and Structure of a Polydopamine Film. Langmuir 2014, 30, 12258–12269. [DOI] [PubMed] [Google Scholar]
- (19).Yu X; Fan H; Wang L; Jin Z Formation of Polydopamine Nanofibers with the Aid of Folic Acid. Angew. Chem., Int. Ed 2014, 53, 12600–12604. [DOI] [PubMed] [Google Scholar]
- (20).Hong S; Na YS; Choi S; Song IT; Kim WY; Lee H Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater 2012, 22, 4711–4717. [Google Scholar]
- (21).Nuzzo RG; Allara DL Adsorption of Bifunctional Organic Disulfides on Gold Surfaces. J. Am. Chem. Soc 1983, 105, 4481–4483. [Google Scholar]
- (22).Love JC; Estroff LA; Kriebel JK; Nuzzo RG; Whitesides GM Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev 2005, 105, 1103–1169. [DOI] [PubMed] [Google Scholar]
- (23).Ulman A Formation and Structure of Self-Assembled Monolayers. Chem. Rev 1996, 96, 1533–1554. [DOI] [PubMed] [Google Scholar]
- (24).Kirkland JJ Porous Thin-Layer Modified Glass Bead Supports for Gas Liquid Chromatography. Anal. Chem 1965, 37, 1458–1461. [Google Scholar]
- (25).Iler RK Multilayers of Colloidal Particles. J. Colloid Interface Sci 1966, 21, 569–594. [Google Scholar]
- (26).Liston EM; Martinu L; Wertheimer MR Plasma Surface Modification of Polymers for Improved Adhesion: A Critical Review. J. Adhes. Sci. Technol 1993, 7, 1091–1127. [Google Scholar]
- (27).Wu CC; Wu CI; Sturm JC; Kahn A Surface Modification of Indium Tin Oxide by Plasma Treatment: An Effective Method to Improve the Efficiency, Brightness, and Reliability of Organic Light Emitting Devices. Appl. Phys. Lett 1997, 70, 1348–1350. [Google Scholar]
- (28).Chu PK; Chen JY; Wang LP; Huang N Plasma-Surface Modification of Biomaterials. Mater. Sci. Eng., R 2002, 36, 143–206. [Google Scholar]
- (29).Mrowczynski R; Coy LE; Scheibe B; Czechowski T; Augustyniak-Jablokow M; Jurga S; Tadyszak K Electron Para-magnetic Resonance Imaging and Spectroscopy of Polydopamine Radicals. J. Phys. Chem. B 2015, 119, 10341–10347. [DOI] [PubMed] [Google Scholar]
- (30).Kim BG; Kim S; Lee H; Choi JW Wisdom from the Human Eye: A Synthetic Melanin Radical Scavenger for Improved Cycle Life of Li–O2 Battery. Chem. Mater 2014, 26, 4757–4764. [Google Scholar]
- (31).Black KC; Yi J; Rivera JG; Zelasko-Leon DC; Messersmith PB Polydopamine-Enabled Surface Functionalization of Gold Nanorods for Cancer Cell-Targeted Imaging and Photo-thermal Therapy. Nanomedicine 2013, 8, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hong S; Lee JS; Ryu J; Lee SH; Lee DY; Kim DP; Park CB; Lee H Bio-Inspired Strategy for On-Surface Synthesis of Silver Nanoparticles for Metal/Organic Hybrid Nanomaterials and LDI-MS Substrates. Nanotechnology 2011, 22, 494020. [DOI] [PubMed] [Google Scholar]
- (33).Hussain MA; Yang M; Lee TJ; Kim JW; Choi BG High Density Decoration of Noble Metal Nanoparticles on Polydop-amine-Functionalized Molybdenum Disulphide. J. Colloid Interface Sci 2015, 451, 216–220. [DOI] [PubMed] [Google Scholar]
- (34).You I; Lee TG; Nam YS; Lee H Fabrication of a Micro-Omnifluidic Device by Omniphilic/Omniphobic Patterning on Nanostructured Surfaces. ACS Nano 2014, 8, 9016–9024. [DOI] [PubMed] [Google Scholar]
- (35).Yu J; Kan Y; Rapp M; Danner E; Wei W; Das S; Miller DR; Chen Y; Waite JH; Israelachvili JN Adaptive Hydrophobic and Hydrophilic Interactions of Mussel Foot Proteins with Organic Thin Films. Proc. Natl. Acad. Sci. U. S. A 2013, 110, 15680–15685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mian SA; Yang LM; Saha LC; Ahmed E; Ajmal M; Ganz E A fundamental Understanding of Catechol and Water Adsorption on a Hydrophilic Silica Surface: Exploring the Underwater Adhesion Mechanism of Mussels on an Atomic Ccale. Langmuir 2014, 30, 6906–6914. [DOI] [PubMed] [Google Scholar]
- (37).Anderson TH; Yu J; Estrada A; Hammer MU; Waite JH; Israelachvili JN The Contribution of DOPA to Substrate-Peptide Adhesion and Internal Cohesion of Mussel-Inspired Synthetic Peptide Films. Adv. Funct. Mater 2010, 20, 4196–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Yu M; Hwang J; Deming TJ Role of L-3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc 1999, 121, 5825–5826. [Google Scholar]
- (39).Kalyanaraman B; Premovic PI; Sealy RC Semiquinone Anion Radicals from Addition of Amino Acids, Peptides, and Proteins to Quinones Derived from Oxidation of Catechols and Catechol-amines. An ESR Spin Stabilization Study. J. Biol. Chem 1987, 262, 11080–11087. [PubMed] [Google Scholar]
- (40).Liu B; Burdine L; Kodadek T Chemistry of Periodate-Mediated Cross-Linking of 3,4-Dihydroxylphenylalanine-Containing Molecules to Proteins. J. Am. Chem. Soc 2006, 128, 15228–15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yang H; Lan Y; Zhu W; Li W; Xu D; Cui J; Shen D; Li G Polydopamine-Coated Nanofibrous Mats as a Versatile Platform for Producing Porous Functional Membranes. J. Mater. Chem 2012, 22, 16994–17001. [Google Scholar]
- (42).Ryou MH; Lee YM; Park JK; Choi JW Mussel-Inspired Polydopamine-Treated Polyethylene Separators for High-Power Li-Ion Batteries. Adv. Mater 2011, 23, 3066–3070. [DOI] [PubMed] [Google Scholar]
- (43).Thakur VK; Vennerberg D; Kessler MR Green Aqueous Surface Modification of Polypropylene for Novel Polymer Nano-composites. ACS Appl. Mater. Interfaces 2014, 6, 9349–9356. [DOI] [PubMed] [Google Scholar]
- (44).You I; Chang Seo Y; Lee H Material-Independent Fabrication of Superhydrophobic Surfaces by Mussel-Inspired Polydopamine. RSC Adv 2014, 4, 10330–10333. [Google Scholar]
- (45).Ma S; Ye Q; Pei X; Wang D; Zhou F Antifouling on Gecko’s Feet Inspired Fibrillar Surfaces: Evolving from Land to Marine and from Liquid Repellency to Algae Resistance. Adv. Mater. Interfaces 2015, 2, 1500257. [Google Scholar]
- (46).Sileika TS; Kim HD; Maniak P; Messersmith PB Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [DOI] [PubMed] [Google Scholar]
- (47).Li P; Cai X; Wang D; Chen S; Yuan J; Li L; Shen J Hemocompatibility and Anti-Biofouling Property Improvement of Poly(ethylene Terephthalate) via Self-Polymerization of Dopamine and Covalent Graft of Zwitterionic Cysteine. Colloids Surf., B 2013, 110, 327–332. [DOI] [PubMed] [Google Scholar]
- (48).Wang W; Cheng W; Tian M; Zou H; Li L; Zhang L Preparation of PET/Ag Hybrid Fibers via a Biomimetic Surface Functionalization Method. Electrochim. Acta 2012, 79, 37–45. [Google Scholar]
- (49).Liang RP; Meng XY; Liu CM; Qiu JD PDMS Microchip Coated with Polydopamine/Gold Nanoparticles Hybrid for Efficient Electrophoresis Separation of Amino Acids. Electrophoresis 2011, 32, 3331–3340. [DOI] [PubMed] [Google Scholar]
- (50).Zhang W; Yang FK; Han Y; Gaikwad R; Leonenko Z; Zhao B Surface and Tribological Behaviors of the Bioinspired Polydopamine Thin Films under Dry and Wet Conditions. Biomacromolecules 2013, 14, 394–405. [DOI] [PubMed] [Google Scholar]
- (51).Kim M; Song KH; Doh J PDMS Bonding to a Bio-Friendly Photoresist via Self-Polymerized Poly(dopamine) Adhesive for Complex Protein Micropatterning inside Microfluidic Channels. Colloids Surf., B 2013, 112, 134–138. [DOI] [PubMed] [Google Scholar]
- (52).Wei Q; Yu B; Wang X; Zhou F Stratified Polymer Brushes from Microcontact Printing of Polydopamine Initiator on Polymer Brush Surfaces. Macromol. Rapid Commun 2014, 35, 1046–1054. [DOI] [PubMed] [Google Scholar]
- (53).Liu J; Ye Q; Yu B; Wang X; Zhou F Contact Printing a Biomimetic Catecholic Monolayer on a Variety of Surfaces and Derivation Reaction. Chem. Commun 2012, 48, 398–400. [DOI] [PubMed] [Google Scholar]
- (54).Kim BH; Lee DH; Kim JY; Shin DO; Jeong HY; Hong S; Yun JM; Koo CM; Lee H; Kim SO Mussel-Inspired Block Copolymer Lithography for Low Surface Energy Materials of Teflon, Graphene, and Gold. Adv. Mater 2011, 23, 5618–5622. [DOI] [PubMed] [Google Scholar]
- (55).Sheng W; Li B; Wang X; Dai B; Yu B; Jia X; Zhou F Brushing Up from ″Anywhere″ under Sunlight: A Universal Surface-Initiated Polymerization from Polydopamine-Coated Surfaces. Chem. Sci 2015, 6, 2068–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Zhang W; Zhou W; Chen Z Graphene/Polydopamine-Modified Polytetrafluoroethylene Microtube for the Sensitive Determination of Three Active Components in Fructus Psoraleae by Online Solid-Phase Microextraction with High-Performance Liquid Chromatography. J. Sep. Sci 2014, 37, 3110–3116. [DOI] [PubMed] [Google Scholar]
- (57).Wei Q; Zhang F; Li J; Li B; Zhao C Oxidant-Induced Dopamine Polymerization for Multifunctional Coatings. Polym. Chem 2010, 1, 1430–1433. [Google Scholar]
- (58).Son HY; Ryu JH; Lee H; Nam YS Silver-Polydopamine Hybrid Coatings of Electrospun Poly(vinyl alcohol) Nanofibers. Macromol. Mater. Eng 2013, 298, 547–554. [Google Scholar]
- (59).Yan J; Huang Y; Miao YE; Tjiu WW; Liu T Polydopamine-Coated Electrospun Poly(vinyl Alcohol)/Poly(acrylic Acid) Membranes as Efficient Dye Adsorbent with Good Recyclability. J. Hazard. Mater 2015, 283, 730–739. [DOI] [PubMed] [Google Scholar]
- (60).Cao C; Tan L; Liu W; Ma J; Li L Polydopamine Coated Electrospun Poly(vinyldiene fluoride) Nanofibrous Membrane as Separator for Lithium-Ion Batteries. J. Power Sources 2014, 248, 224–229. [Google Scholar]
- (61).Wei H; Ren J; Han B; Xu L; Han L; Jia L Stability of Polydopamine and poly(DOPA) Melanin-like Films on the Surface of`Polymer Membranes under Strongly Acidic and Alkaline Conditions. Colloids Surf., B 2013, 110, 22–28. [DOI] [PubMed] [Google Scholar]
- (62).Zhang W; Chen Z Mussel Inspired Polydopamine Function-alized Poly(ether Ether Ketone) Tube for Online Solid-Phase Microextraction–high Prformance Liquid Chromatography and Its Application in Analysis of Protoberberine Alkaloids in Rat Plasma. J. Chromatogr. A 2013, 1278, 29–36. [DOI] [PubMed] [Google Scholar]
- (63).Xi J; Dai W; Yu L Polydopamine Coated SPEEK Membrane for a Vanadium Redox Flow Battery. RSC Adv 2015, 5, 33400–33406. [Google Scholar]
- (64).Li L; Zhu C; Wu Y; Wang J; Zhang T; Liu Y A Conductive Ternary Network of a Highly Stretchable AgNWs/AgNPs Conductor Based on a Polydopamine-Modified Polyurethane Sponge. RSC Adv 2015, 5, 62905–62912. [Google Scholar]
- (65).Pardieu E; Chau NT; Dintzer T; Romero T; Favier D; Roland T; Edouard D; Jierry L; Ritleng V Polydopamine-Coated Open Cell Polyurethane Foams as an Inexpensive, Flexible yet Robust Catalyst Support: A Proof of Concept. Chem. Commun 2016, 52, 4691–4693. [DOI] [PubMed] [Google Scholar]
- (66).Park J; Brust TF; Lee HJ; Lee SC; Watts VJ; Yeo Y Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. ACS Nano 2014, 8, 3347–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Kim M; Kim JS; Lee H; Jang JH Polydopamine-Decorated Sticky, Water-Friendly, Biodegradable Polycaprolactone Cell Carriers. Macromol. Biosci 2016, 16, 738–747. [DOI] [PubMed] [Google Scholar]
- (68).Xie J; Zhong S; Ma B; Shuler FD; Lim CT Controlled Biomineralization of Electrospun Poly(ε-caprolactone) Fibers to Enhance their Mechanical Properties. Acta Biomater 2013, 9, 5698–5707. [DOI] [PubMed] [Google Scholar]
- (69).Jo S; Kang SM; Park SA; Kim WD; Kwak J; Lee H Enhanced Adhesion of Preosteoblasts inside 3DPCL Scaffolds by Polydopamine Coating and Mineralization. Macromol. Biosci 2013, 13, 1389–1395. [DOI] [PubMed] [Google Scholar]
- (70).Tsai WB; Chen WT; Chien HW; Kuo WH; Wang MJ Poly(dopamine) Coating of Scaffolds for Articular Cartilage Tissue Engineering. Acta Biomater 2011, 7, 4187–4194. [DOI] [PubMed] [Google Scholar]
- (71).Xu Q; Kong Q; Liu Z; Zhang J; Wang X; Liu R; Yue L; Cui G Polydopamine-Coated Cellulose Microfibrillated Membrane as High Performance Lithium-Ion Battery Separator. RSC Adv 2014, 4, 7845–7850. [Google Scholar]
- (72).Ye C; Wu Y; Wang Z Modification of Cellulose Paper with Polydopamine as a Thin Film Microextraction Phase for Detection of Nitrophenols in Oil Samples. RSC Adv 2016, 6, 9066–9071. [Google Scholar]
- (73).Feng Y; Zheng Y; Rahman ZU; Wang D; Zhou F; Liu W Paper-Based Triboelectric Nanogenerators and Their Application in Self-Powered Anticorrosion and Antifouling. J. Mater. Chem. A 2016, 4, 18022–18030. [Google Scholar]
- (74).Lu Z; Xiao J; Wang Y; Meng M In Situ Synthesis of Silver Nanoparticles Uniformly Distributed on Polydopamine-Coated Silk Fibers for Antibacterial Application. J. Colloid Interface Sci 2015, 452, 8–14. [DOI] [PubMed] [Google Scholar]
- (75).Hu W; He G; Zhang H; Wu X; Li J; Zhao Z; Qiao Y; Lu Z; Liu Y; Li CM Polydopamine-Functionalization of Graphene Oxide to Enable Dual Signal Amplification for Sensitive Surface Plasmon Resonance Imaging Detection of Biomarker. Anal. Chem 2014, 86, 4488–4493. [DOI] [PubMed] [Google Scholar]
- (76).Guo L; Liu Q; Li G; Shi J; Liu J; Wang T; Jiang G A Mussel-Inspired Polydopamine Coating as a Versatile Platform for the in Situ Synthesis of Graphene-Based Nanocomposites. Nanoscale 2012, 4, 5864–5867. [DOI] [PubMed] [Google Scholar]
- (77).Hu H; Yu B; Ye Q; Gu Y; Zhou F Modification of Carbon Nanotubes with a Nanothin Polydopamine Layer and Polydimethy-lamino-Ethyl Methacrylate Brushes. Carbon 2010, 48, 2347–2353. [Google Scholar]
- (78).Shi C; Deng C; Zhang X; Yang P Synthesis of Highly Water-Dispersible Polydopamine-Modified Multiwalled Carbon Nano-tubes for Matrix-Assisted Laser Desorption/Ionization Mass Spec-trometry Analysis. ACS Appl. Mater. Interfaces 2013, 5, 7770–7776. [DOI] [PubMed] [Google Scholar]
- (79).Pop-Georgievski O; Neykova N; Proks V; Houdkova J; Ukraintsev E; Zemek J; Kromka A; Rypacek,ˇ F. Polydopamine-Modified Nanocrystalline Diamond Thin Films as a Platform for Bio-Sensing Applications. Thin Solid Films 2013, 543, 180–186. [Google Scholar]
- (80).Wei Q; Pei X; Hao J; Cai M; Zhou F; Liu W Surface Modification of Diamond-Like Carbon Film with Polymer Brushes Using a Bio-Inspired Catechol Anchor for Excellent Biological Lubrication. Adv. Mater. Interfaces 2014, 1, 1400035. [Google Scholar]
- (81).Liu J; Li J; Yu B; Ma B; Zhu Y; Song X; Cao X; Yang W; Zhou F Tribological Properties of Self-Assembled Monolayers of Catecholic Imidazolium and the Spin-Coated Films of Ionic Liquids. Langmuir 2011, 27, 11324–11331. [DOI] [PubMed] [Google Scholar]
- (82).Zhang L; Wu J; Wang Y; Long Y; Zhao N; Xu J Combination of Bioinspiration: A General Route to Superhydrophobic Particles. J. Am. Chem. Soc 2012, 134, 9879–9881. [DOI] [PubMed] [Google Scholar]
- (83).Wu Y; Yan M; Cui J; Yan Y; Li C A Multiple-Functional Ag/SiO2/Organic Based Biomimetic Nanocomposite Membrane for High-Stability Protein Recognition and Cell Adhesion/Detachment. Adv. Funct. Mater 2015, 25, 5823–5832. [Google Scholar]
- (84).Wu C; Fan W; Chang J; Xiao Y Mussel-Inspired Porous SiO2 Scaffolds with Improved Mineralization and Cytocompatibility for Drug Delivery and Bone Tissue Engineering. J. Mater. Chem 2011, 21, 18300–18307. [Google Scholar]
- (85).Lynge ME; Ogaki R; Laursen AO; Lovmand J; Sutherland DS; Stadler B Polydopamine/Liposome Coatings and Their Interaction with Myoblast Cells. ACS Appl. Mater. Interfaces 2011, 3, 2142–2147. [DOI] [PubMed] [Google Scholar]
- (86).Lee M; Rho J; Lee DE; Hong S; Choi SJ; Messersmith PB; Lee H Water Detoxification by a Substrate-Bound Catechol-amine Adsorbent. ChemPlusChem 2012, 77, 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Fu Y; Liu L; Zhang L; Wang W Highly Conductive One-Dimensional Nanofibers: Silvered Electrospun Silica Nanofibers via Poly(dopamine) Functionalization. ACS Appl. Mater. Interfaces 2014, 6, 5105–5112. [DOI] [PubMed] [Google Scholar]
- (88).Yang L; Phua SL; Teo JK; Toh CL; Lau SK; Ma J; Lu X A Biomimetic Approach to Enhancing Interfacial Interactions: Polydopamine-Coated Clay as Reinforcement for Epoxy Resin. ACS Appl. Mater. Interfaces 2011, 3, 3026–3032. [DOI] [PubMed] [Google Scholar]
- (89).Zhou WH; Tang SF; Yao QH; Chen FR; Yang HH; Wang XR A Quartz Crystal Microbalance Sensor Based on Mussel-Inspired Molecularly Imprinted Polymer. Biosens. Bioelectron 2010, 26, 585–589. [DOI] [PubMed] [Google Scholar]
- (90).Jia X; Ma ZY; Zhang GX; Hu JM; Liu ZY; Wang HY; Zhou F Polydopamine Film Coated Controlled-Release Multi-element Compound Fertilizer Based on Mussel-Inspired Chemistryry. J. Agric. Food Chem 2013, 61, 2919–2924. [DOI] [PubMed] [Google Scholar]
- (91).Zhang J; Zhang W; Bao T; Chen Z Mussel-Inspired Polydopamine-Assisted Hydroxyapatite as the Stationary Phase for Capillary Electrochromatography. Analyst 2014, 139, 242–250. [DOI] [PubMed] [Google Scholar]
- (92).Ryu J; Ku SH; Lee H; Park CB Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater 2010, 20, 2132–2139. [Google Scholar]
- (93).Liu Z; Qu S; Zheng X; Xiong X; Fu R; Tang K; Zhong Z; Weng J Effect of Polydopamine on the Biomimetic Mineralization of Mussel-Inspired Calcium Phosphate Cement In Vitro. Mater. Sci. Eng., C 2014, 44, 44–51. [DOI] [PubMed] [Google Scholar]
- (94).Li C; Qian Z.-j.; Zhou C; Su W; Hong P; Liu S; He L; Chen Z; Ji H Mussel-Inspired Synthesis of Polydopamine-Function-alized Calcium Carbonate as Reusable Adsorbents for Heavy Metal Ions. RSC Adv 2014, 4, 47848–47852. [Google Scholar]
- (95).Dalsin JL; Lin L; Tosatti S; Voros J; Textor M; Messersmith PB Protein Resistance of Titanium Oxide Surfaces Modified by Biologically Inspired mPEG–DOPA. Langmuir 2005, 21, 640–646. [DOI] [PubMed] [Google Scholar]
- (96).Wang X; Ye Q; Gao T; Liu J; Zhou F Self-Assembly of Catecholic Macroinitiator on Various Substrates and Surface-Initiated Polymerization. Langmuir 2012, 28, 2574–2581. [DOI] [PubMed] [Google Scholar]
- (97).Mao WX; Lin XJ; Zhang W; Chi ZX; Lyu RW; Cao AM; Wan LJ Core-Shell Structured TiO2@Polydopamine for Highly Active Visible-Light Photocatalysis. Chem. Commun 2016, 52, 7122–7125. [DOI] [PubMed] [Google Scholar]
- (98).Ye Q; Gao T; Wan F; Yu B; Pei X; Zhou F; Xue Q Grafting poly(Ionic Liquid) Brushes for Anti-Bacterial and Anti-Biofouling Applications. J. Mater. Chem 2012, 22, 13123–13131. [Google Scholar]
- (99).Ye Q; Wang X; Li S; Zhou F Surface-Initiated Ring-Opening Metathesis Polymerization of Pentadecafluorooctyl-5-nor-bornene-2-carboxylate from Variable Substrates Modified with Sticky Biomimic Initiator. Macromolecules 2010, 43, 5554–5560. [Google Scholar]
- (100).Wang D; Ye Q; Yu B; Zhou F Towards Chemically Bonded P–n Heterojunctions through Surface Initiated Electro-deposition of P-Type Conducting Polymer inside TiO2 Nanotubes. J. Mater. Chem 2010, 20, 6910–6915. [Google Scholar]
- (101).Yan J; Zhou F TiO2 Nanotubes: Structure Optimization for Solar Cells. J. Mater. Chem 2011, 21, 9406–9418. [Google Scholar]
- (102).Ou J; Wang J; Qiu Y; Liu L; Yang S Mechanical Property and Corrosion Resistance of Zirconia/Polydopamine Nanocomposite Multilayer Films Fabricated via a Novel Non-Electrostatic Layer-by-Layer Assembly Technique. Surf. Interface Anal 2011, 43, 803–808. [Google Scholar]
- (103).Mrowczyń́ski R; Jurga-Stopa J; Markiewicz R; Coy EL; Jurga S; Wozniak A Assessment of Polydopamine Coated Magnetic Nanoparticles in Doxorubicin Delivery. RSC Adv 2016, 6, 5936–5943. [Google Scholar]
- (104).Xie A; Zhang K; Wu F; Wang N; Wang Y; Wang M Polydopamine Nanofilms as Visible Light-Harvesting Interfaces for Palladium Nanocrystal Catalyzed Coupling Reactions. Catal. Sci. Technol 2016, 6, 1764–1771. [Google Scholar]
- (105).Ye Q; Wang X; Hu H; Wang D; Li S; Zhou F Polyelectrolyte Brush Templated Multiple Loading of Pd Nano-particles onto TiO2 Nanowires via Regenerative Counterion Exchange–Reduction. J. Phys. Chem. C 2009, 113, 7677–7683. [Google Scholar]
- (106).Kang K; Choi IS; Nam Y A Biofunctionalization Scheme for Neural Interfaces Using Polydopamine Polymer. Biomaterials 2011, 32, 6374–6380. [DOI] [PubMed] [Google Scholar]
- (107).Kim R; Nam Y Electrochemical Layer-by-Layer Approach to Fabricate Mechanically Stable Platinum Black Microelectrodes Using a Mussel-Inspired Polydopamine Adhesive. J. Neural Eng 2015, 12, 026010. [DOI] [PubMed] [Google Scholar]
- (108).Wei N; Jiang Y; Ying Y; Guo X; Wu Y; Wen Y; Yang H Facile Construction of a Polydopamine-Based Hydrophobic Surface for Protection of Metals against Corrosion. RSC Adv 2017, 7, 11528–11536. [Google Scholar]
- (109).Chen S; Chen Y; Lei Y; Yin Y Novel Strategy in Enhancing Stability and Corrosion Resistance for Hydrophobic Functional Films on Copper Surfaces. Electrochem. Commun 2009, 11, 1675–1679. [Google Scholar]
- (110).Feng J-J; Zhang P-P; Wang A-J; Liao Q-C; Xi J-L; Chen J-R One-Step Synthesis of Monodisperse Polydopamine-Coated Silver Core–shell Nanostructures for Enhanced Photo-catalysis. New J. Chem 2012, 36, 148–154. [Google Scholar]
- (111).Yu B; Liu J; Liu S; Zhou F Pdop Layer Exhibiting Zwitterionicity: A Simple Electrochemical Interface for Governing Ion Permeability. Chem. Commun 2010, 46, 5900–5902. [DOI] [PubMed] [Google Scholar]
- (112).Wei Y; Kong J; Yang L; Ke L; Tan HR; Liu H; Huang Y; Sun XW; Lu X; Du H Polydopamine-Assisted Decoration of ZnO Nanorods with Ag Nanoparticles: An Improved Photo-electrochemical Anode. J. Mater. Chem. A 2013, 1, 5045–5052. [Google Scholar]
- (113).Mondin G; Wisser FM; Leifert A; Mohamed-Noriega N; Grothe J; Dorfler S; Kaskel S Metal Deposition by Electroless Plating on Polydopamine Functionalized Micro- and Nanoparticles. J. Colloid Interface Sci 2013, 411, 187–193. [DOI] [PubMed] [Google Scholar]
- (114).Li J; Wen XM; Zhang W; Chen YP; Xiao Y; Xiong CX; Zhu W; Jiang T Reinforcing Epoxy Resin with Polydopamine-Coated Al(OH)3: A Biomimetic Method to Constructing Organic-Inorganic Hybrid Materials. Adv. Mater. Res 2014, 1082, 65–68. [Google Scholar]
- (115).Ye W; Wang D; Zhang H; Zhou F; Liu W Electro-chemical Growth of Flowerlike Gold Nanoparticles on Polydopamine Modified ITO Glass for SERS Application. Electrochim. Acta 2010, 55, 2004–2009. [Google Scholar]
- (116).Yu F; Chen S; Chen Y; Li H; Yang L; Chen Y; Yin Y Experimental and Theoretical Analysis of Polymerization Reaction Process on the Polydopamine Membranes and Its Corrosion Protection Properties for 304 Stainless Steel. J. Mol. Struct 2010, 982, 152–161. [Google Scholar]
- (117).Huang A; Liu Q; Wang N; Caro J Highly Hydrogen Permselective ZIF-8 Membranes Supported on Polydopamine Functionalized Macroporous Stainless-Steel-Nets. J. Mater. Chem. A 2014, 2, 8246–8251. [Google Scholar]
- (118).Yan J; Ye Q; Zhou F Polymer Brushes Assisted Loading of High Density CdS/CdSe Quantum Dots onto TiO2 Nanotubes and the Resulting Photoelectric Performance. RSC Adv 2012, 2, 3978–3985. [Google Scholar]
- (119).Park JP; Do M; Jin HE; Lee SW; Lee H M13 Bacteriophage Displaying DOPA on Surfaces: Fabrication of Various Nanostructured Inorganic Materials without Time-Consuming Screen-ing Processes. ACS Appl. Mater. Interfaces 2014, 6, 18653–18660. [DOI] [PubMed] [Google Scholar]
- (120).Park JP; Choi MJ; Kim SH; Lee SH; Lee H Preparation of Sticky Escherichia Coli through Surface Display of an Adhesive Catecholamine Moiety. Appl. Environ. Microbiol 2014, 80, 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Kang SM; You I; Cho WK; Shon HK; Lee TG; Choi IS; Karp JM; Lee H One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem., Int. Ed 2010, 49, 9401–9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Ponzio F; Payamyar P; Schneider A; Winterhalter M; Bour J; Addiego F; Krafft MP; Hemmerle J; Ball V Polydopamine Films from the Forgotten Air/Water Interface. J. Phys. Chem. Lett 2014, 5, 3436–3440. [DOI] [PubMed] [Google Scholar]
- (123).Hong S; Schaber CF; Dening K; Appel E; Gorb SN; Lee H Air/Water Interfacial Formation of Freestanding, Stimuli-Responsive, Self-Healing Catecholamine Janus-Faced Microfilms. Adv. Mater 2014, 26, 7581–7587. [DOI] [PubMed] [Google Scholar]
- (124).Yang HC; Wu QY; Wan LS; Xu ZK Polydopamine Gradients by Oxygen Diffusion Controlled Autoxidation. Chem. Commun 2013, 49, 10522–10524. [DOI] [PubMed] [Google Scholar]
- (125).Yu B; Wang DA; Ye Q; Zhou F; Liu W Robust Polydopamine Nano/Microcapsules and Their Loading and Release Behavior. Chem. Commun 2009, 6789–6791. [DOI] [PubMed]
- (126).Liu Q; Yu B; Ye W; Zhou F Highly Selective Uptake and Release of Charged Molecules by pH-Responsive Polydopamine Microcapsules. Macromol. Biosci 2011, 11, 1227–1234. [DOI] [PubMed] [Google Scholar]
- (127).Sheng W; Li W; Li B; Li C; Xu Y; Guo X; Zhou F; Jia X Mussel-Inspired Photografting on Colloidal Spheres: A Generalized Self-Template Route to Stimuli-Responsive Hollow Spheres for Controlled Pesticide Release. Macromol. Rapid Commun 2015, 36, 1640–1645. [DOI] [PubMed] [Google Scholar]
- (128).Liu Y; Liu Z; Liu Y; Hu H; Li Y; Yan P; Yu B; Zhou F One-Step Modification of Fabrics with Bioinspired Polydopamine@ Octadecylamine Nanocapsules for Robust and Healable Self-Cleaning Performance. Small 2015, 11, 426–431. [DOI] [PubMed] [Google Scholar]
- (129).Ochs CJ; Hong T; Such GK; Cui J; Postma A; Caruso F Dopamine-Mediated Continuous Assembly of Biodegradable Capsules. Chem. Mater 2011, 23, 3141–3143. [Google Scholar]
- (130).Cui J; Yan Y; Such GK; Liang K; Ochs CJ; Postma A; Caruso F Immobilization and Intracellular Delivery of an Anticancer Drug Using Mussel-Inspired Polydopamine Capsules. Biomacromole-cules 2012, 13, 2225–2228. [DOI] [PubMed] [Google Scholar]
- (131).Ku SH; Ryu J; Hong SK; Lee H; Park CB General Functionalization Route for Cell Adhesion on Non-Wetting Surfaces. Biomaterials 2010, 31, 2535–2541. [DOI] [PubMed] [Google Scholar]
- (132).Luo R; Tang L; Zhong S; Yang Z; Wang J; Weng Y; Tu Q; Jiang C; Huang N In Vitro Investigation of Enhanced Hemocompatibility and Endothelial Cell Proliferation Associated with Quinone-Rich Polydopamine Coating. ACS Appl. Mater. Interfaces 2013, 5, 1704–1714. [DOI] [PubMed] [Google Scholar]
- (133).Yang K; Lee JS; Kim J; Lee YB; Shin H; Um SH; Kim JB; Park KI; Lee H; Cho SW Polydopamine-Mediated Surface Modification of Scaffold Materials for Human Neural Stem Cell Engineering. Biomaterials 2012, 33, 6952–6964. [DOI] [PubMed] [Google Scholar]
- (134).Kandasamy K; Narayanan K; Ni M; Du C; Wan AC; Zink D Polysulfone Membranes Coated with Polymerized 3,4-Dihydroxy-L-Phenylalanine Are a Versatile and Cost-Effective Synthetic Substrate for Defined Long-Term Cultures of Human Pluripotent Stem Cells. Biomacromolecules 2014, 15, 2067–2078. [DOI] [PubMed] [Google Scholar]
- (135).Ku SH; Lee JS; Park CB Spatial Control of Cell Adhesion and Patterning through Mussel-Inspired Surface Modifica-tion by Polydopamine. Langmuir 2010, 26, 15104–15108. [DOI] [PubMed] [Google Scholar]
- (136).Sun K; Xie Y; Ye D; Zhao Y; Cui Y; Long F; Zhang W; Jiang X Mussel-Inspired Anchoring for Patterning Cells Using Polydopamine. Langmuir 2012, 28, 2131–2136. [DOI] [PubMed] [Google Scholar]
- (137).Chien HW; Kuo WH; Wang MJ; Tsai SW; Tsai WB Tunable Micropatterned Substrates Based on Poly(dopamine) Deposition via Microcontact Printing. Langmuir 2012, 28, 5775–5782. [DOI] [PubMed] [Google Scholar]
- (138).Lee HY; Jeong H; Jung IY; Jang B; Seo YC; Lee H; Lee H DhITACT: DNA Hydrogel Formation by Isothermal Amplification of Complementary Target in Fluidic Channels. Adv. Mater 2015, 27, 3513–3517. [DOI] [PubMed] [Google Scholar]
- (139).You I; Kang SM; Lee S; Cho YO; Kim JB; Lee SB; Nam YS; Lee H Polydopamine Microfluidic System toward a Two-Dimensional, Gravity-Driven Mixing Device. Angew. Chem., Int. Ed 2012, 51, 6126–6130. [DOI] [PubMed] [Google Scholar]
- (140).Zhang Z; Zhang J; Zhang B; Tang J Mussel-Inspired Functionalization of Graphene for Synthesizing Ag-Polydopamine-Graphenenanosheets as Antibacterial Materials. Nanoscale 2013, 5, 118–123. [DOI] [PubMed] [Google Scholar]
- (141).Jiang J; Zhu L; Zhu L; Zhang H; Zhu B; Xu Y Antifouling and Antimicrobial Polymer Membranes Based on Bioinspired Polydopamine and Strong Hydrogen-Bonded Poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [DOI] [PubMed] [Google Scholar]
- (142).Chien CY; Tsai WB Poly(dopamine)-Assisted Immobilization of Arg-Gly-Asp Peptides, Hydroxyapatite, and Bone Morphogenic Protein-2 on Titanium to Improve the Osteogenesis of Bone Marrow Stem Cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [DOI] [PubMed] [Google Scholar]
- (143).Kang SM; Hwang NS; Yeom J; Park SY; Messersmith PB; Choi IS; Langer R; Anderson DG; Lee H One-Step Multipurpose Surface Functionalization by Adhesive Catecholamine. Adv. Funct. Mater 2012, 22, 2949–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Li Y; Yang W; Li X; Zhang X; Wang C; Meng X; Pei Y; Fan X; Lan P; Wang C; Li X; Guo Z Improving Osteointegration and Osteogenesis of Three-Dimensional Porous Ti6Al4V Scaffolds by Polydopamine-Assisted Biomimetic Hydroxyapatite Coatingcoating. ACS Appl. Mater. Interfaces 2015, 7, 5715–5724. [DOI] [PubMed] [Google Scholar]
- (145).Zhang X; Wang S; Xu L; Feng L; Ji Y; Tao L; Li S; Wei Y Biocompatible Polydopamine Fluorescent Organic Nanoparticles: Facile Preparation and Cell Imaging. Nanoscale 2012, 4, 5581–5584. [DOI] [PubMed] [Google Scholar]
- (146).Nurunnabi M; Khatun Z; Nafiujjaman M; Lee DG; Lee YK Surface Coating of Graphene Quantum Dots Using Mussel-Inspired Polydopamine for Biomedical Optical Imaging. ACS Appl. Mater. Interfaces 2013, 5, 8246–8253. [DOI] [PubMed] [Google Scholar]
- (147).Lin L-S; Cong Z-X; Cao J-B; Ke K-M; Peng Q-L; Gao J; Yang H-H; Liu G; Chen X Multifunctional Fe3O4@Polydop-amine Core–Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Tao W; Zeng X; Wu J; Zhu X; Yu X; Zhang X; Zhang J; Liu G; Mei L Polydopamine-Based Surface Modification of Novel Nanoparticle-Aptamer Bioconjugates for In Vivo Breast Cancer Targeting and Enhanced Therapeutic Effects. Theranostics 2016, 6, 470–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Liu Y; Ai K; Liu J; Deng M; He Y; Lu L Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photo-thermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater 2013, 25, 1353–1359. [DOI] [PubMed] [Google Scholar]
- (150).Sharker SM; Kim SM; Lee JE; Choi KH; Shin G; Lee S; Lee KD; Jeong JH; Lee H; Park SY Functionalized Biocompatible WO3 Nanoparticles for Triggered and Targeted in Vitro and in Vivo Photothermal Therapy. J. Controlled Release 2015, 217, 211–220. [DOI] [PubMed] [Google Scholar]
- (151).Gullotti E; Park J; Yeo Y Polydopamine-Based Surface Modification for the Development of Peritumorally Activatable Nanoparticles. Pharm. Res 2013, 30, 1956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Sun Y; Deng Y; Ye Z; Liang S; Tang Z; Wei S Peptide Decorated Nano-Hydroxyapatite with Enhanced Bioactivity and Osteogenic Differentiation via Polydopamine Coating. Colloids Surf., B 2013, 111, 107–116. [DOI] [PubMed] [Google Scholar]
- (153).Kim S; Park CB Dopamine-Induced Mineralization of Calcium Carbonate Vaterite Microspheres. Langmuir 2010, 26, 14730–14736. [DOI] [PubMed] [Google Scholar]
- (154).Kim S; Park CB Mussel-Inspired Transformation of CaCO3 to Bone Minerals. Biomaterials 2010, 31, 6628–6634. [DOI] [PubMed] [Google Scholar]
- (155).Yang SH; Hong D; Lee J; Ko EH; Choi IS Artificial Spores: Cytocompatible Encapsulation of Individual Living Cells within Thin, Tough Artificial Shells. Small 2013, 9, 178–186. [DOI] [PubMed] [Google Scholar]
- (156).Yang SH; Kang SM; Lee KB; Chung TD; Lee H; Choi IS Mussel-Inspired Encapsulation and Functionalization of Individual Yeast Cells. J. Am. Chem. Soc 2011, 133, 2795–2797. [DOI] [PubMed] [Google Scholar]
- (157).Zhang C; Yang HC; Wan LS; Liang HQ; Li H; Xu ZK Polydopamine-Coated Porous Substrates as a Platform for Mineralized β-FeOOH Nanorods with Photocatalysis under Sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [DOI] [PubMed] [Google Scholar]
- (158).Ko JW; Kim JH; Park CB Synthesis of Visible Light-Active CeO2 Sheets via Mussel-Inspired CaCO3 Mineralization. J. Mater. Chem. A 2013, 1, 241–245. [Google Scholar]
- (159).Lee M; Kim JU; Lee JS; Lee BI; Shin J; Park CB Mussel-Inspired Plasmonic Nanohybrids for Light Harvesting. Adv. Mater 2014, 26, 4463–4468. [DOI] [PubMed] [Google Scholar]
- (160).Kang SM; Ryou M-H; Choi JW; Lee H Mussel- and Diatom-Inspired Silica Coating on Separators Yields Improved Power and Safety in Li-Ion Batteries. Chem. Mater 2012, 24, 3481–3485. [Google Scholar]
- (161).Wang H; Wu J; Cai C; Guo J; Fan H; Zhu C; Dong H; Zhao N; Xu J Mussel Inspired Modification of Polypropylene Separators by Catechol/Polyamine for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 5602–5608. [DOI] [PubMed] [Google Scholar]
- (162).Ryou M-H; Lee DJ; Lee J-N; Lee YM; Park J-K; Choi JW Excellent Cycle Life of Lithium-Metal Anodes in Lithium-Ion Batteries with Mussel-Inspired Polydopamine-Coated Separators. Adv. Energy Mater 2012, 2, 645–650. [Google Scholar]
- (163).Kim DS; Park YJ A Simple Method for Surface Modification of Carbon by Polydopamine Coating for Enhanced Li– air Batteries. Electrochim. Acta 2014, 132, 297–306. [Google Scholar]
- (164).Yoon TH; Park YJ Polydopamine-Assisted Carbon Nanotubes/Co3O4 Composites for Rechargeable Li-Air Batteries. J. Power Sources 2013, 244, 344–353. [Google Scholar]
- (165).Zhou W; Xiao X; Cai M; Yang L Polydopamine-Coated, Nitrogen-Doped, Hollow Carbon-Sulfur Double-Layered Core-Shell Structure for Improving Lithium-Sulfur Batteries. Nano Lett 2014, 14, 5250–5256. [DOI] [PubMed] [Google Scholar]
- (166).Li B; Chen Y; Ge X; Chai J; Zhang X; Hor TS; Du G; Liu Z; Zhang H; Zong Y Mussel-Inspired One-Pot Synthesis of Transition Metal and Nitrogen Co-Doped Carbon (M/N–C) as Efficient Oxygen Catalysts for Zn-Air Batteries. Nanoscale 2016, 8, 5067–5075. [DOI] [PubMed] [Google Scholar]
- (167).Cao Y; Zhang X; Tao L; Li K; Xue Z; Feng L; Wei Y Mussel-Inspired Chemistry and Michael Addition Reaction for Efficient Oil/Water Separation. ACS Appl. Mater. Interfaces 2013, 5, 4438–4442. [DOI] [PubMed] [Google Scholar]
- (168).Zhu Q; Pan Q Mussel-Inspired Direct Immobilization of Nanoparticles and Application for Oil–Water Separation. ACS Nano 2014, 8, 1402–1409. [DOI] [PubMed] [Google Scholar]
- (169).Yang H-C; Liao K-J; Huang H; Wu Q-Y; Wan L-S; Xu Z-K Mussel-Inspired Modification of a Polymer Membrane for Ultra-High Water Permeability and Oil-in-Water Emulsion Separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar]
- (170).Ma ZY; Jia X; Zhang GX; Hu JM; Zhang XL; Liu ZY; Wang HY; Zhou F pH-Responsive Controlled-Release Fertilizer with Water Retention via Atom Transfer Radical Polymerization of Acrylic Acid on Mussel-Inspired Initiator. J. Agric. Food Chem 2013, 61, 5474–5482. [DOI] [PubMed] [Google Scholar]
- (171).Zhu B; Edmondson S Polydopamine-Melanin Initiators for Surface-Initiated ATRP. Polymer 2011, 52, 2141–2149. [Google Scholar]
- (172).Wu H; Kong J; Yao X; Zhao C; Dong Y; Lu X Polydopamine-Assisted Attachment of β-Cyclodextrin on Porous Electrospun Fibers for Water Purification under Highly Basic Condition. Chem. Eng. J 2015, 270, 101–109. [Google Scholar]
- (173).Liu R; Mahurin SM; Li C; Unocic RR; Idrobo JC; Gao H; Pennycook SJ; Dai S Dopamine as a Carbon Source: the Controlled Synthesis of Hollow Carbon Spheres and Yolk-Structured Carbon Nanocomposites. Angew. Chem., Int. Ed 2011, 50, 6799–6802. [DOI] [PubMed] [Google Scholar]
- (174).Kong J; Yee WA; Yang L; Wei Y; Phua SL; Ong HG; Ang JM; Li X; Lu X Highly Electrically Conductive Layered Carbon Derived from Polydopamine and Its Functions in SnO2-Based Lithium Ion Battery Anodes. Chem. Commun 2012, 48, 10316–10318. [DOI] [PubMed] [Google Scholar]
- (175).Zhou D; Yang L; Yu L; Kong J; Yao X; Liu W; Xu Z; Lu X Fe/N/C Hollow Nanospheres by Fe(III)-Dopamine Complex-ation-Assisted One-Pot Doping as Nonprecious-Metal Electrocatalysts for Oxygen Reduction. Nanoscale 2015, 7, 1501–1509. [DOI] [PubMed] [Google Scholar]
- (176).McCloskey BD; Park HB; Ju H; Rowe BW; Miller DJ; Chun BJ; Kin K; Freeman BD Influence of Polydopamine Deposition Conditions on Pure Water Flux and Foulant Adhesion Resistance of Reverse Osmosis, Ultrafiltration, and Microfiltration Membranes. Polymer 2010, 51, 3472–3485. [Google Scholar]
- (177).Jiang J-H; Zhu L-P; Li X-L; Xu Y-Y; Zhu B-K Surface Modification of PE Porous Membranes Based on the Strong Adhesion of Polydopamine and Covalent Immobilization of Heparin. J. Membr. Sci 2010, 364, 194–202. [Google Scholar]
- (178).Kasemset S; Lee A; Miller DJ; Freeman BD; Sharma MM Effect of Polydopamine Deposition Conditions on Fouling Resistance, Physical Properties, and Permeation Properties of Reverse Osmosis Membranes in Oil/Water Separation. J. Membr. Sci 2013, 425–426, 208–216. [Google Scholar]
- (179).Mrowczynski R; Bunge A; Liebscher J Polydopamine-An Organocatalyst Rather than an Innocent Polymer. Chem. - Eur. J 2014, 20, 8647–8653. [DOI] [PubMed] [Google Scholar]
- (180).Kanyong P; Rawlinson S; Davis J Fabrication and Electrochemical Characterization of Polydopamine Redox Polymer Modified Screen-Printed Carbon Electrode for the Detection of Guanine. Sens. Actuators, B 2016, 233, 528–534. [Google Scholar]
- (181).Liu X; Cao J; Li H; Li J; Jin Q; Ren K; Ji J Mussel-Inspired Polydopamine: A Biocompatible and Ultrastable Coating for Nanoparticles in Vivo. ACS Nano 2013, 7, 9384–9395. [DOI] [PubMed] [Google Scholar]
- (182).Fei B; Qian B; Yang Z; Wang R; Liu WC; Mak CL; Xin JH Coating Carbon Nanotubes by Spontaneous Oxidative Polymerization of Dopamine. Carbon 2008, 46, 1795–1797. [Google Scholar]
- (183).Han G; Zhang S; Li X; Widjojo N; Chung T-S Thin Film Composite Forward Osmosis Membranes Based on Polydopamine Modified Polysulfone Substrates with Enhancements in Both Water Flux and Salt Rejection. Chem. Eng. Sci 2012, 80, 219–231. [Google Scholar]
- (184).Ball V; Del Frari D; Toniazzo V; Ruch D Kinetics of Polydopamine Film Deposition as a Function of pH and Dopamine Concentration: Insights in the Polydopamine Deposition Mechanism. J. Colloid Interface Sci 2012, 386, 366–372. [DOI] [PubMed] [Google Scholar]
- (185).Bernsmann F; Ball V; Addiego F; Ponche A; Michel M; Gracio JJ; Toniazzo V; Ruch D Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [DOI] [PubMed] [Google Scholar]
- (186).Ball V Impedance Spectroscopy and Zeta Potential Titration of Dopa-Melanin Films Produced by Oxidation of Dopamine. Colloids Surf., A 2010, 363, 92–97. [Google Scholar]
- (187).Della Vecchia NF; Luchini A; Napolitano A; D’Errico G; Vitiello G; Szekely N; d’Ischia M; Paduano L Tris Buffer Modulates Polydopamine Growth, Aggregation, and Paramagnetic Properties. Langmuir 2014, 30, 9811–9818. [DOI] [PubMed] [Google Scholar]
- (188).Lee M; Lee SH; Oh IK; Lee H Microwave-Accelerated Rapid, Chemical Oxidant-Free, Material-Independent Surface Chem-istry of Poly(dopamine). Small 2017, 13, 1600443. [DOI] [PubMed] [Google Scholar]
- (189).Ponzio F; Barthes J; Bour J; Michel M; Bertani P; Hemmerle J; d’Ischia M; Ball V Oxidant Control of Polydopamine Surface Chemistry in Acids: A Mechanism-Based Entry to Super-hydrophilic-Superoleophobic Coatings. Chem. Mater 2016, 28, 4697–4705. [Google Scholar]
- (190).Du X; Li L; Li J; Yang C; Frenkel N; Welle A; Heissler S; Nefedov A; Grunze M; Levkin PA UV-Triggered Dopamine Polymerization: Control of Polymerization, Surface Coating, and Photopatterning. Adv. Mater 2014, 26, 8029–8033. [DOI] [PubMed] [Google Scholar]
- (191).Du X; Li L; Behboodi-Sadabad F; Welle A; Li J; Heissler S; Zhang H; Plumere N; Levkin PA Bio-inspired strategy for controlled dopamine polymerization in basic solutions. Polym. Chem 2017, 8, 2145–2151. [Google Scholar]
- (192).Shafiq Z; Cui J; Pastor-Perez L; San Miguel V; Gropeanu RA; Serrano C; del Campo A Bioinspired Underwater Bonding and Debonding on Demand. Angew. Chem 2012, 124, 4408–4411. [DOI] [PubMed] [Google Scholar]
- (193).Nijhuis AW; van den Beucken JJ; Boerman OC; Jansen JA; Leeuwenburgh SC 1-Step Versus 2-Step Immobilization of Alkaline Phosphatase and Bone Morphogenetic Protein-2 onto Implant Surfaces Using Polydopamine. Tissue Eng., Part C 2013, 19, 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Mrowczyń́ski R; Markiewicz R; Liebscher J Chemistry of Polydopamine Analogues. Polym. Int 2016, 65, 1288–1299. [Google Scholar]
- (195).Xi Z-Y; Xu Y-Y; Zhu L-P; Wang Y; Zhu B-K A Facile Method of Surface Modification for Hydrophobic Polymer Mem-branes Based on the Adhesive Behavior of poly(DOPA) and Poly(dopamine). J. Membr. Sci 2009, 327, 244–253. [Google Scholar]
- (196).Kuang J; Guo JL; Messersmith PB High Ionic Strength Formation of DOPA-Melanin Coating for Loading and Release of Cationic Antimicrobial Compounds. Adv. Mater. Interfaces 2014, 1, 1400145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Hong S; Kim J; Na YS; Park J; Kim S; Singha K; Im GI; Han DK; Kim WJ; Lee H Poly(norepinephrine): Ultrasmooth Material-Independent Surface Chemistry and Nanodepot for Nitric Oxide. Angew. Chem., Int. Ed 2013, 52, 9187–9191. [DOI] [PubMed] [Google Scholar]
- (198).Jeon YJ; Kang SM Chemically Stable Poly-(norepinephrine) Coatings on Solid Substrates by Post-Oxidation. Polym. Degrad. Stab 2013, 98, 1271–1273. [Google Scholar]
- (199).Jiang Y; Wang Y; Wang H; Zhou L; Gao J; Zhang Y; Zhang X; Wang X; Li J Facile Immobilization of Enzyme on Three Dimensionally Ordered Macroporous Silica via a Biomimetic Coating. New J. Chem 2015, 39, 978–984. [Google Scholar]
- (200).Park M; Shin M; Kim E; Lee S; Park KI; Lee H; Jang J-H The Promotion of Human Neural Stem Cells Adhesion Using Bioinspired Poly(norepinephrine) Nanoscale Coating. J. Nanomater 2014, 2014, 1–10. [Google Scholar]
- (201).Kang SM; Rho J; Choi IS; Messersmith PB; Lee H Norepinephrine: Material-Independent, Multifunctional Surface Mod-ification Reagent. J. Am. Chem. Soc 2009, 131, 13224–13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Chen J; Liang RP; Wu LL; Qiu JD One-Step Preparation and Application of Mussel-Inspired Poly(norepinephrine)-Coated Polydimethylsiloxane Microchip for Separation of Chiral Compounds. Electrophoresis 2016, 37, 1676–1684. [DOI] [PubMed] [Google Scholar]
- (203).Yang D; Wang X; Ai Q; Shi J; Jiang Z Performance Comparison of Immobilized Enzyme on the Titanate Nanotube Surfaces Modified by Poly(dopamine) and Poly(norepinephrine). RSC Adv 2015, 5, 42461–42467. [Google Scholar]
- (204).Chen J; Liang RP; Wang XN; Qiu JD A Norepinephrine Coated Magnetic Molecularly Imprinted Polymer for Simultaneous Multiple Chiral Recognition. J. Chromatogr. A 2015, 1409, 268–276. [DOI] [PubMed] [Google Scholar]
- (205).Taskin MB; Xu R; Zhao H; Wang X; Dong M; Besenbacher F; Chen M Poly(norepinephrine) as a Functional Bio-Interface for Neuronal Differentiation on Electrospun Fibers. Phys. Chem. Chem. Phys 2015, 17, 9446–9453. [DOI] [PubMed] [Google Scholar]
- (206).Hong J-Y; Yu X; Bak BM; Pang C; Park HS Bio-Inspired Functionalization and Redox Charge Transfer of Graphene Oxide Sponges for Pseudocapacitive Electrodes. Carbon 2015, 83, 71–78. [Google Scholar]
- (207).Napolitano l.; Memoli S; Nappi AJ; d’Ischia M; Prota G 5-S-Cysteinyldopa, a Diffusible Product of Melanocyte Activity, Is an Efficient Inhibitor of HydroxylationOxidation Reactions Induced by the Fenton System. Biochim. Biophys. Acta, Gen. Subj 1996, 1291, 75–82. [DOI] [PubMed] [Google Scholar]
- (208).Napolitano A; Palumbo A; d’Ischia M Oxidation of the Neurotoxin 6-Nitrodopamine and Related 4-Nitrocatechols Under Biomimetic Conditions. Tetrahedron 2000, 56, 5941–5945. [Google Scholar]
- (209).Kohri M; Kohma H; Shinoda Y; Yamauchi M; Yagai S; Kojima T; Taniguchi T; Kishikawa K A colorless Functional Polydopamine Thin Layer as a Basis for Polymer Capsules. Polym. Chem 2013, 4, 2696–2702. [Google Scholar]
- (210).Glass P; Chung H; Washburn NR; Sitti M Enhanced Reversible Adhesion of Dopamine Methacrylamide-Coated Elastomer Microfibrillar Structures under Wet Conditions. Langmuir 2009, 25, 6607–6612. [DOI] [PubMed] [Google Scholar]
- (211).Wang X; Ye Q; Liu J; Liu X; Zhou F Low Surface Energy Surfaces from Self-Assembly of Perfluoropolymer with Sticky Func-tional Groups. J. Colloid Interface Sci 2010, 351, 261–266. [DOI] [PubMed] [Google Scholar]
- (212).Wu Y; Liu Z; Liang Y; Pei X; Zhou F; Xue Q Photoresponsive Superhydrophobic Coating for Regulating Boundary Slippage. Soft Matter 2014, 10, 5318–5324. [DOI] [PubMed] [Google Scholar]
- (213).Kurzhals S; Gal N; Zirbs R; Reimhult E Controlled Aggregation and Cell Uptake of Thermoresponsive Polyoxazoline-Grafted Superparamagnetic Iron Oxide Nanoparticles. Nanoscale 2017, 9, 2793–2805. [DOI] [PubMed] [Google Scholar]
- (214).Lassenberger A; Scheberl A; Stadlbauer A; Stiglbauer A; Helbich T; Reimhult E Individually Stabilized, Superparamagnetic Nanoparticles with Controlled Shell and Size Leading to Exceptional Stealth Properties and High Relaxivities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Amstad E; Gehring AU; Fischer H; Nagaiyanallur VV; Hahner G; Textor M; Reimhult E Influence of Electronegative Substituents on the Binding Affinity of Catechol-Derived Anchors to Fe3O4 Nanoparticles. J. Phys. Chem. C 2011, 115, 683–691. [Google Scholar]
- (216).Gal N; Lassenberger A; Herrero-Nogareda L; Scheberl A; Charwat V; Kasper C; Reimhult E Interaction of Size-Tailored PEGylated Iron Oxide Nanoparticles with Lipid Membranes and Cells. ACS Biomater. Sci. Eng 2017, 3, 249–259. [DOI] [PubMed] [Google Scholar]
- (217).Morgese G; Shirmardi Shaghasemi B; Causin V; Zenobi-Wong M; Ramakrishna SN; Reimhult E; Benetti EM Next-Generation Polymer Shells for Inorganic Nanoparticles are Highly Compact, Ultra-Dense, and Long-Lasting Cyclic Brushes. Angew. Chem., Int. Ed 2017, 56, 4507–4511. [DOI] [PubMed] [Google Scholar]
- (218).Ye Q; Zhou F; Liu W Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev 2011, 40, 4244–4258. [DOI] [PubMed] [Google Scholar]
- (219).Zeng R; Luo Z; Zhou D; Cao F; Wang Y A Novel PEG Coating Immobilized onto Capillary through Polydopamine Coating for Separation of Proteins in CE. Electrophoresis 2010, 31, 3334–3341. [DOI] [PubMed] [Google Scholar]
- (220).Xu LQ; Yang WJ; Neoh K-G; Kang E-T; Fu GD Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar]
- (221).Liu X; Barizuddin S; Shin W; Mathai CJ; Gangopadhyay S; Gillis KD Microwell Device for Targeting Single Cells to Electrochemical Microelectrodes for High-Throughput Amperometric Detection of Quantal Exocytosis. Anal. Chem 2011, 83, 2445–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Tsai WB; Chien CY; Thissen H; Lai JY Dopamine-Assisted Immobilization of Poly(ethylene imine) Based Polymers for Control of Cell-Surface Interactions. Acta Biomater 2011, 7, 2518–2525. [DOI] [PubMed] [Google Scholar]
- (223).Pop-Georgievski O; Popelka S; Houska M; Chvostova D; Proks V; Rypacek F Poly(ethylene oxide) Layers Grafted to Dopamine-Melanin Anchoring Layer: Stability and Resistance to Protein Adsorption. Biomacromolecules 2011, 12, 3232–3242. [DOI] [PubMed] [Google Scholar]
- (224).Miller DJ; Araujo PA; Correia PB; Ramsey MM; Kruithof JC; van Loosdrecht MC; Freeman BD; Paul DR; Whiteley M; Vrouwenvelder JS Short-Term Adhesion and Long-Term Biofouling Testing of Polydopamine and Poly(ethylene glycol) Surface Modifications of Membranes and Feed Spacers for Biofouling Control. Water Res 2012, 46, 3737–3753. [DOI] [PubMed] [Google Scholar]
- (225).Poh CK; Shi Z; Tan XW; Liang ZC; Foo XM; Tan HC; Neoh KG; Wang W Cobalt Chromium Alloy with Immobilized BMP Peptide for Enhanced Bone Growth. J. Orthop. Res 2011, 29, 1424–1430. [DOI] [PubMed] [Google Scholar]
- (226).Lee H; Rho J; Messersmith PB Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater 2009, 21, 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (227).Ham HO; Liu Z; Lau KH; Lee H; Messersmith PB Facile DNA Immobilization on Surfaces through a Catecholamine Polymer. Angew. Chem 2011, 123, 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Zhu LP; Jiang JH; Zhu BK; Xu YY Immobilization of Bovine Serum Albumin onto Porous Polyethylene Membranes Using Strongly Attached Polydopamine as a Spacer. Colloids Surf., B 2011, 86, 111–118. [DOI] [PubMed] [Google Scholar]
- (229).Chen Z; Li Q; Chen J; Luo R; Maitz MF; Huang N Immobilization of Serum Albumin and Peptide Aptamer for EPC on Polydopamine Coated Titanium Surface for Enhanced in-Situ Self-Endothelialization. Mater. Sci. Eng., C 2016, 60, 219–229. [DOI] [PubMed] [Google Scholar]
- (230).Amoozgar Z; Goldberg MS Surface Modulation of Polymeric Nanocarriers Enhances the Stability and Delivery of Proteins and Small Molecules. Nanomedicine 2017, 12, 729–743. [DOI] [PubMed] [Google Scholar]
- (231).Cho HJ; Madhurakkat Perikamana SK; Lee JH; Lee J; Lee KM; Shin CS; Shin H Effective Immobilization of BMP-2 Mediated by Polydopamine Coating on Biodegradable Nanofibers for Enhanced In Vivo Bone Formation. ACS Appl. Mater. Interfaces 2014, 6, 11225–11235. [DOI] [PubMed] [Google Scholar]
- (232).Lee SJ; Lee D; Yoon TR; Kim HK; Jo HH; Park JS; Lee JH; Kim WD; Kwon IK; Park SA Surface Modification of 3D-Printed Porous Scaffolds via Mussel-Inspired Polydopamine and Effective Immobilization of rhBMP-2 to Promote Osteogenic Differentiation for Bone Tissue Engineering. Acta Biomater 2016, 40, 182–191. [DOI] [PubMed] [Google Scholar]
- (233).Yang X; Zhu L; Tada S; Zhou D; Kitajima T; Isoshima T; Yoshida Y; Nakamura M; Yan W; Ito Y Mussel-Inspired Human Gelatin Nanocoating for Creating Biologically Adhesive Surfaces. Int. J. Nanomed 2014, 9, 2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Rivera JG; Messersmith PB Polydopamine-Assisted Immobilization of Trypsin onto Monolithic Structures for Protein Digestion: Sample Preparation. J. Sep. Sci 2012, 35, 1514–1520. [DOI] [PubMed] [Google Scholar]
- (235).Liu K; Wang C; Ma J; Shi G; Yao X; Fang H; Song Y; Wang J Janus Effect of Antifreeze Proteins on Ice Nucleation. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 14739–14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (236).Hu Y; Dan W; Xiong S; Kang Y; Dhinakar A; Wu J; Gu Z Development of Collagen/Polydopamine Complexed Matrix as Mechanically Enhanced and Highly Biocompatible Semi-Natural Tissue Engineering Scaffold. Acta Biomater 2017, 47, 135–148. [DOI] [PubMed] [Google Scholar]
- (237).Sun G; Chung TS; Jeyaseelan K; Armugam A Stabilization and Immobilization of Aquaporin Reconstituted Lipid Vesicles for Water Purification. Colloids Surf., B 2013, 102, 466–471. [DOI] [PubMed] [Google Scholar]
- (238).Park HJ; Yang K; Kim MJ; Jang J; Lee M; Kim DW; Lee H; Cho SW Bio-Inspired Oligovitronectin-Grafted Surface for Enhanced Self-Renewal and Long-Term Maintenance of Human Pluripotent Stem Cells under Feeder-Free Conditions. Biomaterials 2015, 50, 127–139. [DOI] [PubMed] [Google Scholar]
- (239).Kang J; Sakuragi M; Shibata A; Abe H; Kitajima T; Tada S; Mizutani M; Ohmori H; Ayame H; Son TI; Aigaki T; Ito Y Immobilization of Epidermal Growth Factor on Titanium and Stainless Steel Surfaces via Dopamine Treatment. Mater. Sci. Eng., C 2012, 32, 2552–2561. [Google Scholar]
- (240).Song IT; Lee M; Lee H; Han J; Jang JH; Lee MS; Koh GY; Lee H PEGylation and HAylation via Catechol: α-Amine-Specific Reaction at N-Terminus of Peptides and Proteins. Acta Biomater 2016, 43, 50–60. [DOI] [PubMed] [Google Scholar]
- (241).Liu R; Guo Y; Odusote G; Qu F; Priestley RD Core-Shell Fe3O4 Polydopamine Nanoparticles Serve Multipurpose as Drug Carrier, Catalyst Support and Carbon Adsorbent. ACS Appl. Mater. Interfaces 2013, 5, 9167–9171. [DOI] [PubMed] [Google Scholar]
- (242).Joddar B; Albayrak A; Kang J; Nishihara M; Abe H; Ito Y Sustained Delivery of siRNA from Dopamine-Coated Stainless Steel Surfaces. Acta Biomater 2013, 9, 6753–6761. [DOI] [PubMed] [Google Scholar]
- (243).Wei W; Yu J; Gebbie MA; Tan Y; Martinez Rodriguez NR; Israelachvili JN; Waite JH Bridging Adhesion of Mussel-Inspired Peptides: Role of Charge, Chain Length, and Surface Type. Langmuir 2015, 31, 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Kim S; Faghihnejad A; Lee Y; Jho Y; Zeng H; Hwang DS Cation–π interaction in DOPA-deficient mussel adhesive protein mfp-1. J. Mater. Chem. B 2015, 3, 738–743. [DOI] [PubMed] [Google Scholar]
- (245).Lim C; Huang J; Kim S; Lee H; Zeng H; Hwang DS Nanomechanics of Poly(catecholamine) Coatings in Aqueous Solutions. Angew. Chem., Int. Ed 2016, 55, 3342–3346. [DOI] [PubMed] [Google Scholar]
- (246).Bae KH; Lee K; Kim C; Park TG Surface Functionalized Hollow Manganese Oxide Nanoparticles for Cancer Targeted siRNA Delivery and Magnetic Resonance Imaging. Biomaterials 2011, 32, 176–184. [DOI] [PubMed] [Google Scholar]
- (247).Ball V Composite Materials and Films Based on Melanins, Polydopamine, and Other Catecholamine-Based Materials. Biomimetics 2017, 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Dyke JC; Hu H; Lee DJ; Ko CC; You W The Role of Temperature in Forming Sol–gel Biocomposites Containing Polydop-amine. J. Mater. Chem. B 2014, 2, 7704–7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (249).Kim H; Jalili R; Spinks GM; Wallace GG; Kim SJ High-Strength Graphene and Polyacrylonitrile Composite Fiber Enhanced by Surface Coating with Polydopamine. Compos. Sci. Technol 2017, 149, 280–285. [Google Scholar]
- (250).Liu Y; Fang Y; Liu X; Wang X; Yang B Mussel-Inspired Modification of Carbon Fiber via Polyethyleneimine/Polydopamine Co-Deposition for the Improved Interfacial Adhesion. Compos. Sci. Technol 2017, 151, 164–173. [Google Scholar]
- (251).losterman L; Ahmad Z; Viswanathan V; Bettinger CJ Synthesis and Measurement of Cohesive Mechanics in Polydopamine Nanomembranes. Adv. Mater. Interfaces 2017, 4, 1700041. [Google Scholar]
- (252).Hu H; Dyke JC; Bowman BA; Ko CC; You W Investigation of Dopamine Analogues: Synthesis, Mechanistic Under-standing, and Structure-Property Relationship. Langmuir 2016, 32, 9873–9882. [DOI] [PubMed] [Google Scholar]
- (253).Kang X; Cai W; Zhang S; Cui S Revealing the Formation Mechanism of Insoluble Polydopamine by Using a Simplified Model System. Polym. Chem 2017, 8, 860–864. [Google Scholar]
- (254).Wu L; Feng H; Guo D; Zheng B Measuring the Adhesion Strength of a Thin Film to a Substrate by Centrifugation. RSC Adv 2014, 4, 60002–60006. [Google Scholar]
- (255).Lee BP; Dalsin JL; Messersmith PB Synthesis and Gelation of DOPA-Modified Poly(ethylene Glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [DOI] [PubMed] [Google Scholar]
- (256).Togashi T; Takami S; Kawakami K; Yamamoto H; Naka T; Sato K; Abe K; Adschiri T Continuous Hydrothermal Synthesis of 3,4-dihydroxyhydrocinnamic acid-Modified Magnetite Nanopar-ticles with Stealth-Functionality against Immunological Response. J. Mater. Chem 2012, 22, 9041–9045. [Google Scholar]
- (257).Contreras Rodríguez AR; Saiz-Poseu J; García-Pardo J; García B; Lorenzo J; Ojea-Jimenez I; Komilis D; Sedo J; Busque F; Sanchez A; Ruiz-Molina D; Font X Biocompatible Polydop-amine-Like Particles for the Removal of Heavy Metals at Extremely Low Concentrations. RSC Adv 2016, 6, 40058–40066. [Google Scholar]
- (258).Novio F; Ruiz-Molina D Hydrophobic Coordination Polymer Nanoparticles and Application for Oil–water Separation. RSC Adv 2014, 4, 15293–15296. [Google Scholar]
- (259).Garcia B; Saiz-Poseu J; Gras-Charles R; Hernando J; Alibes R; Novio F; Sedo J; Busque F; Ruiz-Molina D Mussel-Inspired Hydrophobic Coatings for Water-Repellent Textiles and Oil Removal. ACS Appl. Mater. Interfaces 2014, 6, 17616–17625. [DOI] [PubMed] [Google Scholar]
- (260).Saiz-Poseu J; Alcon I; Alibes R; Busque F; Faraudo J; Ruiz-Molina D Self-Assembly of Alkylcatechols on HOPG Inves-tigated by Scanning Tunneling Microscopy and Molecular Dynamics Simulations. CrystEngComm 2012, 14, 264–271. [Google Scholar]
- (261).Saiz-Poseu J; Faraudo J; Figueras A; Alibes R; Busque F; Ruiz-Molina D Switchable Self-Assembly of a Bioinspired Alkyl Catechol at a Solid/Liquid Interface: Competitive Interfacial, Non-covalent, and Solvent Interactions. Chem. - Eur. J 2012, 18, 3056–3063. [DOI] [PubMed] [Google Scholar]
- (262).Saiz-Poseu J; Sedo J; Garcia B; Benaiges C; Parella T; Alibes R; Hernando J; Busque F; Ruiz-Molina D Versatile Nanostructured Materials via Direct Reaction of Functionalized Catechols. Adv. Mater 2013, 25, 2066–2070. [DOI] [PubMed] [Google Scholar]
- (263).Guardingo M; Bellido E; Miralles-Lluma R; Faraudo J; Sedo J; Tatay S; Verdaguer A; Busque F; Ruiz-Molina D Bioinspired Catechol-Terminated Self-Assembled Monolayers with Enhanced Adhesion Properties. Small 2014, 10, 1594–1602. [DOI] [PubMed] [Google Scholar]
- (264).Ejima H; Richardson JJ; Liang K; Best JP; van Koeverden MP; Such GK; Cui J; Caruso F One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineer-ing. Science 2013, 341, 154–157. [DOI] [PubMed] [Google Scholar]
- (265).Ejima H; Richardson JJ; Caruso F Metal-Phenolic Networks as a Versatile Platform to Engineer Nanomaterials and Biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar]
- (266).Guo J; Ping Y; Ejima H; Alt K; Meissner M; Richardson JJ; Yan Y; Peter K; von Elverfeldt D; Hagemeyer CE; Caruso F Engineering Multifunctional Capsules through the Assembly of Metal-Phenolic Networks. Angew. Chem., Int. Ed 2014, 53, 5546–5551. [DOI] [PubMed] [Google Scholar]
- (267).Kim S; Gim T; Kang SM Versatile, Tannic Acid-Mediated Surface PEGylation for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2015, 7, 6412–6416. [DOI] [PubMed] [Google Scholar]
- (268).Lee J; Cho H; Choi J; Kim D; Hong D; Park JH; Yang SH; Choi IS Chemical Sporulation and Germination: Cytoprotective Nanocoating of Individual Mammalian Cells with a Degradable Tannic Acid-FeIII Complex. Nanoscale 2015, 7, 18918–18922. [DOI] [PubMed] [Google Scholar]
- (269).Park JH; Kim K; Lee J; Choi JY; Hong D; Yang SH; Caruso F; Lee Y; Choi IS A Cytoprotective and Degradable Metal-Polyphenol Nanoshell for Single-Cell Encapsulation. Angew. Chem., Int. Ed 2014, 53, 12420–12425. [DOI] [PubMed] [Google Scholar]
- (270).Oh DX; Prajatelistia E; Ju SW; Jeong Kim H; Baek SJ; Joon Cha H; Ho Jun S; Ahn JS; Soo Hwang D A Rapid, Efficient, and Facile Solution for Dental Hypersensitivity: The Tannin–iron Complex. Sci. Rep 2015, 5, 10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Kim HJ; Kim D-G; Yoon H; Choi Y-S; Yoon J; Lee J-C Polyphenol/FeIII Complex Coated Membranes Having Multifunc-tional Properties Prepared by a One-Step Fast Assembly. Adv. Mater. Interfaces 2015, 2, 1500298. [Google Scholar]
- (272).Yang Z; Yang Y; Yan W; Tu Q; Wang J; Huang N Construction of Polyfunctional Coatings Assisted by Gallic Acid to Facilitate Co-Immobilization of Diverse Biomolecules. ACS Appl. Mater. Interfaces 2013, 5, 10495–10501. [DOI] [PubMed] [Google Scholar]
- (273).Xiong K; Qi P; Yang Y; Li X; Qiu H; Li X; Shen R; Tu Q; Yang Z; Huang N Facile Immobilization of Vascular Endothelial Growth Factor on a Tannic Acid-Functionalized Plasma-Polymerized Allylamine Coating Rich in Quinone Groups. RSC Adv 2016, 6, 17188–17195. [Google Scholar]
- (274).Sileika TS; Barrett DG; Zhang R; Lau KH; Messersmith PB Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem., Int. Ed 2013, 52, 10766–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Barrett DG; Sileika TS; Messersmith PB Molecular Diversity in Phenolic and Polyphenolic Precursors of Tannin-Inspired Nanocoatings. Chem. Commun 2014, 50, 7265–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Abouelmagd SA; Meng F; Kim BK; Hyun H; Yeo Y Tannic Acid-Mediated Surface Functionalization of Polymeric Nano-particles. ACS Biomater. Sci. Eng 2016, 2, 2294–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Bai G; Ma S; Qie R; Liu Z; Shi Y; Li C; Wang R; Guo X; Zhou F; Jia X UV-Triggered Surface-Initiated Polymerization from Colorless Green Tea Polyphenol-Coated Surfaces. Macromol. Rapid Commun 2016, 37, 1256–1261. [DOI] [PubMed] [Google Scholar]
- (278).Ball V; Meyer F Deposition Kinetics and Electrochemical Properties of Tannic Acid on Gold and Silica. Colloids Surf., A 2016, 491, 12–17. [Google Scholar]
- (279).Behboodi-Sadabad F; Zhang H; Trouillet V; Welle A; Plumere N; Levkin PA UV-Triggered Polymerization, Deposition, and Patterning of Plant Phenolic Compounds. Adv. Funct. Mater 2017, 27, 1700127. [Google Scholar]
- (280).Das C; Jain B; Krishnamoorthy K Phenols from Green Tea as a Dual Functional Coating to Prepare Devices for Energy Storage and Molecular Separation. Chem. Commun 2015, 51, 11662–11664. [DOI] [PubMed] [Google Scholar]
- (281).Fang Y; Gonuguntla S; Soh S Universal Nature-Inspired Coatings for Preparing Noncharging Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 32220–32226. [DOI] [PubMed] [Google Scholar]
- (282).Geissler S; Barrantes A; Tengvall P; Messersmith PB; Tiainen H Deposition Kinetics of Bioinspired Phenolic Coatings on Titanium Surfaces. Langmuir 2016, 32, 8050–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (283).Hu Z; Berry RM; Pelton R; Cranston ED One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nano-crystals Using Plant Polyphenols. ACS Sustainable Chem. Eng 2017, 5, 5018–5026. [Google Scholar]
- (284).Huang S; Zhang Y; Shi J; Huang W Superhydrophobic Particles Derived from Nature-Inspired Polyphenol Chemistry for Liquid Marble Formation and Oil Spills Treatment. ACS Sustainable Chem. Eng 2016, 4, 676–681. [Google Scholar]
- (285).Iacomino M; Paez JI; Avolio R; Carpentieri A; Panzella L; Falco G; Pizzo E; Errico ME; Napolitano A; Del Campo A; d’Ischia M Multifunctional Thin Films and Coatings from Caffeic Acid and a Cross-Linking Diamine. Langmuir 2017, 33, 2096–2102. [DOI] [PubMed] [Google Scholar]
- (286).Kim JY; Lee H; Park T; Park J; Kim MH; Cho H; Youn W; Kang SM; Choi IS Artificial Spores: Cytocompatible Coating of Living Cells with Plant-Derived Pyrogallol. Chem. - Asian J 2016, 11, 3183–3187. [DOI] [PubMed] [Google Scholar]
- (287).Lee JS; Lee JS; Lee MS; An S; Yang K; Lee K; Yang HS; Lee H; Cho S-W Plant Flavonoid-Mediated Multifunctional Surface Modification Chemistry: Catechin Coating for Enhanced Osteogenesis of Human Stem Cells. Chem. Mater 2017, 29, 4375–4384. [Google Scholar]
- (288).Li J; Wu S; Wu C; Qiu L; Zhu G; Cui C; Liu Y; Hou W; Wang Y; Zhang L; Teng IT; Yang HH; Tan W Versatile Surface Engineering of Porous Nanomaterials with Bioinspired Polyphenol Coatings for Targeted and Controlled Drug Delivery. Nanoscale 2016, 8, 8600–8606. [DOI] [PubMed] [Google Scholar]
- (289).Lim M-Y; Shin H; Shin DM; Lee S-S; Lee J-C Poly(vinyl alcohol) Nanocomposites Containing Reduced Graphene Oxide Coated with Tannic Acid for Humidity Sensor. Polymer 2016, 84, 89–98. [Google Scholar]
- (290).Roman MJ; Decker EA; Goddard JM Biomimetic Polyphenol Coatings for Antioxidant Active Packaging Applications. Colloid Interface Sci. Commun 2016, 13, 10–13. [Google Scholar]
- (291).Song W; Tao S; Yu Y; Du X; Wang S Preparing Magnetic Multicomponent Catalysts via a Bio-Inspired Assembly for Heteroge-neous Reactions. RSC Adv 2016, 6, 69909–69918. [Google Scholar]
- (292).Wang H; Wang C; Tao S; Qiu J; Yu Y; Gu M Biomimetic Preparation of Hybrid Porous Adsorbents for Efficiently Purifying Complex Wastewater. ACS Sustainable Chem. Eng 2016, 4, 992–998. [Google Scholar]
- (293).Wang Z; Xu C; Li X; Liu Z In situ green synthesis of Ag nanoparticles on tea polyphenols-modified graphene and their catalytic reduction activity of 4-nitrophenol. Colloids Surf., A 2015, 485, 102–110. [Google Scholar]
- (294).Watanabe H; Fujimoto A; Nishida J; Ohishi T; Takahara A Biobased Polymer Coating Using Catechol Derivative Urushiol. Langmuir 2016, 32, 4619–4623. [DOI] [PubMed] [Google Scholar]
- (295).Wu C; Li T; Liao C; Li L; Yang J Tea Polyphenol-Inspired Tannic Acid-Treated Polypropylene Membrane as a Stable Separator for Lithium–Oxygen Batteries. J. Mater. Chem. A 2017, 5, 12782–12786. [Google Scholar]
- (296).Yeon DK; Ki SH; Choi J; Kang SM; Cho WK Formation of Turmeric-Based Thin Films: Universal, Transparent Coatings. Langmuir 2017, 33, 3639–3646. [DOI] [PubMed] [Google Scholar]
- (297).Zhan K; Ejima H; Yoshie N Antioxidant and Adsorption Properties of Bioinspired Phenolic Polymers: A Comparative Study of Catechol and Gallol. ACS Sustainable Chem. Eng 2016, 4, 3857–3863. [Google Scholar]
- (298).Zhang S; Wu L; Deng F; Zhao D; Zhang C; Zhang C Hydrophilic Modification of PVDF Porous Membrane via a Simple Dip-Coating Method in Plant Tannin Solution. RSC Adv 2016, 6, 71287–71294. [Google Scholar]
- (299).Zhang S; Jiang Z; Wang X; Yang C; Shi J Facile Method To Prepare Microcapsules Inspired by Polyphenol Chemistry for Efficient Enzyme Immobilization. ACS Appl. Mater. Interfaces 2015, 7, 19570–19578. [DOI] [PubMed] [Google Scholar]
- (300).Zhang X; Lv Y; Yang HC; Du Y; Xu ZK Polyphenol Coating as an Interlayer for Thin-Film Composite Membranes with Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2016, 8, 32512–32519. [DOI] [PubMed] [Google Scholar]
- (301).Zhang X; Ren P-F; Yang H-C; Wan L-S; Xu Z-K Co-Deposition of Tannic Acid and Diethlyenetriamine for Surface Hydrophilization of Hydrophobic Polymer Membranes. Appl. Surf. Sci 2016, 360, 291–297. [Google Scholar]
- (302).Zhang Y; Baekgaard-Laursen M; Mouritzen SA; Lynge ME; Schattling PS; Danial M; Postma A; Stadler B. Tannic Acid and Cholesterol-Dopamine as Building Blocks in Composite Coatings for Substrate-Mediated Drug Delivery. Polym. Int 2016, 65, 1306–1314. [Google Scholar]
- (303).Zhou B; Hu X; Zhu J; Wang Z; Wang X; Wang M Release Properties of Tannic Acid from Hydrogen Bond Driven Antioxidative Cellulose Nanofibrous Films. Int. J. Biol. Macromol 2016, 91, 68–74. [DOI] [PubMed] [Google Scholar]
- (304).Zelasko-Leon DC; Fuentes CM; Messersmith PB MUC1-Targeted Cancer Cell Photothermal Ablation Using Bio-inspired Gold Nanorods. PLoS One 2015, 10, e0128756. [DOI] [PMC free article] [PubMed] [Google Scholar]


