Abstract
Context
Orbital tissues in thyroid-associated ophthalmopathy exhibit particular reactivity and undergo characteristic remodeling. Mechanisms underlying these changes have remained largely unexplained. Studies have characterized orbital connective tissues and derivative fibroblasts to gain insights into local manifestations of a systemic autoimmune syndrome.
Evidence Acquisition
A systematic search of PubMed was undertaken for studies related to thyroid-associated ophthalmopathy (TAO), orbital fibroblasts, and fibrocytes involved in pathogenesis.
Evidence Synthesis
Orbital tissues display marked cellular heterogeneity. Fibroblast subsets, putatively derived from multiple precursors, inhabit the orbit in TAO. Among them are cells displaying the CD34+CXC chemokine receptor 4+collagen I+ phenotype, identifying them as fibrocytes, derived from the monocyte lineage. Their unique presence in the TAO orbit helps explain the tissue reactivity and characteristic remodeling that occurs in the disease. Their unanticipated expression of several proteins traditionally thought to be thyroid gland specific, including the TSH receptor and thyroglobulin, may underlie orbital involvement in Graves disease. Although no currently available information unambiguously establishes that CD34+ orbital fibroblasts originate from circulating fibrocytes, inferences from animal models of lung disease suggest that they derive from bone marrow. Further studies are necessary to determine whether fibrocyte abundance and activity in the orbit determine the clinical behavior of TAO.
Conclusion
Evidence supports a role for fibrocytes in the pathogenesis of TAO. Recognition of their presence in the orbit now allows development of therapies specifically targeting these cells that ultimately could allow the restoration of immune tolerance within the orbit and perhaps systemically.
Literature pertaining to the putative involvement of bone marrow–derived fibrocytes in thyroid-associated ophthalmopathy is reviewed.
The orbit in Graves disease (GD) exhibits remarkable reactivity (1). In the most severe cases, thyroid-associated ophthalmopathy (TAO) can distort tissue architecture, cause dysfunction, and impair vision. Much of the functional disruption associated with TAO results from end-stage fibrosis. Typically, the course of TAO can be parsed into two clinically recognizable phases, as described by Rundle and Wilson (2). Initially, it presents as a constellation of signs and symptoms attributable to inflammation and localized edema that can extend beyond the anatomic boundaries of the orbit to the upper face. This active phase usually lasts from 1 to 3 years. It gives way to the chronic/stable phase in which the disease ceases changing and when processes, such as fat expansion, muscle enlargement, and fibrosis, are thought to be irreversible. Underlying this characteristic pattern of tissue remodeling appear to be orbital fibroblasts (OFs), which are cells possessing unusual phenotypic attributes. The nature and derivation of OFs have long been speculated about but their precise lineage has remained uncertain. They are generally considered to be neural crest in origin (3). Besides residential OFs, fibroblasts within the orbit could also result from epithelial–mesenchymal transitions (4) or represent cells infiltrating from other anatomic regions, most notably the bone marrow (5, 6).
OFs derived from orbital tissues manifesting TAO exhibit particular cellular heterogeneity and exaggerated responses to many molecular factors in vitro. They hereafter are referred to as GD-OFs. Their unusual characteristics were initially recognized nearly 50 years ago when Sisson et al. (7) began cultivating OFs in vitro. These investigators determined that the release of glycosaminoglycans and glucose utilization in GD-OFs were enhanced when cocultured with lymphocytes (8). These findings remained largely unembellished for nearly two decades before Bahn et al. (9) proposed that cultured GD-OFs might be exploited as a model for studying TAO. Around this same time, Hiromatsu et al. (10) were cultivating OFs and comparing their susceptibility to antibody-dependent cell-mediated cytotoxicity with that of muscle cells. Weetman et al. (11) conducted immunohistochemical analysis of TAO orbital tissues and concluded that the interstitial cells were the likely immune targets in the disease. A number of studies have been performed subsequently examining the ultrastructure of GD-OFs (12, 13), their morphologies (14), and their biochemical attributes (15). Furthermore, it has become clear that GD-OFs derived from orbital fat diverge from those associated with extraocular muscles (14, 16). Of note, GD-OFs express the functional TSH receptor (TSHR) (17, 18). Levels of this receptor are very low compared with those found on thyroid epithelial cells but can be enhanced with their differentiation into adipocytes (19).
Many Aspects of Tissue Remodeling in TAO Can Be Directly Linked to the Phenotypes of GD-OFs
One of the dominant characteristics of fibroblasts in general and GD-OFs in particular concerns their capacity to generate substantial amounts of glycosaminoglycans (20). A cardinal feature of TAO is the disordered accumulation of these macromolecules in the orbit and upper face (20, 21). This material is largely hyaluronan (HA), the most abundant, nonsulfated glycosaminoglycan and one that lacks a core protein (22). HA associates with cellular receptors, CD44 (23) and the receptor for HA-mediated motility (24, 25), and through these interactions initiates cellular responses. It plays a key role in the orbital tissue remodeling and expansion occurring in TAO (26). This can be attributed to its impact on vascular permeability, water content, and the chemoattractant properties of its fragments (27). Its rheological properties include an enormous capacity for water binding. Chain length is an important determinant of the biological impact that HA exerts within tissues (28). Short-chain HA can elicit responses in a wide range of target cell types (29, 30).
Among the early, pivotal insights into the mechanisms underlying HA generation was the subcellular localization of its synthesis to the plasma membrane (31). Subsequently, three mammalian HA synthase (HAS) isoenzymes were identified and cloned (32, 33). The gene encoding uridine 5′-diphosphate (UDP) glucose dehydrogenase was also cloned (34). This enzyme catalyzes the oxidation of UDP glucose to UDP glucuronic acid upstream from the HAS proteins (34). Gene promoters for these enzymes have been cloned (35, 36). HA is produced in the orbit primarily by GD-OFs (20), cells that express all three HAS isoenzymes. HAS2 is the most abundant and appears to dominate HA synthesis in these cells (37). UDP glucose dehydrogenase is also expressed by GD-OFs and can be induced by IL-1β (34, 36). HA synthesis is enhanced in GD-OFs by several cytokines, including IFN-γ (38), leukoregulin (39), CD40 ligand (CD154) (40), and IL-1β (37). A recent study disclosed that IGF-I could also induce HA synthesis in GD-OFs (41) and could skew generation toward high–molecular mass HA in perimysial GD-OFs (42). In contrast, one report found that the induction by IGF-I of HAS2 mRNA in GD-OFs occurred only in the presence of a MAPK inhibitor (43). Forkhead transcription factors have a potentially important role in regulating HA synthesis in these cells (44).
Fat expansion frequently contributes to tissue remodeling in TAO and in some cases predominates in the development of proptosis (45). Adipogenic potential of GD-OFs appears to underlie, at least in part, expansion of orbital tissue (46, 47). These cells express peroxisome proliferator–activated receptor γ (PPARγ). When this receptor is activated, GD-OFs undergo differentiation into triglyceride-accumulating adipocytes (14, 46, 48). Once differentiated, they express elevated levels of TSHR compared with undifferentiated cells (19). It is currently thought that the combination of HA accumulation and adipogenic activity of GD-OF accounts for expansion of orbital connective tissue in TAO.
GD-OFs as Sources of Proinflammatory Cytokines
GD-OFs generate several cytokines that appear to play roles in TAO (1). Many of these have been detected in affected orbital tissues or were found to be generated in activated GD-OFs in vitro. Cytokines detected in situ in TAO include TNF-α, IL-1α, IL-6, IL-8, IL-10, IL-12, IL-13, and IFN-γ (49, 50). Several of these are more highly expressed in active vs stable disease and include IL-1β, IL-6, IL-8, and IL-10 (50). That study also suggested a predominance of T helper (Th)1 cytokines in active TAO. Both IFN-γ and TNF-α induce B cell activating factor in GD-OFs, an induction that appears to be more robust than in cells cultivated from healthy orbital tissues (51). IL-1β induces both IL-16 and RANTES in GD-OFs and in so doing enhances the release of T cell migration-promoting activity (52). The same actions are true for IGF-I and immunoglobulins from patients with GD (53, 54). Additionally, IL-1β, IL-1α, IL-6, IL-1 receptor antagonist, and TGF-β are inducible in GD-OFs (55–57).
GD-OFs Express Molecular Machinery to Generate Arachidonic Acid Derivatives
The repertoire of proinflammatory genes expressed and highly inducible in GD-OFs includes those encoding biosynthetic enzymes for generating prostanoids and eicosanoids. In the cytokine-activated state, GD-OFs produce substantial levels of prostaglandin E2 (PGE2) (58) and 15-hydroxyeicosatetraenoic acid (59). This results from active arachidonic acid generation and from the robust induction of several enzymes. CD40 ligand, leukoregulin, and IL-1β induce high levels of prostaglandin endoperoxide H synthase-2 (PGHS-2), the inflammatory cyclooxygenase (15, 40), and glutathione-dependent PGE2 synthase (60). Both specific and nonselective cyclooxygenase inhibitors and glucocorticoids attenuate the cytokine-dependent PGE2 synthesis in GD-OFs. A recent report suggests that sphingosine-1-phosphate (S1P) pathways are involved in mediating the induction of PGHS-2 in GD-OFs (61). That report suggests that the five S1P receptor subtypes are differentially expressed in tissues from patients with TAO compared with healthy orbital tissues. Levels of S1P1, S1P2, and S1P3 receptors are elevated in TAO whereas those for S1P4 and S1P5 are higher in healthy tissues. PGE2 can drive the T cell skew toward a Th2 phenotype and can enhance immunoglobulin synthesis by B cells (62, 63). When engaged with the Th2 cytokine, IL-4, these cells express 15-lipoxygenase-1 (59).
Understanding the heterogeneity of OFs in TAO
GD-OFs are considerably more diverse with regard to their cellular phenotypes in vitro than are fibroblast cultures from healthy orbits and those from other tissues such as skin. Among the first cell markers used to distinguish fibroblast populations was the surface molecule CD90 [thymocyte antigen 1 (Thy-1)], the natural ligand for which remains uncertain but whose role in lymphocyte and thymocyte behavior appears vast (64, 65). Although the proportion of Thy-1+ and Thy-1− cells differs among individual donors, most cultures derived from orbital fat can be bisected on the basis of cells displaying that protein (66, 67). In striking contrast, perimysial fibroblasts cultivated from extraocular muscles uniformly display Thy-1 (14). When Thy-1+ and Thy-1− GD-OFs were compared side by side, distinct functional differences emerged. Following separation into pure Thy-1+ and Thy-1− subsets, both cell populations generate IL-6 when activated by CD40 ligand or IL-1β (67). In contrast, Thy-1+ fibroblasts express considerably higher levels of PGHS-2 and generate more PGE2. Thy-1− fibroblasts synthesize and release more IL-8 under similar culture conditions. Thy-1+ cells can differentiate into myofibroblasts when treated with TGF-β whereas Thy-1− fibroblasts accumulate cytoplasmic triglycerides when exposed to PPARγ agonists such as 15-deoxy-Δ12,14-PGJ2 or ciglitazone (68). Thus, GD-OFs comprise discrete populations of cells with distinct markers and specialized capacities for biosynthesis and terminal differentiation. An as yet unresolved question concerns whether the different cell types within the TAO orbit derive from a common progenitor or whether some or all GD-OFs originate outside the orbit.
Fibrocytes Play Important Roles in Wound Repair and Fibrosis
Fibrocytes represent a unique, fibroblast-like cell type first described by Bucala et al. (5). They exhibit a CD45+CD34+CXC chemokine receptor (CXCR)4+collagen I+ phenotype, the constellation of markers that allows them to be distinguished from other cells with similar morphologies (69). They are relatively rare among circulating mononuclear cells and emanate from the bone marrow where they derive from progenitor cells of the monocyte lineage (69). Their abundance increases in certain diseases such as systemic sclerosis (70) and GD (71, 72). Fibrocytes traffic to sites of tissue injury as a consequence of chemokine networks such as CXC chemokine ligand 12/CXCR4 (73), CC chemokine ligand (CCL)12/CC chemokine receptor (CCR)2 (74), CCL21/CCR7 (75), and CCL3/CCR5 ligand/receptor cognates (76). Important differences in the particular chemokines used for fibrocyte trafficking appear to exist in human beings when compared with mice. They enter injured tissues where they can produce collagen and other extracellular matrix constituents as well as many physiologically important cytokines (77). Several factors appear to determine whether fibrocytes engage in tissue remodeling or exhibit a particular inflammatory phenotype. They are involved in immune responses and host defense. They express major histocompatibility class II (MHC-II) constitutively, as well as an array of costimulatory molecules (78, 79). They also engage in bidirectional crosstalk with T cells. Fibrocytes can prime T cells and depend on contact with lymphocytes that support their differentiation from CD14+ monocytes (6). In mice, fibrocyte differentiation from CD11b+CD155+Gr1+ monocytes is dependent on CD4+ T cells (80). In general, cytokines such as INF-γ and IL-12 (Th1) inhibit fibrocyte differentiation from monocytes, whereas Th2 cytokines, such as IL-4 and IL-13, enhance that process (81). They participate in the fibrosis associated with several disease processes, including experimental bleomycin-induced lung fibrosis (76). Much of what we currently understand about fibrocyte biology comes from studies of lung fibrosis in mouse models (82). These models are currently being used to identify strategies for minimizing the morbidity associated with fibrocyte-directed fibrosis (83–85).
Fibrocytes exhibit remarkable developmental plasticity and can differentiate into multiple cell types, depending on the intracellular signaling pathways that become activated. These events, in turn, result from extracellular molecular cues coming from their cellular neighborhood. Fibrocytes undergo adipogenesis when exposed to PPARγ agonists (86). In contrast, TGF-β and endothelin 1 drive differentiation toward the myofibroblast phenotype while inhibiting adipogenesis. It is the myofibroblast phenotype, particularly the expression of smooth muscle actin, which conveys the contractile qualities of wound healing associated with fibrocytes. PPARγ activation disrupts the stress-activated protein kinase/Jun N-terminal kinase signaling initiated by TGF-β. Thus, the molecular niche surrounding fibrocytes in the tissues where they infiltrate critically determines their differentiation pathway.
Fibrocytes have been implicated in several disease processes such as those associated with idiopathic pulmonary fibrosis (87). The abundance of CD45−collagen I+ fibrocytes was found to be elevated in the blood of patients with stable idiopathic pulmonary fibrosis (87). Levels were further increased, up to 10-fold, above those found in stable disease during acute exacerbations. Other comparators, such as patients with adult respiratory distress syndrome, were found to have normal fibrocyte levels (87). Survival of patients with more abundant fibrocytes was considerably shorter than that of individuals with lower levels, suggesting that relative fibrocyte frequency may have predictive value for clinical outcome. Nephrogenic systemic fibrosis occurs in individuals manifesting dermal hardening and thickening associated with renal insufficiency (88). Typically, the affected dermal tissues are infiltrated with fibrocytes, evidence of mucin deposition and thickened collagen bundles. Systemic sclerosis can also be associated with increased levels of fibrocytes (89).
Identifying Fibrocytes as Discrete, Putative Progenitors of a GD-OF Subset in TAO
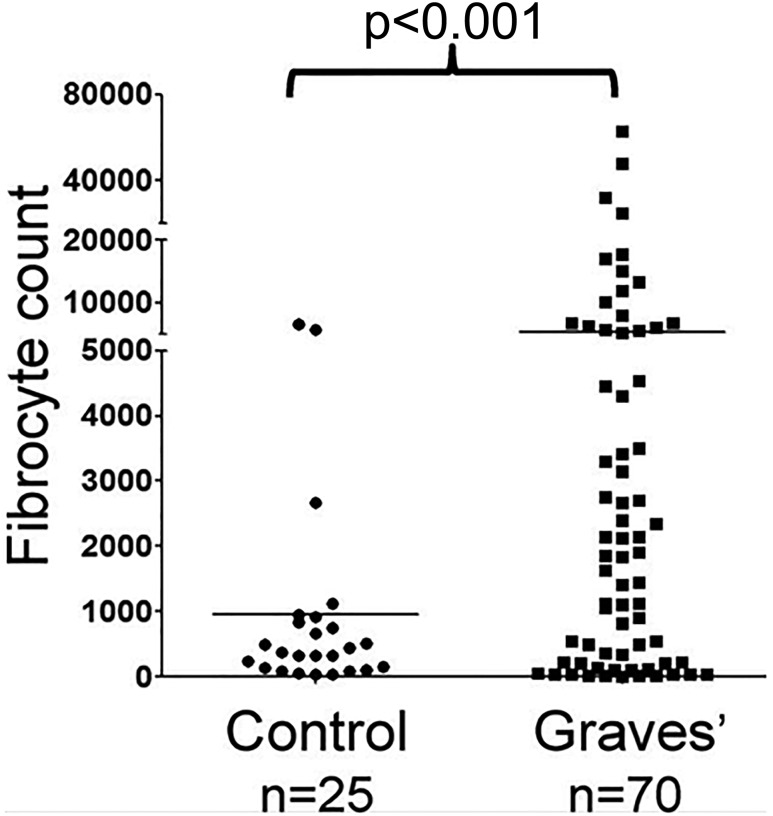
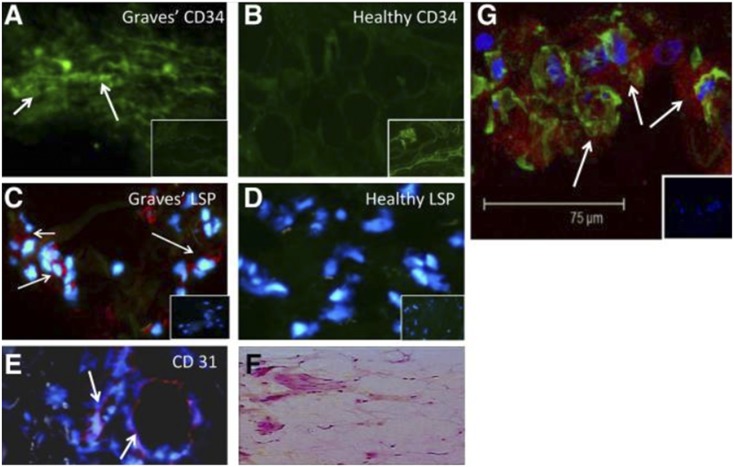
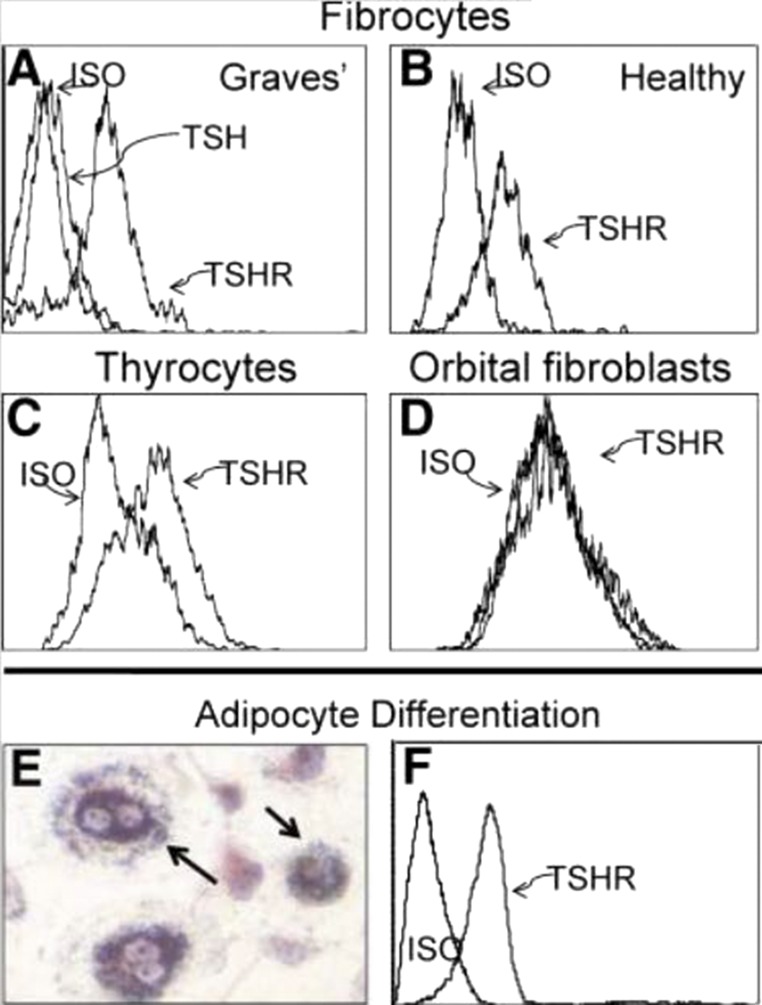
Fibrocytes have been implicated in the development of multiple ocular-related diseases (90). Among these, diabetic retinopathy development may be mediated at least in part by fibrocytes (91). In that process, fibrovascular membrane formation may result from their activities. Bone marrow–derived cells contributing to the myofibroblast population in corneal wounds (92) exhibit features suggestive of their derivation from fibrocytes. In GD, they have been identified as orbit-infiltrating cells (71). Circulating CD34+ fibrocytes become considerably more abundant in patients with GD than in healthy individuals (Fig. 1). Furthermore, it appeared that patients with severe, active TAO exhibited among the highest levels of circulating fibrocytes. CD34+ cells have been identified in situ in deep TAO orbital fat. A subpopulation of GD-OFs cultivated from those tissues display CD34 (Fig. 2) (71). A similar fraction of cells failed to surface display CD34, and thus the population of GD-OFs can be subdivided into two discrete subsets with identical morphologies. Whereas the GD-OF population comprises the mixture of CD34+ and CD34− cells (∼50% CD34+ OFs), those from healthy orbital tissues are uniformly CD34− fibroblasts. It has yet to be established whether CD34+ OFs infiltrate the orbit from the circulation or arise from another source. Among CD34+ OFs are those that strongly express TSHR. Levels of TSHR are substantially higher in fibrocytes cultivated from blood than in GD-OFs (Fig. 3) and the receptor is considerably more abundant on CD34+ OFs than CD34− OFs (93). It is functional in both fibrocytes and GD-OFs; TSH and thyroid-stimulating immunoglobulins (TSIs) induce expression of several cytokines, such as IL-6. TSH induces TNF-α in fibrocytes, a response that is essentially absent in GD-OFs (94). Additionally, signaling initiated through TSHR also results in an induction of IL-1β, IL-1 receptor antagonist, and IL-12 (57, 95, 96). Subjecting fibrocytes to adipogenic culture conditions further enhances TSHR expression. Fibrocyte signaling pathways downstream from TSHR crosstalk with the CXC chemokine ligand 12/CXCR4 network (97), suggesting the potential for TSH/TSI to influence cell trafficking. Fang et al. (98) have reported potentially important interactions between CCR6+ Th17 cells and fibrocytes. Circulating Th17 cells are more abundant in TAO and the IL-17 receptor is expressed at higher levels in TAO-derived fibrocytes (99).
Figure 1.
Increased frequency of CD34+ fibrocyte generation from the peripheral blood mononuclear cells of 70 patients with GD compared with 25 healthy control donors. P < 0.001. Reproduced with permission from Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010; 95(1):430-438.
Figure 2.
CD34+lymphocyte-specific protein-1 (LSP-1)+TSHR+ fibrocytes can be identified in the orbital tissue of patients with TAO but are absent in tissues from healthy donors. (A) CD34 expression (arrows, green; fluorescein isothiocyanate) in TAO-derived tissue (inset shows negative control staining). (B) Absent CD34 expression in healthy tissue (inset shows positive staining control). (C) LSP-1 expression in TAO-derived tissue (red, arrows; nuclei counterstained with 4′,6′-diamidino-2-phenylindole; inset shows negative control). (D) Absence of LSP-1 expression in healthy tissue (inset shows negative control). (E) CD31 expression in disease-derived tissue is limited to vascular endothelium (red, arrows). (F) Hematoxylin and eosin–stained consecutive thin sections of the same orbital tissue (original magnification, ×40). (G) Fibrocytes present in orbital tissue from patients with TAO coexpress CD34 and TSHR as demonstrated by confocal microscopy. Thin-sectioned tissue from a donor with TAO was stained with anti-CD34 (green) and anti-TSHR (red) antibodies. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole. The inset contains a negative staining control. Reproduced with permission from Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010; 95(1):430-438.
Figure 3.
(A and B) Fibrocytes cultivated from peripheral blood mononuclear cells express high levels of TSHR, regardless of whether they derive from (A) patients with GD or (B) healthy donors. (C) TSHR levels are comparable with those found on primary human thyroid epithelial cells. (D) Undifferentiated OFs fail to express TSHR. (E) Fibrocytes differentiated into adipocytes accumulate intracellular lipid droplets staining with Oil Red O. (F) TSHR levels on fibrocytes remain elevated after differentiation. ISO, isotype control. Reproduced with permission from Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010; 95(1):430-438.
In addition to TSHR, fibrocytes and GD-OFs express high levels of IGF-I receptor (IGF-IR) (100). Inhibiting this receptor activity with the therapeutic monoclonal antibody teprotumumab reduces the surface display of both TSHR and IGF-IR. Furthermore, teprotumumab and another IGF-IR inhibitory monoclonal antibody, 1H7, attenuate the actions of both TSH and IGF-I (41, 95, 100).
Fibrocytes Promiscuously Express Autoantigens
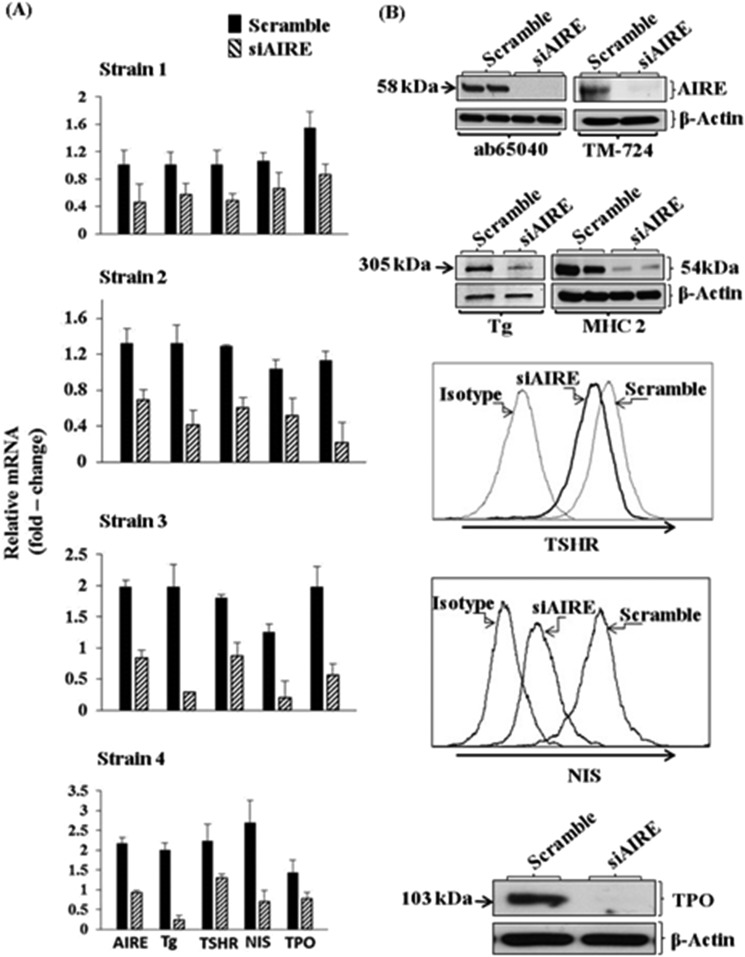
Fibrocytes exhibit a characteristic repertoire of gene expression that includes proteins identified as autoantigens in endocrine autoimmune diseases. They express islet antigen 2 and islet cell autoantigen 69, two self-antigens associated with type I diabetes mellitus (101). Relevant to GD, fibrocytes express not only TSHR but also thyroglobulin (Tg), thyroperoxidase, and sodium iodide symporter (93, 102), proteins initially thought to be restricted to the thyroid gland (93). Their expression is dependent on the noncanonical transcription factor autoimmune regulator protein (AIRE) (Fig. 4). Knocking down AIRE expression with targeting small interfering RNA (siRNA) depresses thyroid protein levels (102). Moreover, fibrocytes from an individual with autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy/dysplasia type 1 syndrome, who harbored a loss-of-function AIRE mutation, were found to express significantly lower levels of these proteins than cells from an unaffected first-degree relative. Preliminary studies suggest that the expression of islet antigen 2 and islet cell autoantigen 69 in fibrocytes may be independent of AIRE (101).
Figure 4.
Interference with AIRE expression knocks down levels of AIRE, Tg, TSHR, sodium iodide symporter (NIS), and thyroperoxidase (TPO). (A) Fibrocytes from four different donors were transfected either with scrambled (control) siRNA or that directed against AIRE. RNA was harvested and subjected to real-time PCR. Data are expressed as mean ± SD. (B) Fibrocyte cultures were transfected with either scrambled siRNA or AIRE-targeting siRNA, and Tg protein was quantified by labeling with [35S]methionine (40 µCi/mL) and then immunoprecipitated. TSHR and NIS were quantified by flow cytometry. Levels of TPO were assessed by Western blotting. Adapted with permission from Fernando R, Lu Y, Atkins SJ, et al. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab 2014; 99(7):E1236-E1244.
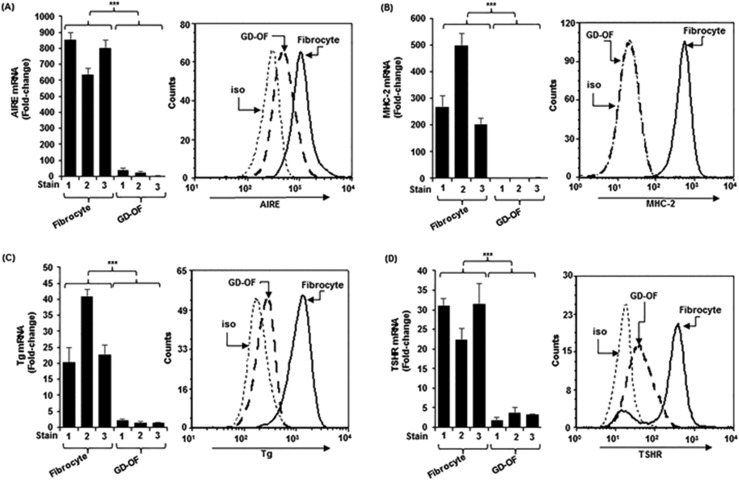
In contrast to the relatively high levels of thyroid proteins and AIRE expression in fibrocytes, those proteins are either undetectable in GD-OFs or the levels are extremely low (Fig. 5) (93, 102). When separated into pure CD34+ OF and CD34− OF subsets, their levels increase substantially in CD34+ OFs (93, 102). Furthermore, the amplitude of cytokine induction by TSH is considerably higher in pure CD34+ OFs when compared with the mixed population of GD-OFs (94, 103). Thus, CD34− OFs express an inhibitory factor that reduces the expression of several thyroid-related proteins and AIRE as well as cytokine induction by TSH in CD34+ OFs (93, 94, 103).
Figure 5.
(A–D) Higher levels of (A) AIRE, (B) MHC-II, (C) Tg, and (D) TSHR in fibrocytes than OFs from patients with GD (GD-OFs). (Left panels) RNA was reverse transcribed and subjected to real-time PCR. (Right panels) Cells were stained with the labeled antibodies for flow cytometry. mRNA data were normalized to their respective glyceraldehyde-3-phosphate dehydrogenase levels and are expressed as the mean ± SD. Data from flow analysis denote fluorescence intensity compared with isotype controls. ***P < 0.001. iso, isotype control. Reproduced with permission from Fernando R, Diniz Grisolia AB, Lu Y, et al. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol 2018; 200(12):3942-3949. Copyright 2018 The American Association of Immunologists, Inc.
Is Slit2 an Endogenous Fibrocyte Regulatory Factor Within the Orbit in TAO?
Finding that CD34− OFs exert a strong modulatory influence on their CD34+ OF counterparts provoked inquiry into the identity and characteristics of this inhibitory activity. That factor appeared to be soluble and released from cell monolayers (93). It could be conveyed to CD34+ OFs and circulating fibrocytes by covering those cells with conditioned medium from CD34− OFs (94). Several candidates were considered, including known soluble coactivational molecules involved in the interplay between fibroblasts and lymphocytes (103–106). Proteins exerting influence on cell migration and development were also considered. The axon guidance glycoprotein Slit2 (107, 108) plays critical roles in normal development of the mammalian central nervous system (109). Slit2 imposes the integrity of the midline and enforces the proper organization of the brain. It belongs to a family of three secreted glycoprotein orthologs (110) that bind to receptors known as Roundabouts (ROBOs) (111, 112). ROBOs are transmembrane receptors distributed widely within the central nervous system and in other tissues as well. Slit2 has been shown to also play roles outside the nervous system, such as regulating the inflammatory response (113). It inhibits monocyte chemotaxis through actions mediated by ROBO1 (114) and modulates immune reactivity. Recent evidence suggests that Slit2 can attenuate the differentiation of monocytes into fibrocytes (115). Those pivotal studies indicated that Slit2 generated by fibroblasts surrounding injured lung tissues helps determine the magnitude of fibrosis. Thus, it may represent an important governor of end-stage tissue remodeling and scar formation.
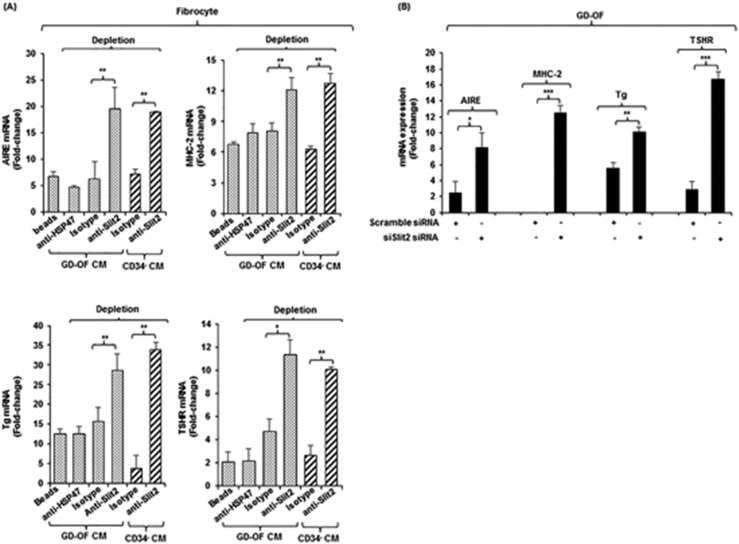
Given the effects of Slit2 on fibrocyte differentiation, examination of its potential impact on the expression of immune- and thyroid-related genes in these cells was undertaken. Slit2 was found to be expressed by GD-OFs, activity that localized specifically to CD34− OFs (103). The protein is highly inducible by TSH and M22, a monoclonal TSI (116), through both transcriptional and posttranscriptional mechanisms. Medium conditioned by CD34− OFs substantially reduces levels of AIRE, Tg, TSHR, and MHC-II when incubated with fibrocytes. Levels of expression can be restored by specifically adsorbing out Slit2 (Fig. 6) (103). Recombinant human Slit2 mimics the actions of the CD34− OF conditioned medium and does so by downregulating the transcription of genes encoding AIRE and Tg (103). Thus, Slit2 appears to represent at least one factor that is expressed and released by CD34− OFs and that may modulate the proinflammatory phenotype of orbit-infiltrating fibrocytes in TAO (Fig. 7).
Figure 6.
Depleting medium conditioned by GD-OFs of Slit2 restores fibrocyte gene expression. (A) Conditioned media from GD-OFs and CD34− OFs were incubated with uncoated beads or beads coated with anti-Slit2 (100 µg), anti-HSP47 (100 µg), or isotype IgG (100 µg). Fibrocyte monolayers were incubated with these media for 5 d. (B) Knocking down Slit2 with specific siRNA enhances gene expression in GD-OFs. GD-OFs were transfected with either scrambled (control) siRNA (3 µg) or Slit2-specific siRNA (3 µg) and incubated 3 d. RNA was extracted, reversed transcribed, and cDNAs were subjected to real-time PCR for the targets indicated. Values were normalized to their respective glyceraldehyde-3-phosphate dehydrogenase levels and expressed as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. Reproduced with permission from Fernando R, Diniz Grisolia AB, Lu Y, et al. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol 2018; 200(12):3942-3949. Copyright 2018 The American Association of Immunologists, Inc.
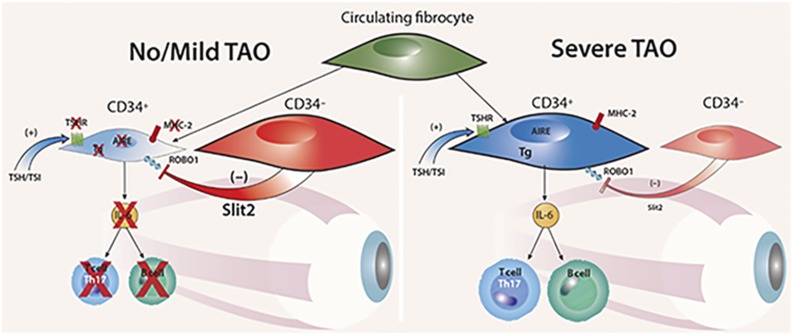
Figure 7.
Theoretical model for the modulatory role of Slit2 on intraorbital pathogenesis of TAO. When CD34− OF-derived Slit2 predominates, the inflammatory phenotype of CD34+ OFs is downregulated and the patient with GD either fails to manifest TAO or the disease is mild (left panel). When CD34+ OFs dominate the fibroblast population and the impact of Slit2 is inadequate, TAO is severe (right panel). Reproduced with permission from Fernando R, Diniz Grisolia AB, Lu Y, et al. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol 2018; 200(12):3942-3949. Copyright 2018 The American Association of Immunologists, Inc.
Fibrocytes and Their Putative Derivative CD34+ OFs as Potential Therapeutic Targets in TAO
Given their potential role in the pathogenesis of TAO, fibrocytes might be targeted as a therapeutic strategy. This approach would in theory aim at interrupting both the inflammatory responses and tissue remodeling. The underlying conceptual premise of targeting fibrocytes hinges on their putative regulation of the quality and duration of the tissue disruption and repair. They express several antigens specific to thyroid autoimmunity (71, 93, 102), efficiently present antigens to T cells, display critical coactivational molecules such as CD80 and CD86 (78, 117), and generate an extensive array of cytokines (97, 118, 119). Thus, they could be considered a “switchboard” for the immune responses occurring within the TAO orbit. Recognizing their generation of extracellular matrix molecules, including glycosaminoglycans, fibronectin, and collagen, strongly suggests that modifying these biosynthetic activities could mitigate several adverse consequences of the disease. It is therefore possible that reducing their abundance or behavior, either in circulation or while inhabiting orbital tissue, could alter the disease course and outcome. The endgame would be to reduce disease morbidity and the need for rehabilitative ocular surgeries.
Several factors have been shown to retard fibrocyte differentiation besides Slit2. These factors could prove useful as treatments for TAO. Among them, serum amyloid p (SAP) has been characterized as a fibrocyte-inhibitory agent (120), the actions of which are mediated through Fcγ receptors that are distinct from those blocking neutrophil adhesion (121). A role for SAP in vivo in modulating tissue reactivity was demonstrated in SAP knockout mice (122). These mice exhibit persistent inflammation and fibrosis following administration of the lung fibrosis-promoting agent, bleomycin, when compared with wild-type controls. Very recently, IL-4 was shown to markedly enhance fibrocyte differentiation, an activity that could be inhibited by the store-operated Ca2+ entry channel blocker SKF-96365 (123). SAP could block this enhancement by IL-4, apparently utilizing a mechanism mediated through the Ca2+ channel. Inhibitors of C-type lectin dendritic cell–specific intracellular adhesion molecule 3–grabbing nonintegrin might also be effective in attenuating fibrocyte differentiation (124). A recent report from Ko et al. (125) provided evidence that blocking the synthesis of S1P and its receptor in GD-OFs could attenuate collagen, fibronectin, and smooth muscle actin synthesis/expression as well as the induction of several metalloproteases by TGF-β. Although not localizing the effects to the CD34+ OF subpopulation, these findings suggest that inhibiting this pathway could be related to the activities in fibrocyte-derived cells. Should this prove to be the case, the actions of this agent could prove cell type specific.
Alternatively, fibrocyte activity could be attenuated by interrupting TSHR/IGF-IR postreceptor signaling. Several inhibitory small molecules targeting TSHR have exhibited activity both in vitro and in small animal models (126–129). Monoclonal antibodies that antagonize TSHR activity have also been described (130, 131, 132). It remains possible that one or more of these or similar agents will emerge as well tolerated and effective in treating TAO. Because these molecules should also attenuate the actions of pathogenic TSIs on the thyroid gland and therefore potentially correct hyperthyroidism, they might represent “all-in-one” treatment options for GD. Fibrocyte activity appears to be susceptible to downregulation by specific inhibition of IGF-IR. We have found that immunoglobulins from patients with GD and IGF-I can induce the expression of RANTES and IL-16 in GD-OFs (53, 54). Those and subsequent studies disclosed overexpression of IGF-IR in GD-OFs and T cells and increased abundance of IGF-IR+ B cells in GD (54, 133, 134). Furthermore, Tsui et al. (135) reported that IGF-IR and TSHR form physical and functional protein complexes and that inhibition of IGF-IR activity can block signaling downstream from TSHR. These observations represent an important rationale for the use of teprotumumab in treating active TAO. Teprotumumab is a fully human IgG1 that inhibits IGF-IR (136). It has proven safe and effective in a recently completed placebo-controlled clinical trial in moderate to severe, active TAO (137). Cytokine induction by TSIs and TSH in fibrocytes and GD-OFs can be reduced substantially by teprotumumab in vitro (100).
Conclusions
Fibrocytes and derivative CD34+ OFs appear to be actively involved in the pathogenesis of TAO as a consequence of their presence in the circulation and orbit. Their repertoire of expressed genes and cellular responses to pathogenic signals insinuates them in the development of this disease. Within tissues, they interact with residential CD34− OFs that condition their behavior. As dominant effectors of pathogenic processes, they appear to possess potential as therapeutic targets. Many aspects of fibrocyte involvement in TAO remain uncertain. Among the most important is whether their relative abundance when compared with CD34− OFs changes as disease activity diminishes, in more severe disease, or in response to therapy. Another relates to whether differences in the phenotype of fibrocytes from one individual to another might underlie the divergent clinical courses of TAO observed in patients. In any event, recognition of fibrocytes and their derivatives as components of orbital biology in health and disease should prove of great importance as we further clarify the process underlying TAO and strive to develop targeted therapies.
Acknowledgments
The expert support in preparing this manuscript provided by Darla Kroft is gratefully acknowledged. The author is indebted to Linda Polonsky for helpful editorial suggestions.
Financial Support: This work was supported in part by National Institutes of Health Grants EY008976, EY11708, DK063121, and 5UMIA110557, Core Center for Research Grant EY007003 from the National Eye Institute, an unrestricted grant from Research to Prevent Blindness, and by funding from the Bell Charitable Family Foundation.
Disclosure Summary: The author has been issued patents covering his inventions concerning the use of IGF-I receptor inhibitors as therapy in Graves’ disease. These patents are held by University of California Los Angeles School of Medicine and Los Angeles Biomedical Research Institute.
Glossary
Abbreviations:
- AIRE
autoimmune regulator protein
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CXCR
CXC chemokine receptor
- GD
Graves disease
- GD-OF
orbital fibroblast from an orbit manifesting TAO
- HA
hyaluronan
- HAS
hyaluronan synthase
- IGF-IR
IGF-I receptor
- MHC-II
major histocompatibility class II
- OF
orbital fibroblast
- PGE2
prostaglandin E2
- PGHS-2
prostaglandin endoperoxide H synthase-2
- PPARγ
peroxisome proliferator–activated receptor γ
- ROBO
Roundabout
- SAP
serum amyloid P
- siRNA
small interfering RNA
- S1P
sphingosine-1-phosphate
- TAO
thyroid-associated ophthalmopathy
- Tg
thyroglobulin
- Th
T helper
- Thy-1
thymocyte antigen-1
- TSHR
thyrotropin receptor
- TSI
thyroid-stimulating immunoglobulin
- UDP
uridine 5′-diphosphate
References
- 1. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1735–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5(3–4):177–194. [PubMed] [Google Scholar]
- 3. Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979;29(1):27–43. [DOI] [PubMed] [Google Scholar]
- 4. Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa AE. Epstein–Barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial–mesenchymal transition and cancer progression. Front Oncol. 2018;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 6. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–7562. [DOI] [PubMed] [Google Scholar]
- 7. Sisson JC, Spaugh BI, Vanderburg JA. Functional aspects of fibroblasts derived from the retrobulbar tissue of man. Exp Eye Res. 1970;10(2):201–206. [DOI] [PubMed] [Google Scholar]
- 8. Sisson JC. Stimulation of glucose utilization and glycosaminoglycans production by fibroblasts derived from retrobulbar tissue. Exp Eye Res. 1971;12(3):285–292. [DOI] [PubMed] [Google Scholar]
- 9. Bahn RS, Gorman CA, Woloschak GE, David CS, Johnson PM, Johnson CM. Human retroocular fibroblasts in vitro: a model for the study of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1987;65(4):665–670. [DOI] [PubMed] [Google Scholar]
- 10. Hiromatsu Y, Fukazawa H, How J, Wall JR. Antibody-dependent cell-mediated cytotoxicity against human eye muscle cells and orbital fibroblasts in Graves’ ophthalmopathy—roles of class II MHC antigen expression and gamma-interferon action of effector and target cells. Clin Exp Immunol. 1987;70(3):593–603. [PMC free article] [PubMed] [Google Scholar]
- 11. Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves’ ophthalmopathy. Clin Exp Immunol. 1989;75(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- 12. Henrikson RC, Smith TJ. Ultrastructure of cultured human orbital fibroblasts. Cell Tissue Res. 1994;278(3):629–631. [DOI] [PubMed] [Google Scholar]
- 13. Smith TJ, Wang HS, Hogg MG, Henrikson RC, Keese CR, Giaever I. Prostaglandin E2 elicits a morphological change in cultured orbital fibroblasts from patients with Graves ophthalmopathy. Proc Natl Acad Sci USA. 1994;91(11):5094–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith TJ, Koumas L, Gagnon A, Bell A, Sempowski GD, Phipps RP, Sorisky A. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87(1):385–392. [DOI] [PubMed] [Google Scholar]
- 15. Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine-target cell interactions. Proc Natl Acad Sci USA. 1998;95(15):8904–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kusner LL, Young A, Tjoe S, Leahy P, Kaminski HJ. Perimysial fibroblasts of extraocular muscle, as unique as the muscle fibers. Invest Ophthalmol Vis Sci. 2010;51(1):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3(4):297–300. [DOI] [PubMed] [Google Scholar]
- 18. Mengistu M, Lukes YG, Nagy EV, Burch HB, Carr FE, Lahiri S, Burman KD. TSH receptor gene expression in retroocular fibroblasts. J Endocrinol Invest. 1994;17(6):437–441. [DOI] [PubMed] [Google Scholar]
- 19. Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84(7):2557–2562. [DOI] [PubMed] [Google Scholar]
- 20. Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10(3):366–391. [DOI] [PubMed] [Google Scholar]
- 21. Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120(3):380–386. [DOI] [PubMed] [Google Scholar]
- 22. Hascall VC. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. [DOI] [PubMed] [Google Scholar]
- 23. Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. [DOI] [PubMed] [Google Scholar]
- 24. Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277(7):4589–4592. [DOI] [PubMed] [Google Scholar]
- 25. Turley EA, Naor D. RHAMM and CD44 peptides—analytic tools and potential drugs. Front Biosci. 2012;17(1):1775–1794. [DOI] [PubMed] [Google Scholar]
- 26. Smith TJ, Bahn RS, Gorman CA. Hormonal regulation of hyaluronate synthesis in cultured human fibroblasts: evidence for differences between retroocular and dermal fibroblasts. J Clin Endocrinol Metab. 1989;69(5):1019–1023. [DOI] [PubMed] [Google Scholar]
- 27. Winkler CW, Foster SC, Matsumoto SG, Preston MA, Xing R, Bebo BF, Banine F, Berny-Lang MA, Itakura A, McCarty OJ, Sherman LS. Hyaluronan anchored to activated CD44 on central nervous system vascular endothelial cells promotes lymphocyte extravasation in experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287(40):33237–33251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cowman MK. Hyaluronan and hyaluronan fragments. Adv Carbohydr Chem Biochem. 2017;74:1–59. [DOI] [PubMed] [Google Scholar]
- 29. Schommer NN, Muto J, Nizet V, Gallo RL. Hyaluronan breakdown contributes to immune defense against group A Streptococcus. J Biol Chem. 2014;289(39):26914–26921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98(10):2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philipson LH, Schwartz NB. Subcellular localization of hyaluronate synthetase in oligodendroglioma cells. J Biol Chem. 1984;259(8):5017–5023. [PubMed] [Google Scholar]
- 32. Spicer AP, Augustine ML, McDonald JA. Molecular cloning and characterization of a putative mouse hyaluronan synthase. J Biol Chem. 1996;271(38):23400–23406. [DOI] [PubMed] [Google Scholar]
- 33. Spicer AP, Olson JS, McDonald JA. Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J Biol Chem. 1997;272(14):8957–8961. [DOI] [PubMed] [Google Scholar]
- 34. Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273(39):25117–25124. [DOI] [PubMed] [Google Scholar]
- 35. Monslow J, Williams JD, Guy CA, Price IK, Craig KJ, Williams HJ, Williams NM, Martin J, Coleman SL, Topley N, Spicer AP, Buckland PR, Davies M, Bowen T. Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J Biol Chem. 2004;279(20):20576–20581. [DOI] [PubMed] [Google Scholar]
- 36. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286(27):24487–24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1β in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84(11):4079–4084. [DOI] [PubMed] [Google Scholar]
- 38. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991;72(5):1169–1171. [DOI] [PubMed] [Google Scholar]
- 39. Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268(2 Pt 1):C382–C388. [DOI] [PubMed] [Google Scholar]
- 40. Cao HJ, Wang HS, Zhang Y, Lin HY, Phipps RP, Smith TJ. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273(45):29615–29625. [DOI] [PubMed] [Google Scholar]
- 41. Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89(10):5076–5080. [DOI] [PubMed] [Google Scholar]
- 42. Ma R, Li Q, Wang Z, Yuan Y, Gan L, Qian J. Modulation of hyaluronan polymer size regulates proliferation of perimysial fibroblasts in thyroid eye disease. Biochem Biophys Res Commun. 2018;496(4):1376–1381. [DOI] [PubMed] [Google Scholar]
- 43. Zhang L, Grennan-Jones F, Lane C, Rees DA, Dayan CM, Ludgate M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97(2):653–662. [DOI] [PubMed] [Google Scholar]
- 44. Zhang L, Ji QH, Ruge F, Lane C, Morris D, Tee AR, Dayan CM, Ludgate M. Reversal of pathological features of Graves’ orbitopathy by activation of forkhead transcription factors, FOXOs. J Clin Endocrinol Metab. 2016;101(1):114–122. [DOI] [PubMed] [Google Scholar]
- 45. Papageorgiou KI, Ang M, Chang SH, Kohn J, Martinez S, Goldberg RA. Aesthetic considerations in upper eyelid retraction surgery. Ophthal Plast Reconstr Surg. 2012;28(6):419–423. [DOI] [PubMed] [Google Scholar]
- 46. Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81(9):3428–3431. [DOI] [PubMed] [Google Scholar]
- 47. Crisp M, Starkey KJ, Lane C, Ham J, Ludgate M. Adipogenesis in thyroid eye disease. Invest Ophthalmol Vis Sci. 2000;41(11):3249–3255. [PubMed] [Google Scholar]
- 48. Starkey K, Heufelder A, Baker G, Joba W, Evans M, Davies S, Ludgate M. Peroxisome proliferator-activated receptor-γ in thyroid eye disease: contraindication for thiazolidinedione use? J Clin Endocrinol Metab. 2003;88(1):55–59. [DOI] [PubMed] [Google Scholar]
- 49. Heufelder AE, Bahn RS. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves’ ophthalmopathy. Eur J Clin Invest. 1993;23(1):10–17. [DOI] [PubMed] [Google Scholar]
- 50. Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves’ ophthalmopathy patients. Clin Endocrinol (Oxf). 2003;58(3):280–287. [DOI] [PubMed] [Google Scholar]
- 51. Tang F, Chen X, Mao Y, Wan S, Ai S, Yang H, Liu G, Zou Y, Lin M, Dan L. Orbital fibroblasts of Graves’ orbitopathy stimulated with proinflammatory cytokines promote B cell survival by secreting BAFF. Mol Cell Endocrinol. 2017;446:1–11. [DOI] [PubMed] [Google Scholar]
- 52. Sciaky D, Brazer W, Center DM, Cruikshank WW, Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol. 2000;164(7):3806–3814. [DOI] [PubMed] [Google Scholar]
- 53. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942–950. [DOI] [PubMed] [Google Scholar]
- 54. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348–6354. [DOI] [PubMed] [Google Scholar]
- 55. Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34+ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013;98(7):2783–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li B, Smith TJ. PI3K/AKT pathway mediates induction of IL-1RA by TSH in fibrocytes: modulation by PTEN. J Clin Endocrinol Metab. 2014;99(9):3363–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li B, Smith TJ. Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J Clin Endocrinol Metab. 2014;99(4):E625–E633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cao HJ, Smith TJ. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999;277(6 Pt 1):C1075–C1085. [DOI] [PubMed] [Google Scholar]
- 59. Chen B, Tsui S, Boeglin WE, Douglas RS, Brash AR, Smith TJ. Interleukin-4 induces 15-lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves disease. Evidence for anatomic site-selective actions of Th2 cytokines. J Biol Chem. 2006;281(27):18296–18306. [DOI] [PubMed] [Google Scholar]
- 60. Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355–16364. [DOI] [PubMed] [Google Scholar]
- 61. Seo Y, Chae MK, Han SA, Lee EJ, Lee JH, Yoon JS. Sphingosine-1-phosphate is involved in inflammatory reactions in patients with Graves’ orbitopathy. Inflamm Res. 2017;66(6):535–545. [DOI] [PubMed] [Google Scholar]
- 62. Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146(1):108–113. [PubMed] [Google Scholar]
- 63. Roper RL, Conrad DH, Brown DM, Warner GL, Phipps RP. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J Immunol. 1990;145(8):2644–2651. [PubMed] [Google Scholar]
- 64. Johnson R, Lancki DW, Fitch FW. Accessory molecules involved in antigen-mediated cytolysis and lymphokine production by cytotoxic T lymphocyte subsets. I. Identification of functions for the T cell surface molecules Ly-6C and Thy-1. J Immunol. 1993;151(6):2986–2999. [PubMed] [Google Scholar]
- 65. Hueber AO, Raposo G, Pierres M, He HT. Thy-1 triggers mouse thymocyte apoptosis through a bcl-2-resistant mechanism. J Exp Med. 1994;179(3):785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smith TJ, Sempowski GD, Wang HS, Del Vecchio PJ, Lippe SD, Phipps RP. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80(9):2620–2625. [DOI] [PubMed] [Google Scholar]
- 67. Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32(2):477–485. [DOI] [PubMed] [Google Scholar]
- 68. Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, Reilkoff RA, Bucala RJ, Herzog EL. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90(6):812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Douglas RS, Afifiyan NF, Hwang CJ, Chong K, Haider U, Richards P, Gianoukakis AG, Smith TJ. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol. 2015;11(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103(38):14098–14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ishida Y, Kimura A, Kondo T, Hayashi T, Ueno M, Takakura N, Matsushima K, Mukaida N. Essential roles of the CC chemokine ligand 3-CC chemokine receptor 5 axis in bleomycin-induced pulmonary fibrosis through regulation of macrophage and fibrocyte infiltration. Am J Pathol. 2007;170(3):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 78. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94(12):6307–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grab DJ, Lanners H, Martin LN, Chesney J, Cai C, Adkisson HD, Bucala R. Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol Med. 1999;5(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 80. Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Göbel N, Talke Y, Schweda F, Mack M. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106(42):17892–17897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen W, Pilling D, Gomer RH. Dietary NaCl affects bleomycin-induced lung fibrosis in mice. Exp Lung Res. 2017;43(9–10):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hamblin MJ, Eberlein M, Black K, Hallowell R, Collins S, Chan-Li Y, Horton MR. Lovastatin inhibits low molecular weight hyaluronan induced chemokine expression via LFA-1 and decreases bleomycin-induced pulmonary fibrosis. Int J Biomed Sci. 2014;10(3):146–157. [PMC free article] [PubMed] [Google Scholar]
- 84. Karhadkar TR, Pilling D, Cox N, Gomer RH. Sialidase inhibitors attenuate pulmonary fibrosis in a mouse model. Sci Rep. 2017;7(1):15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sato S, Shinohara S, Hayashi S, Morizumi S, Abe S, Okazaki H, Chen Y, Goto H, Aono Y, Ogawa H, Koyama K, Nishimura H, Kawano H, Toyoda Y, Uehara H, Nishioka Y. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir Res. 2017;18(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-β and peroxisome proliferator-activated receptor γ. J Biol Chem. 2007;282(31):22910–22920. [DOI] [PubMed] [Google Scholar]
- 87. Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O’Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(7):588–594. [DOI] [PubMed] [Google Scholar]
- 88. Deng A, Martin DB, Spillane A, Chwalek J, St Surin-Lord S, Brooks S, Petrali J, Sina B, Gaspari A, Kao G. Nephrogenic systemic fibrosis with a spectrum of clinical and histopathological presentation: a disorder of aberrant dermal remodeling. J Cutan Pathol. 2010;37(2):204–210. [DOI] [PubMed] [Google Scholar]
- 89. Brunasso AM, Massone C. Update on the pathogenesis of scleroderma: focus on circulating progenitor cells. F1000 Res. 2016;5:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang F, Liu K, Zhao H, He Y. The emerging role of fibrocytes in ocular disorders. Stem Cell Res Ther. 2018;9(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tamaki K, Usui-Ouchi A, Murakami A, Ebihara N. Fibrocytes and fibrovascular membrane formation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016;57(11):4999–5005. [DOI] [PubMed] [Google Scholar]
- 92. Singh V, Jaini R, Torricelli AA, Santhiago MR, Singh N, Ambati BK, Wilson SE. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp Eye Res. 2014;121:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fernando R, Atkins S, Raychaudhuri N, Lu Y, Li B, Douglas RS, Smith TJ. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109(19):7427–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lu Y, Atkins SJ, Fernando R, Trierweiler A, Mester T, Grisolia ABD, Mou P, Novaes P, Smith TJ. CD34− orbital fibroblasts from patients with thyroid-associated ophthalmopathy modulate TNF-α expression in CD34+ fibroblasts and fibrocytes. Invest Ophthalmol Vis Sci. 2018;59(6):2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013;8(9):e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu T, Mester T, Gupta S, Sun F, Smith TJ, Douglas RS. Thyrotropin and CD40L stimulate interleukin-12 expression in fibrocytes: implications for pathogenesis of thyroid-associated ophthalmopathy. Thyroid. 2016;26(12):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fernando R, Atkins SJ, Smith TJ. Intersection of chemokine and TSH receptor pathways in human fibrocytes: emergence of CXCL-12/CXCR4 cross talk potentially relevant to thyroid-associated ophthalmopathy. Endocrinology. 2016;157(10):3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fang S, Huang Y, Liu X, Zhong S, Wang N, Zhao B, Li Y, Sun J, Wang Y, Zhang S, Gu P, Zhou H, Li B, Fan X. Interaction between CCR6+ Th17 cells and CD34+ fibrocytes promotes inflammation: implications in Graves’ orbitopathy in chinese population. Invest Ophthalmol Vis Sci. 2018;59(6):2604–2614. [DOI] [PubMed] [Google Scholar]
- 99. Fang S, Huang Y, Wang S, Zhang Y, Luo X, Liu L, Zhong S, Liu X, Li D, Liang R, Miranda P, Gu P, Zhou H, Fan X, Li B. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibroblasts in TAO. J Clin Endocrinol Metab. 2016;101(8):2955–2965. [DOI] [PubMed] [Google Scholar]
- 100. Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J Clin Endocrinol Metab. 2014;99(9):E1635–E1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fernando R, Vonberg A, Atkins SJ, Pietropaolo S, Pietropaolo M, Smith TJ. Human fibrocytes express multiple antigens associated with autoimmune endocrine diseases. J Clin Endocrinol Metab. 2014;99(5):E796–E803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99(7):E1236–E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fernando R, Grisolia ABD, Lu Y, Atkins S, Smith TJ. Slit2 modulates the inflammatory phenotype of orbit-infiltrating fibrocytes in Graves’ disease. J Immunol. 2018;200(12):3942–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274(3 Pt 1):C707–C714. [DOI] [PubMed] [Google Scholar]
- 105. Zhang Y, Cao HJ, Graf B, Meekins H, Smith TJ, Phipps RP. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160(3):1053–1057. [PubMed] [Google Scholar]
- 106. Gianoukakis AG, Smith TJ. Recent insights into the pathogenesis and management of thyroid-associated ophthalmopathy. Curr Opin Endocrinol Diabetes Obes. 2008;15(5):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96(6):795–806. [DOI] [PubMed] [Google Scholar]
- 108. Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96(6):771–784. [DOI] [PubMed] [Google Scholar]
- 109. Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chédotal A. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22(3):463–473. [DOI] [PubMed] [Google Scholar]
- 110. Katoh Y, Katoh M. Comparative genomics on SLIT1, SLIT2, and SLIT3 orthologs. Oncol Rep. 2005;14(5):1351–1355. [PubMed] [Google Scholar]
- 111. Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11(1):13–21. [DOI] [PubMed] [Google Scholar]
- 112. Ypsilanti AR, Chedotal A. Roundabout receptors. Adv Neurobiol. 2014;8:133–164. [DOI] [PubMed] [Google Scholar]
- 113. Kanellis J, Garcia GE, Li P, Parra G, Wilson CB, Rao Y, Han S, Smith CW, Johnson RJ, Wu JY, Feng L. Modulation of inflammation by Slit protein in vivo in experimental crescentic glomerulonephritis. Am J Pathol. 2004;165(1):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen B, Blair DG, Plisov S, Vasiliev G, Perantoni AO, Chen Q, Athanasiou M, Wu JY, Oppenheim JJ, Yang D. Cutting edge: bone morphogenetic protein antagonists Drm/Gremlin and Dan interact with Slits and act as negative regulators of monocyte chemotaxis. J Immunol. 2004;173(10):5914–5917. [DOI] [PubMed] [Google Scholar]
- 115. Pilling D, Zheng Z, Vakil V, Gomer RH. Fibroblasts secrete Slit2 to inhibit fibrocyte differentiation and fibrosis. Proc Natl Acad Sci USA. 2014;111(51):18291–18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17(10):923–938. [DOI] [PubMed] [Google Scholar]
- 117. Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005;77(6):923–933. [DOI] [PubMed] [Google Scholar]
- 118. Liu Y, Qingjuan S, Gao Z, Deng C, Wang Y, Guo C. Circulating fibrocytes are involved in inflammation and leukocyte trafficking in neonates with necrotizing enterocolitis. Medicine (Baltimore). 2017;96(26):e7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gillespie EF, Raychaudhuri N, Papageorgiou KI, Atkins SJ, Lu Y, Charara LK, Mester T, Smith TJ, Douglas RS. Interleukin-6 production in CD40-engaged fibrocytes in thyroid-associated ophthalmopathy: involvement of Akt and NF-κB. Invest Ophthalmol Vis Sci. 2012;53(12):7746–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171(10):5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cox N, Pilling D, Gomer RH. Distinct Fcγ receptors mediate the effect of serum amyloid P on neutrophil adhesion and fibrocyte differentiation. J Immunol. 2014;193(4):1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pilling D, Gomer RH. Persistent lung inflammation and fibrosis in serum amyloid P component (APCs−/−) knockout mice. PLoS One. 2014;9(4):e93730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhong JN, Lan L, Chen YF, Huang G, He GZ, Yang J, Gao YD. IL-4 and serum amyloid P inversely regulate fibrocyte differentiation by targeting store-operated Ca2+ channels. Pharmacol Rep. 2018;70(1):22–28. [DOI] [PubMed] [Google Scholar]
- 124. Borrok MJ, Kiessling LL. Non-carbohydrate inhibitors of the lectin DC-SIGN. J Am Chem Soc. 2007;129(42):12780–12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ko J, Chae MK, Lee JH, Lee EJ, Yoon JS. Sphingosine-1-phosphate mediates fibrosis in orbital fibroblasts in Graves’ orbitopathy. Invest Ophthalmol Vis Sci. 2017;58(5):2544–2553. [DOI] [PubMed] [Google Scholar]
- 126. Neumann S, Eliseeva E, McCoy JG, Napolitano G, Giuliani C, Monaco F, Huang W, Gershengorn MC. A new small-molecule antagonist inhibits Graves’ disease antibody activation of the TSH receptor. J Clin Endocrinol Metab. 2011;96(2):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149(12):5945–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Neumann S, Pope A, Geras-Raaka E, Raaka BM, Bahn RS, Gershengorn MC. A drug-like antagonist inhibits thyrotropin receptor-mediated stimulation of cAMP production in Graves’ orbital fibroblasts. Thyroid. 2012;22(8):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Neumann S, Nir EA, Eliseeva E, Huang W, Marugan J, Xiao J, Dulcey AE, Gershengorn MC. A selective TSH receptor antagonist inhibits stimulation of thyroid function in female mice. Endocrinology. 2014;155(1):310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, Summerhayes S, Harris R, Reeve M, Coco G, Zanchetta R, Chen S, Furmaniak J, Smith BR. A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid. 2008;18(7):735–746. [DOI] [PubMed] [Google Scholar]
- 131. Evans M, Sanders J, Tagami T, Sanders P, Young S, Roberts E, Wilmot J, Hu X, Kabelis K, Clark J, Holl S, Richards T, Collyer A, Furmaniak J, Smith BR. Monoclonal autoantibodies to the TSH receptor, one with stimulating activity and one with blocking activity, obtained from the same blood sample. Clin Endocrinol (Oxf). 2010;73(3):404–412. [DOI] [PubMed] [Google Scholar]
- 132. Furmaniak J, Sanders J, Rees Smith B. Blocking type TSH receptor antibodies. Auto Immun Highlights. 2012;4(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178(5):3281–3287. [DOI] [PubMed] [Google Scholar]
- 134. Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. B cells from patients with Graves’ disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. J Immunol. 2008;181(8):5768–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kurzrock R, Patnaik A, Aisner J, Warren T, Leong S, Benjamin R, Eckhardt SG, Eid JE, Greig G, Habben K, McCarthy CD, Gore L. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16(8):2458–2465. [DOI] [PubMed] [Google Scholar]
- 137. Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]