Abstract
Context
Quantitative studies of the short-term feedback of testosterone (T) on luteinizing hormone (LH) secretion in healthy men are relatively rare. Such studies require the shutting down of endogenous T secretion and the imposition of experimentally controlled IV T addback.
Objective
To evaluate whether pulsatile and continuous T delivery confers equivalent negative feedback on LH secretion.
Design
This was a placebo-controlled, blinded, and prospectively randomized crossover study comprising 16 healthy men [age range 23 to 54 years and a body mass index (BMI) between 22.3 and 34.2 kg/m2]. Subjects received ketoconazole to block endogenous T secretion and received continuous or 90-minute pulses of IV T addback.
Setting
The study was performed in a Clinical Translational Research Unit.
Interventions
Subjects underwent 14 hours of blood sampling at 10-minute intervals, with a bolus IV injection of 33 ng/kg gonadotropin-releasing hormone (GnRH).
Main Outcome Measures
Log-transformed LH and T concentration ratios before and after GnRH administration.
Results
Despite higher T concentrations during pulsatile T feedback, LH concentrations and secretion rates, whether driven by endogenous or exogenous GnRH, were similar to those during continuous T infusion, indicating diminished pulsatile T feedback. Feedback correlated negatively with BMI. Under controlled T feedback, basal but not pulsatile LH secretion correlated negatively with CT-estimated visceral fat mass.
Conclusion
Feedback by pulsatile T delivery has diminished inhibitory strength compared with continuous infusion. Feedback is negatively correlated with BMI.
Keywords: human, LH, body composition, feedback, bioavailable testosterone
Luteinizing hormone (LH) secretion by the pituitary gland is regulated by hypothalamic kisspeptin and gonadotropin-releasing hormone (GnRH) neurons [1]. Kisspeptin stimulates the pulsatile release of GnRH into the hypophyseal-portal blood system, stimulating gonadotropes in the pituitary gland [2]. As a result of pulsatile stimulation by GnRH, LH is synthesized and secreted in a pulsatile fashion. Leydig cells exposed to pulsatile LH drive respond with increased testosterone (T) synthesis and pulsatile T secretion into the spermatic vein [3]. An essential part of regulation of the adenohypophysis is negative feedback by hormones secreted by the ultimate target organ(s), e.g., cortisol, thyroxine, T, and estradiol (E2) and IGF-I. Generally, more than one site acts as the feedback receiver. Thus, beyond specific pituitary cells (e.g., gonadotrope, corticotrope, somatotrope, or thyrotrope), one or more hypothalamic centers mediate restraining effects of feedback.
Clinical investigations of T-mediated feedback are relatively few and contradictory in inference [4]. For instance, in aging men, various reports have described normal, blunted, or heightened androgen-dependent inhibition of gonadotropin production [5–7]. Discrepancies among studies may reflect the following: (i) the use of a pharmacological dose or type of androgen, (ii) the intramuscular route of T repletion, (iii) trans-scrotal T administration, which forces marked 5α-reduction of T, (iv) confounding by time-varying concentrations of T and LH, and (v) continuous IV administration of androgen, whereas pulsatile T secretion is physiological.
Feedback studies of T on LH secretion in patients with untreated primary (gonadal) hypogonadism are fairly straightforward, in contrast to studies in healthy men. For quantitating feedback strength, endogenous T secretion should be inhibited and exogenous IV T delivery achieved in the physiological range [8, 9]. Moreover, in normal men, the T concentration profile is pulsatile, with ~90-minute intervals between pulses, superimposed on a nonpulsatile component and a diurnal variation, with highest T levels in the early morning but not in all subjects [3, 10, 11]. Furthermore, the measurement of total T might be less informative than bioactive T, especially when sex hormone-binding globulin (SHBG) levels are elevated or diminished [2, 9]. In addition, only one paper examined influence of the infusion pattern of T on feedback [8]. In this study, in six young men, LH concentrations were more suppressed during continuous than pulsatile T administration.
The goal of this study was to quantify feedback in relation to the infused T pattern (pulsatile vs nonpulsatile) with the hypotheses that (i) feedback by T pulses is less efficient, (ii) T feedback operates partly at the pituitary level, and (iii) T feedback is controlled, in part, by body composition.
1. Methods
A. Subjects
This investigator-initiated pilot study does not qualify as a clinical trial, as it is an acute physiological examination of mechanism without any health-related or behavioral outcomes per se. Sixteen healthy, ambulatory, community-dwelling men (mean age 35 years, range 23 to 54 years) participated in two overnight Clinical Translational Unit (CRU)-based studies each. The body mass index (BMI) was 28.0 (range 22.3 to 34.2 kg/m2). Volunteers were recruited by newspaper advertisements, local posters, the Clinical Trials Center web page, and community (general and minority) bulletin boards. This was an investigator-initiated US Food and Drug Administration-reviewed, prospectively randomized, double-blind, placebo-controlled study. Each subject completed a continuous vs pulsatile T-infusion regimen in randomized order, at least 10 days apart. To enforce controlled T-feedback inhibition in a near-physiological manner, the following combined experimental regimen was designed: oral administration of the steroidogenic inhibitor, ketoconazole (KTCZ), with a replacement dose of dexamethasone (DEX) as glucocorticoid, suppresses testicular and adrenal steroidogenesis overnight. The latter was established in an unpublished Mayo Clinic study comprising 40 healthy men, mean age 47 years (range 19 to 73 years) and BMI 26.7 kg/m2 (range 20 to 34.4 kg/m2). LH levels were raised overnight to 6.42 ± 0.42 IU/L after 800 mg KTCZ orally at 20:00 compared with 3.99 ± 0.33 IU/L after placebo (P < 0.0001), whereas T concentrations decreased by >90%, from 711 ± 18 to 61 ± 3.3 ng/dL (P < 0.0001), and rose to baseline levels during IV infusion of serum consecutive pulses of T or continuous IV T infusion. Both sessions ended with a bolus IV injection of GnRH to test pituitary inhibition.
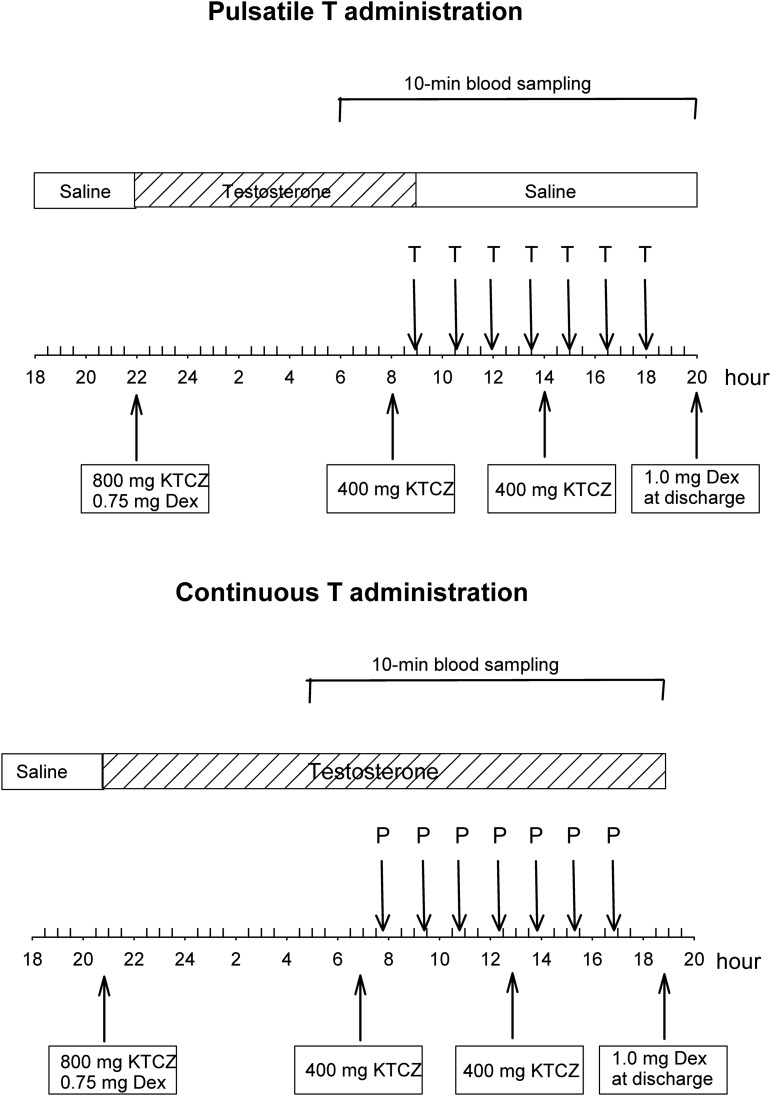
Volunteers arrived at the unit in the evening for admission. At 18:00 to 20:00 on the night of admission, two IV catheters were placed in (contralateral) antecubital veins to allow simultaneous infusions and blood sampling. At 22:00, 800 mg KTCZ and 0.75 mg DEX were given orally with a nondairy snack. KTCZ (400 mg) was administered again at 08:00 and 14:00 on the following day, and DEX (1.0 mg) was given after completing all sampling (at discharge). Importantly, this administered DEX dose does not interfere with LH secretion [12]. Constant IV infusion of T (5.0 mg over 22 hours) was initiated with the oral loading dose of KTCZ given at 22:00 and maintained until 09:00 and thus, either continued or replaced by pulsatile T infusions. The latter comprised a total of seven consecutive IV pulses of T (250 μg), each delivered as a 45-minute square wave as one every 90 minutes. Pulsatile T injections thus ended at 18:00 (Evening 2). In both sessions, an IV injection of GnRH was administered at 16:30 (33 ng/kg IV bolus) to stimulate LH secretion. Blood samples (1.5 mL) were withdrawn every 10 minutes for 14 hours, beginning at 06:00 (180 minutes before starting IV pulses of T) until 20:00 (85 samples per session) to measure LH and T. A diagram of the experimental design is shown in Fig. 1.
Figure 1.
Schema of the experimental design comprising KTCZ blockade of androgen and cortisol secretion, DEX addback of glucocorticoid, and continuous or pulsatile administration of T from 09:00 on. Blood sampling at 10-min intervals took place from 06:00 until 20:00 at discharge. P, placebo.
Supper (the evening of CRU admission), snacks (nondairy at 22:00, 08:00, and 14:00, with oral KTCZ administration), breakfast, lunch, and supper of day 2 were provided. Subjects were allowed to ambulate to the lavatory.
B. Criteria for Inclusion
The protocol was approved by Mayo Institutional Review Board. Witnessed voluntary, written consent was obtained before study enrollment. A medical history, physical examination, and screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal.
C. Criteria for Exclusion
Exclusion criteria included recent use of psychotropic or neuroactive drugs; morbid obesity; abnormal laboratory test results; drug or alcohol abuse, psychosis, depression, mania, or severe anxiety; acute or chronic organ-system disease; endocrinopathy or anabolic steroid use; nightshift work or recent (1 month) more than three time zones transmeridian travel; acute weight change (loss or gain of >2 kg in 6 weeks); unwillingness to provide written, informed consent; history or suspicion of prostatic disease [elevated prostate-specific antigen (>4.0 ng/mL)], indeterminate nodule or mass, or obstructive uropathy; history of carcinoma (excluding localized basal cell carcinoma, removed or surgically treated with no recurrence); and history of thrombotic arterial disease (stroke, transient ischemic attack, myocardial infarction, angina) or deep-vein thrombophlebitis.
D. Analytical Methods
D-1. Assays
LH, follicle-stimulating hormone (FSH), and prolactin (PRL) were determined in duplicate using an automated, two-site monoclonal immunochemiluminesce assay with a sensitivity for LH and FSH of 0.20 IU/L (First International Reference Preparation) and PRL 0.2 μg/L and respective median inter- and intra-assay coefficients of variation (CVs) of 5.5 and 8.5%, 4.7 and 5.2%, and 4.1 and 5.6% on a Dxl 800 automated system (Beckman Instruments, Chaska, MN) [13–15]. Crossreactivity with thyroid-stimulating hormone, α-subunits, or free β-subunits is <0.1%. Total T concentrations were assessed by liquid chromatography-tandem mass spectrometry (Agilent Technologies, Santa Clara, CA). Free and bioavailable hormone concentrations were calculated, as described previously [11]. E2 was likewise measured by mass spectrometry. Interassay CVs for E2 are 10.8% at 0.29 pg/mL and 5.1% at 32 pg/mL. SHBG was quantified by a solid-phase chemiluminescent assay on the Siemens Immulite 2000 Automated Immunoassay System (Siemens Healthcare Diagnostics, Deerfield, IL) [16]. Interassay CVs for SHBG are 4.0% at 5.4 nM and 5.9% at 74 nM.
E. Visceral and Total Fat Mass
Intra-abdominal and total fat mass were estimated by single-slice abdominal CT scan at L3-L4 [17].
E-1. Calculations
The LH and T concentration time series were subjected to biexponential deconvolution analysis using independently determined estimates of two-compartment LH and T disappearance kinetics [12]. The complete 11-hour time series was used to estimate LH secretion before (7.5 hours) and after (3.5 hours) stimulation with GnRH. The primary analytical outcome was the ratio of serum concentrations of LH/T, an indicator of feedback during pulsatile or continuous T administration, before and after administration of GnRH. For statistical purposes, the LH/T ratios, within 90-minute bins, were averaged. Secondarily, we applied a Matlab-based program designated to quantify the unobserved dose-response function that implicitly couples feedback-paired hormone output: here, suppression of instantaneous (calculated) LH secretion rates by pulsatile or continuous bioavailable T concentrations [18, 19]. In addition, approximate entropy (ApEn) was applied to the time series as a sensitive (>90%) and specific (>95%) measure of feedback-conferred pattern orderliness [20].
F. Statistical Assessment
The hypothesis is that the pulsatile mode of T addback is less efficient in feedback of (suppressing) endogenously driven (spontaneous) and GnRH-infused (exogenously forced) LH secretion in healthy men. Here, the statistical endpoint is the logarithm of the LH/T ratio (total, free, and bioavailable T) during spontaneous secretion and after GnRH administration. Analyses included two-way repeated-measures ANOVA across both study occasions. Linear regression tools were used. P values of 0.05 and less were considered significant. Data are shown as means ± SEM, unless mentioned otherwise. Statistical calculations were performed with Systat version 13 (Systat Software, San Jose, CA).
2. Results
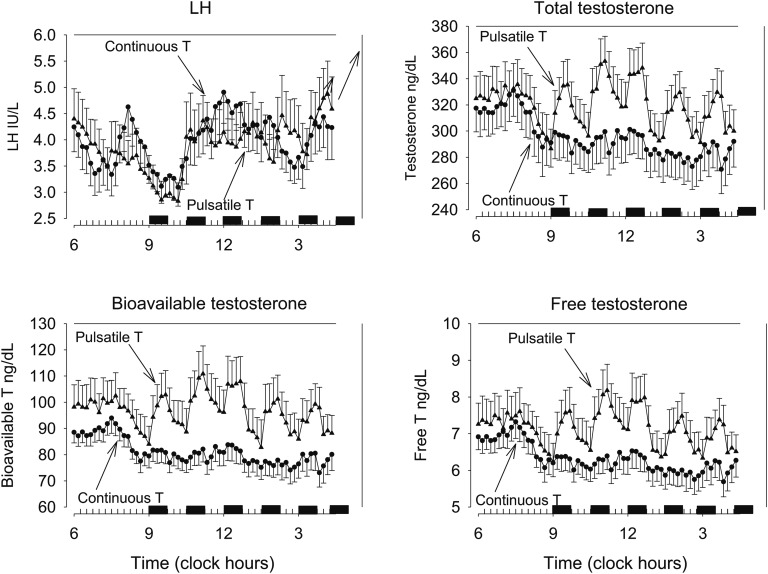
Serum levels of PRL, E2, SHBG, LH, FSH, and T were all within normal limits for the age range of 23 to 54 years. There were no dropouts. Infusion protocols were identical during the first 3 hours of sampling (between 06:00 and 09:00). Here, LH concentrations were similar: viz., 3.8 ± 0.4 and 3.9 ± 0.4 IU/L during pulsatile and continuous T delivery (P = 0.83), as were total T concentrations, viz., 321 ± 17 ng/dL and 313 ± 18 ng/dL, respectively (P = 0.54). Before the T infusions started, a temporary rise in LH levels was present in both study sessions, matching a decrease in T levels. During the 7.5-hour period between 09:00 and 16:30, when T pulses every 90 minutes replaced continuous T infusion (see Methods), the group means of the 10-minute sampled serum concentrations of LH and total T, as well as (calculated) free T and bioavailable T, are shown in Fig. 2. Inspection of the graphs suggested no clear difference in LH levels, but total, free, and bioavailable T levels were higher during pulsatile T administration. LH secretion and total T secretion during this interval were quantified by deconvolution analysis. The results are displayed in Table 1. There were no differences in LH secretion parameters between the two distinct T-infusion schemes. Specifically, basal, pulsatile, and total LH secretion, as well as LH pulse frequency and LH half-life, were not changed by the way of T administration. Average LH concentrations between 09:00 and 16:30 were 4.0 ± 0.3 IU/L during continuous and 3.9 ± 0.4 IU/L during pulsatile T infusion (P = 0.60). T administered in pulses resulted in higher (calculated) T influx in the circulation than continuous T infusion (Table 1). Calculated pulsatile T influx (sum of infused T and the endogenous T contribution) was almost twofold larger, and total T influx was 32% higher. Mean total T concentrations were 288 ± 17 ng/dL and 318 ± 16 ng/dL during continuous and pulsatile T administration, respectively (P = 0.009). Free T concentrations were 6.15 ± 0.38 and 7.12 ± 0.56 ng/dL, respectively (P = 0.008), and bioavailable T concentrations were 79 ± 4.7 ng/dL and 96 ± 8.4 ng/dL (P = 0.023).
Figure 2.
Profiles of 10-min sampled serum concentrations of LH and total, free, and bioavailable T in 16 healthy men. Data are shown as means ± SEM. Two different study sessions were done in each subject. After a baseline interval of continuous IV T infusion, seven consecutive, 45-min square-wave T pulses (pulsatile infusion) were given IV at 90-min intervals starting at 09:00, or continuous IV T infusion was continued from 09:00 onward.
Table 1.
Deconvolution Analysis of the LH and T Profiles During 7.5 H of Continuous and Pulsatile T Infusions
| Continuous T | Pulsatile T | P Value | |
|---|---|---|---|
| LH | |||
| Pulse frequency, no./7.5 h | 3.6 ± 0.34 | 3.3 ± 0.23 | 0.32 |
| Fast half- life, min | 6.93 | 6.93 | 1.0 |
| Long half- life, min | 57 ± 4.4 | 62 ± 4.3 | 0.38 |
| Mode, min | 13 ± 0.7 | 13 ± 0.9 | 0.94 |
| Basal secretion, IU/L/7.5 h | 29.2 ± 3.9 | 28.9 ± 5.6 | 0.95 |
| Pulsatile secretion, IU/L/7.5 h | 25.6 ± 2.3 | 23.3 ± 2.4 | 0.39 |
| Total secretion, U/L/7.5 h | 54.8 ± 5.8 | 52.2 ± 7.1 | 0.62 |
| Mean pulse mass, U/L | 7.7 ± 0.8 | 7.4 ± 0.8 | 0.76 |
| T | |||
| Pulse frequency, no./7.5 h | 5.4 ± 0.4 | 6.2 ± 0.4 | 0.26 |
| Fast half- life, min | 1.4 | 1.4 | 1.0 |
| Long half- life, min | 18.5 ± 1.1 | 15.0 ± 0.4 | 0.016 |
| Mode, min | 11.9 ± 1.5 | 13.1 ± 1.6 | 0.64 |
| Basal secretion, IU/L/7.5 h | 7070 ± 500 | 9050 ± 550 | 0.002 |
| Pulsatile secretion, IU/L/7.5 h | 530 ± 54 | 1020 ± 160 | 0.008 |
| Total secretion, U/L/7.5 h | 7600 ± 520 | 10,070 ± 630 | 0.001 |
| Mean pulse mass, U/L | 99 ± 8.2 | 162 ± 18.5 | 0.003 |
Data are shown as means ± SEM. Statistical comparison between the two groups was done with the two-tailed Student t test for paired data. P < 0.01 was considered significant.
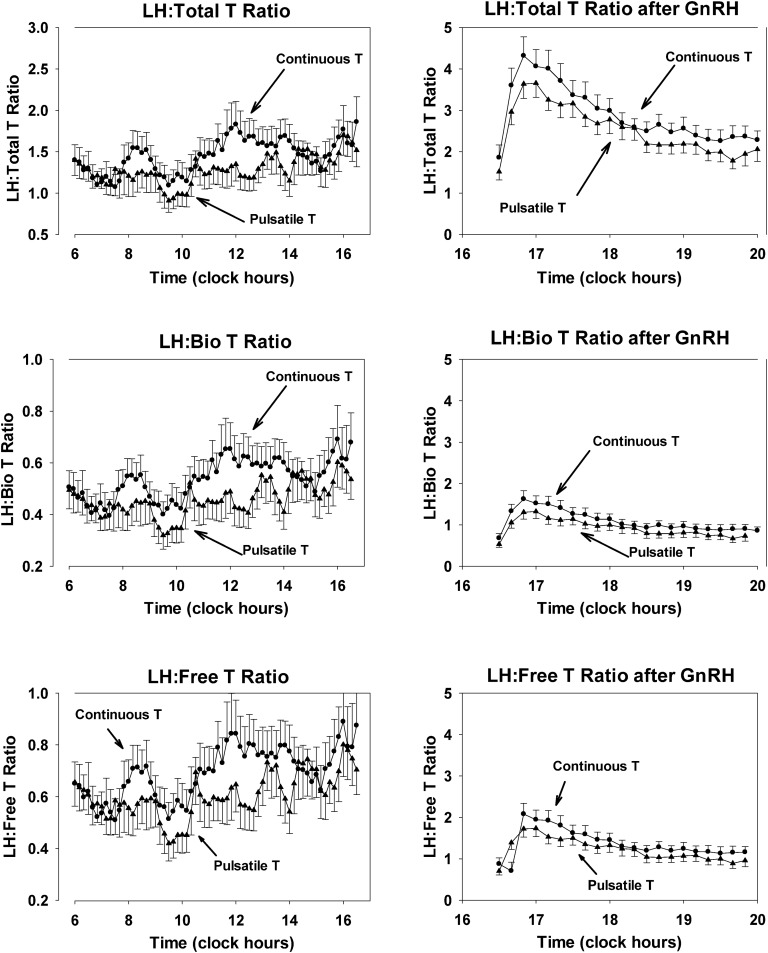
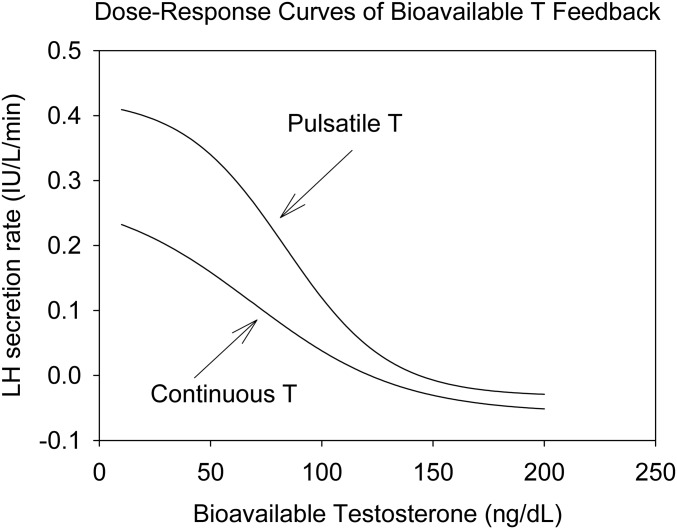
Feedback strength was evaluated by the LH/T ratio. The ratios of LH to total T, free T, and bioavailable T concentrations during pulsatile and continuous T infusions are depicted in Fig. 3. By visual inspection, the LH/T ratios during continuous T administration were larger than during pulsatile T infusion, predicting more feedback. Ratios were evaluated across successive 90-minute bins, based on the T pulse interval of 90 minutes. Results for the log-transformed ratios of LH to bioavailable T are listed in Table 2. Log-transformed ratios of LH to total and free T showed consistently lower values during pulsatile than continuous T infusions, denoting lesser pulsatile T feedback on LH. After GnRH administration, the pulsatile mass of LH, calculated by deconvolution, was 33.9 ± 3.3 IU/L/3 hours during continuous T administration, and 31.8 ± 4.2 IU/L/3 hours during pulsatile T infusion (P = 0.62). Mean post-GnRH serum LH concentrations were 7.5 ± 0.6 IU/L and 7.3 ± 0.6 IU/L, respectively (P = 0.59), and LH peaks were 19.1 ± 1.8 IU/L and 22.9 ± 2.6 IU/L, respectively (P = 0.28). Thus, after GnRH administration, with the consideration of the different T levels, T feedback also was stronger during continuous rather than pulsatile T infusion (Fig. 3, right). The calculated dose-response relation between LH and bioavailable T concentrations is shown in Fig. 4. The T/LH feedback dose-response relationship during infused T pulses was shifted upward and to the right, compared with continuous T infusion, indicating diminished feedback of bioavailable T on LH secretion. ApEn for LH was similar under both experimental conditions: during continuous T 1.097 ± 0.085 and during T pulses 1.019 ± 0.074 (P = 0.26), as were values for cross-ApEn (between T and LH), namely, 0.134 ± 0.007 and 0.120 ± 0.007, respectively (P = 0.18).
Figure 3.
LH/T ratios during the period between (left) 06:00 and 16:30 and (right) after GnRH injection. Data are shown as means ± SEM. LH is measured in international units per liter and T in nanograms per deciliter. To avoid very small numbers, the ratio LH/T was multiplied by 100, LH/bioavailable T by 10, and LH/free T by one. Bio, bioavailable.
Table 2.
Ninety-Minute Means of the Logarithmic Ratio of LH and Bioavailable T (Bio T) During Continuous and Pulsatile T Infusion
| Log (LH/Bio T) | Continuous | Pulsatile | P Value |
|---|---|---|---|
| Bin1 | 2.39 ± 0.16 | 2.39 ± 0.14 | 0.98a |
| Bin2 | 2.51 ± 0.14 | 2.31 ± 0.16 | 0.10a |
| Bin3b | 2.41 ± 0.12 | 2.16 ± 0.14 | 0.018 |
| Bin4 | 2.62 ± 0.15 | 2.38 ± 0.15 | 0.036 |
| Bin5 | 2.73 ± 0.12 | 2.42 ± 0.14 | 0.014 |
| Bin6 | 2.67 ± 0.12 | 2.41 ± 0.19 | 0.05 |
| Bin7 | 2.63 ± 0.15 | 2.46 ± 0.15 | 0.04 |
| Bin8 | 3.48 ± 0.11 | 3.28 ± 0.11 | 0.012 |
| Bin9 | 3.23 ± 0.11 | 3.04 ± 0.13 | 0.011 |
| Bin10 | 3.12 ± 0.12 | 2.86 ± 0.15 | 0.02 |
Data are means ± SEM. Comparisons were made by paired Student t test. The duration of the bins was 90 min and synchronized with the pulsatile injections.
During Bin1–Bin2 (first 3 h of study), T was infused continuously in both groups.
During inclusive Bin3–Bin10, T was infused continuously or in pulses. Duration of the bins is 90 min, except Bin10 (duration of 30 min).
Figure 4.
Completed dose-response curves of bioavailable T feedback on LH secretion rate. With increasing T concentrations, LH secretion rates diminish. Note the difference in curves. At any T concentration during (right curve) pulsatile T infusion, LH secretion is higher than that during (left curve) continuous infusions, indicating reduced feedback. The curves were constructed by four-parameter logistic regression (see Methods).
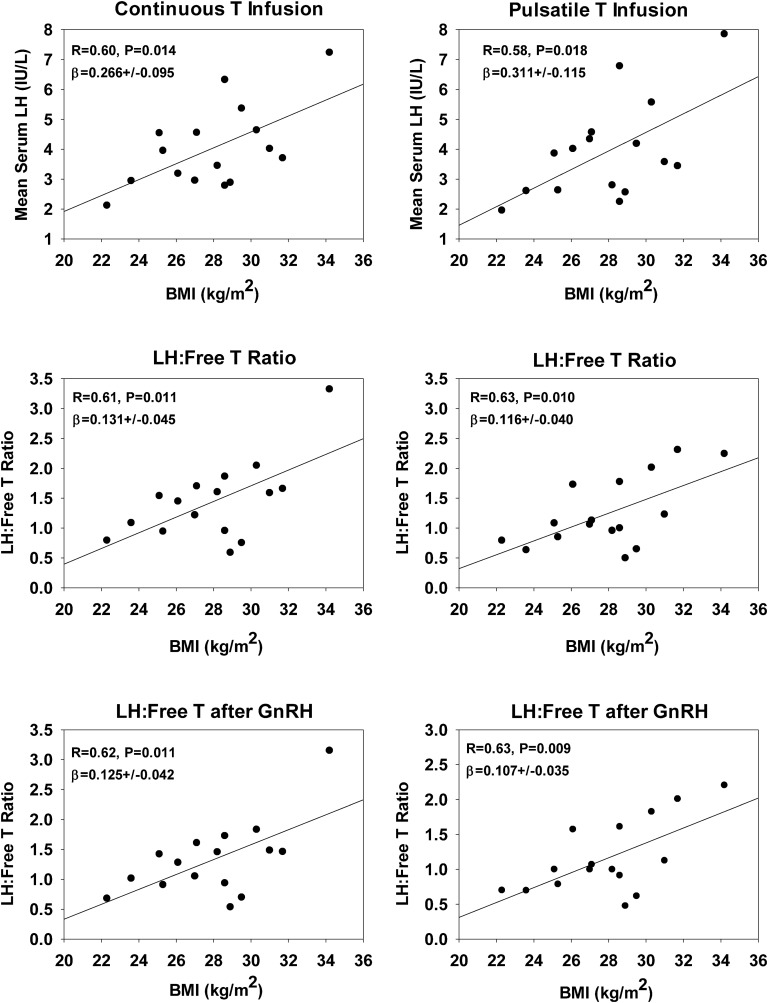
There was a nonsignificant trend between single baseline serum LH concentrations and BMI (R = +0.46, P = 0.075). Baseline serum E2 was unrelated to BMI (R = 0.36, P = 0.18), as was total T (R = 0.10, P = 0.71). However, bioavailable T correlated significantly with BMI (R = −0.51, P = 0.045). Mean serum LH concentration between 09:00 and 16:30 was positively correlated with BMI: during continuous T infusion, the correlation coefficient was 0.60, P = 0.014, and during pulsatile T infusion, R = 0.58, P = 0.018, but total T concentrations were unrelated to BMI (Fig. 5, top). The corresponding regression slopes did not differ by T infusion type (P = 0.65). Results for LH/free T ratios before and after GnRH administration are plotted in Fig. 5. Comparable regression results were found for LH/total T and LH/bioavailable T ratios and BMI (data not shown).
Figure 5.
Linear regressions of mean LH concentrations (before and after GnRH injection) and LH/free T ratios (y-axes) under controlled T feedback on BMI (x-axes). The ratios were calculated from the mean concentrations of LH and free T during the periods from 09:00 to 16:30 and after GnRH injection.
Pulsatile LH secretion, estimated by deconvolution analysis (time period between 09:00 and 16:30), was not related to visceral fat or total fat area. However, basal (nonpulsatile) LH secretion correlated negatively with visceral fat area during both continuous and pulsatile T delivery (R = −0.62, P = 0.023, β (slope) = −10.3 ± 3.9; R = −0.67, P = 0.012, β (slope) = −9.2 ± 3.0, respectively). Furthermore, BMI was not related to pulsatile LH secretion under either T feedback mode (R values 0.002 and 0.49, P values 0.99 and 0.06 under pulsatile and continuous T infusion, respectively). None of the statistical analyses showed age as a significant (co)variate in this nonaging study design.
3. Discussion
In this prospective randomized, blinded, placebo-controlled crossover study, healthy adult male subjects were treated with KTCZ to control T levels by T infusions. This procedure allowed us to quantitate T feedback on LH secretion. The inhibitory effect of T on LH secretion has been known for almost five decades [2], but no study in healthy humans has attempted to quantify T feedback under strictly controlled conditions [21]. Feedback by T modulates LH pulse frequency, pulse amplitude, and pattern regularity (ApEn), as demonstrated in healthy adults by reversible Leydig cell inhibition with KTCZ [5]. Aside from our approach of inhibition of T (and cortisol) secretion, such studies could, in principle, also be performed in castrates and patients with primary (testicular) Leydig cell failure, although comorbidities might confound interpretation. With the reduction of endogenous T secretion, to a large extent (>85%), we investigated whether an imposed pulsatile T feedback pattern has a comparable inhibitory effect on LH secretion as continuously infused T. The influence of feedback hormone patterns on feedback in T–LH and cortisol–adrenocorticotropic hormone axes has rarely been investigated under experimentally controlled conditions.
Principal outcomes of this study include the following: (i) pulsatile T is less effective than continuous T delivery in suppression of LH secretion, (ii) T feedback is reduced by BMI, and (iii) basal and total, but not pulsatile, LH secretion, estimated by deconvolution analysis is negatively correlated to visceral fat and total abdominal fat area, calculated from a single CT slice at L3-L4.
During the first 3 hours of blood sampling, when only continuous T infusion was used on both occasions, mean LH and T concentrations were identical. When T pulses were administered as 45-minute square-wave infusions at 90-minute intervals, T concentration profiles became visually different with distinct T pulses, which were readily quantified by deconvolution analysis. T concentration profiles during continuous T infusion exhibited very low-level T pulsatility, which might be attributed to some residual endogenous T secretion. Under the androgen-restraint protocols, LH secretion was similar (54.8 vs 52.2 IU/L in 7.5 hours), notwithstanding twofold larger T pulses, suggesting diminished feedback by pulsatile T delivery. Lesser feedback by T pulses on LH was inferred from diminished LH/T ratios of total, free, and bioavailable T. In addition to the model-free LH/T ratio approach, feedback quantitated by the four-parameter logistic regression model affirmed differences between pulsatile and continuous T feedback modes (Fig. 4).
The hypothalamic-pituitary-gonadal axis consists of hypothalamic kisspeptin neurons, which activate GnRH neurons. In turn, pulsatile GnRH, released into hypophyseal portal blood, induces pulsatile LH (and FSH) secretion by pituitary gonadotropes, which drive pulsatile T secretion by Leydig cells into the spermatic vein [3]. In this highly coordinated system, both E2 and T act as feedback signals. GnRH neurons lack estrogen receptor α receptors, in contrast to kisspeptin neurons that mediate such feedback [22]. Feedback on LH secretion has been studied in men with central hypogonadism lacking GnRH signaling [23, 24]. In these patients, gonadal T secretion can be restored by pulsatile IV administration of GnRH. In one study, both T and E2, given in a high dose, decreased LH secretion under exogenous GnRH [18]. In another study, endogenous T secretion was blocked by KTCZ [24]. Addback of T did not decrease LH, in contrast to E2, thus pointing to the direct inhibitory effect of E2 on LH secretion. This observation is corroborated by observations on perifused monkey pituitary cells. In this system, the stimulating effect on LH by GnRH was blocked by E2 but not by T or DHT [25]. These observations corroborate an analysis in young men, showing that aromatization mediates T short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH secretion [26]. In the current study, a strictly comparable effect of the T administration mode on spontaneous and GnRH-stimulated secretion was observed. However, this result does not necessarily point to an inhibitory action of T or E2 at the pituitary level.
It is not yet clear why pulsatile T infusion is less effective in the inhibition of LH secretion (whether GnRH is injected) than constant T delivery. Only one other study explored the efficacy of pulsatile vs continuous T addback (in a comparable infused dose of 8 mg in 24 hours) on LH secretion in six medically androgen-deprived men [8]. Continuous T infusion resulted in considerably higher free T levels than pulsatile T administration, notwithstanding the strictly identical total doses. In that study, neither mode of IV T infusion normalized serum LH concentrations, possibly as a result of inhibition of aromatization by KTCZ [8, 27, 28]. Although T feedback was not quantified in that study, mean LH was suppressed to a greater extent during continuous rather than pulsatile T infusion. A major limitation there was that T levels during continuous T addback were roughly threefold higher. In the current study, the addback of T was started at the time of steroidogenic inhibition, so that LH levels remained within the physiological range before T pulses were started. A second major difference is that the study by Zwart et al. [8] used a 1-minute bolus T injection vs a 45-minute square-wave infusion here. This infusion scheme simulates the pulsatile T pattern present in the spermatic vein in healthy men [29]. Age and body composition impact hypothalamic systems, especially the growth hormone–IGF-I axis, where age and obesity greatly diminish growth hormone secretion [30]. Studies on age and the gonadal system have revealed decreased T (free T) secretion with advancing age, as a result of various factors, including diminished LH secretion [31]. However, other studies suggest that LH secretion is unchanged or increased as men grow older [32–34]. The current study did not set out to assess any age effect. Studies on the effects of BMI (generally, statistically used as a categorical variable in three classes) have shown a negative influence on total T, free T (nine of 14 studies), and SHBG (13 of 14 studies) [35]. Two studies found a negative relation between LH and BMI [36, 37], but nine did not. The current study, in contrast, disclosed a positive relation between BMI and mean LH concentrations under controlled T feedback, based on the mean of 45 samples, rather than a single morning specimen. Statistically, similar slopes of LH on BMI were found in both experimental sessions. In contrast, analyses in massively obese subjects (BMI > 40 kg/m2) disclosed diminished LH secretion, as a result of attenuated LH pulse amplitude, which was partly corrected by the administration of testolactone, an aromatase-inhibiting drug [38–40]. Thus, the BMI range may be critically important in affecting T, SHBG, and E2 levels. In addition, the present data show that body composition (abdominal visceral fat or BMI) likely affects T feedback.
The average feedback strength (LH/T ratio) during the 7.5-hour sampling period, before and 3 hours after administration of GnRH, was positively related to BMI during both sessions (continuous T and T pulses). This finding implies less T feedback at increased BMI.
This might be a compensatory mechanism to decreased LH secretion in severe obesity. One of the metrics to quantify feedback strength is ApEn. In the current experiment, ApEn did not differ by T infusion type, possibly because LH levels were well within the normal range for each individual [19, 41]. Cross-ApEn between T and LH was also very strong in both sessions, as evidenced by the remarkable low values.
Pulsatile or episodic secretion of pituitary hormones has been recognized for several decades. It is assumed that ultradian rhythmicity prevents downregulation of the target organs. Nevertheless, clinical studies on this subject are scarce. A major discovery recently is that the pulsatility of cortisol is required for normal emotional and cognitive responses in man [42]. Although speculative, the clinical significance of pulsatile feedback, as occurs with T and cortisol, may prevent too strong a negative signal on the adrenocorticotropic hormone or LH when ongoing secretion is required.
Limitations of the study include the number of included subjects and the rather narrow age range. In addition, we cannot exclude the possibility that a longer sampling period could influence outcomes. Furthermore, this complicated study did not investigate different doses of T administration. Finally, we did not measure serum E2 concentrations during T addback so that any contributing effect of circulating E2 is unknown. On the other hand, the contribution of E2, generated locally by the anterior pituitary gland and/or hypothalamic neurons on feedback, is not well established in men.
Potentially, the DEX dose used for compensation of the acute blocking of cortisol secretion by KTCZ might interfere with LH secretion per se. However, a fourfold higher dose of DEX, administered to healthy men for 8 days, did not diminish 24-hour LH secretion or the response to GnRH [43]. A possible role of adrenal androgens, which are also suppressed by KTCZ, should be considered. The natural models for this question are patients with Cushing or Addison disease. Reports on gonadotropic function in Cushing patients are rare and conflicting. One study reported hypogonadotropism in 33% of male patients (defined by T levels <9.9 nM) and in 11% of women, defined by early menopause [44]. On the other hand, a postal survey of 269 women in the Norwegian Addison Registry disclosed a lower birth rate in patients compared with the general population (confidence interval 0.52 to 0.86), but the limitation of this observational study is that no corrections were made for diminished sexual activity [45]. Another clinical study [46] demonstrated that LH secretion was diminished in patients with Addison disease during short-term withdrawal of glucocorticoid substitution but not during eucortisolism. This was a carefully controlled study, and the conclusions are applicable for our study [46]. Therefore, it seems reasonable to infer that short-term decreased adrenal androgen secretion in the presence of sufficient glucocorticoids does not greatly impact LH secretion. If there were an impact, it would be similar in all groups that received KTCZ.
In summary, pulsatile T is less effective as a negative-feedback signal on LH secretion than continuous T administration, as demonstrated by two independent methods. This difference in feedback strength occurs, probably in part, at the pituitary level, but the data do not exclude hypothalamic involvement. Visceral and total abdominal fat by CT correlated negatively with basal and total LH secretion but not with pulsatile secretion. Feedback strength decreased with higher BMI, both without and with exogenous GnRH stimulation. These data could indicate that physiologically pulsatile and pharmacologically continuous T feedback has unequal, suppressive effects on the central gonadal axis in men.
Acknowledgments
We thank Jill Smith for support of manuscript preparation, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementation of the protocol. Content is solely the responsibility of the authors and does not necessarily represent the official views of any federal institution.
Financial Support: This work was supported, in part, by Grants R01 AG019695, R01 DK073148, R01 AG029362, R01 AG031763, and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). Matlab versions of ApEn and deconvolution methodology are available from J.D.V. (veldhuis.johannes@mayo.edu). The project described was supported by UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS) and 60NANB10D005Z from the National Institute of Standards and Technology.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ApEn
approximate entropy
- BMI
body mass index
- CV
coefficient of variation
- DEX
dexamethasone
- E2
estradiol
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- KTCZ
ketoconazole
- LH
luteinizing hormone
- PRL
prolactin
- SHBG
sex hormone-binding globulin
- T
testosterone
References and Notes
- 1. Liu PY, Veldhuis JD. Hypothalamo-pituitary unit, testis, and male accessory organs Yen & Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management: Eighth Edition. Amsterdam, The Netherlands: Elsevier; 2018:285–300. [Google Scholar]
- 2. Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114(47):E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foresta C, Bordon P, Rossato M, Mioni R, Veldhuis JD. Specific linkages among luteinizing hormone, follicle-stimulating hormone, and testosterone release in the peripheral blood and human spermatic vein: evidence for both positive (feed-forward) and negative (feedback) within-axis regulation. J Clin Endocrinol Metab. 1997;82(9):3040–3046. [DOI] [PubMed] [Google Scholar]
- 4. Finkelstein JS, Whitcomb RW, O’Dea LS, Longcope C, Schoenfeld DA, Crowley WF Jr. Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 1991;73(3):609–620. [DOI] [PubMed] [Google Scholar]
- 5. Veldhuis JD, Zwart AD, Iranmanesh A. Neuroendocrine mechanisms by which selective Leydig cell castration unleashes increased pulsatile LH release. Am J Physiol. 1997;272(2 Pt 2):R464–R474. [DOI] [PubMed] [Google Scholar]
- 6. Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A. Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab. 1987;64(1):68–73. [DOI] [PubMed] [Google Scholar]
- 7. Winters SJ, Sherins RJ, Troen P. The gonadotropin-suppressive activity of androgen is increased in elderly men. Metabolism. 1984;33(11):1052–1059. [DOI] [PubMed] [Google Scholar]
- 8. Zwart AD, Iranmanesh A, Veldhuis JD. Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab. 1997;82(7):2062–2069. [DOI] [PubMed] [Google Scholar]
- 9. Veldhuis JD. Recent insights into neuroendocrine mechanisms of aging of the human male hypothalamic-pituitary-gonadal axis. J Androl. 1999;20(1):1–17. [PubMed] [Google Scholar]
- 10. Winters SJ, Troen P. Testosterone and estradiol are co-secreted episodically by the human testis. J Clin Invest. 1986;78(4):870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spratt DI, Crowley WF Jr. Pituitary and gonadal responsiveness is enhanced during GnRH-induced puberty. Am J Physiol. 1988;254(5 Pt 1):E652–E657. [DOI] [PubMed] [Google Scholar]
- 12. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RRID:AB_2750984 http://antibodyregistry.org/search.php?q=AB_2750984. Beckman Coulter, Cat. #33510, 2018.
- 14.RRID:AB_2750983 http://antibodyregistry.org/search.php?q=AB_2750983. Beckman Coulter, Cat. #33520, 2018.
- 15.RRID:AB_2750985 http://antibodyregistry.org/search.php?q=AB_2750985. Beckman Coulter, Cat. #33530, 2018.
- 16.RRID:AB_2750986 http://antibodyregistry.org/search.php?q=AB_2750986. Siemens, Cat. #L2SH12, 2018.
- 17. Vahl N, Jorgensen JO, Skjaerback C, Veldhuis JD, Orskov H, Christiansen J. Abdominal adiposity rather than age and sex predicts the mass and patterned regularity of growth hormone secretion in mid-life healthy adults. Am J Physiol. 1997;272:E1108–E1116. [DOI] [PubMed] [Google Scholar]
- 18. Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol Regul Integr Comp Physiol. 2001;280(6):R1755–R1771. [DOI] [PubMed] [Google Scholar]
- 19. Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci USA. 2001;98(7):4028–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veldhuis JD, Straume M, Iranmanesh A, Mulligan T, Jaffe C, Barkan A, Johnson ML, Pincus S. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol Regul Integr Comp Physiol. 2001;280(3):R721–R729. [DOI] [PubMed] [Google Scholar]
- 21. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwata K, Kunimura Y, Matsumoto K, Ozawa H. Effect of androgen on Kiss1 expression and luteinizing hormone release in female rats. J Endocrinol. 2017;233(3):281–292. [DOI] [PubMed] [Google Scholar]
- 23. Bagatell CJ, Dahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl. 1994;15(1):15–21. [PubMed] [Google Scholar]
- 24. Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF Jr, Hayes FJ. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93(3):784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawakami S, Winters SJ. Regulation of lutenizing hormone secretion and subunit messenger ribonucleic acid expression by gonadal steroids in perifused pituitary cells from male monkeys and rats. Endocrinology. 1999;140(8):3587–3593. [DOI] [PubMed] [Google Scholar]
- 26. Schnorr JA, Bray MJ, Veldhuis JD. Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH and FSH secretion in young men. J Clin Endocrinol Metab. 2001;86(6):2600–2606. [DOI] [PubMed] [Google Scholar]
- 27. Wouters W, De Coster R, Goeminne N, Beerens D, van Dun J. Aromatase inhibition by the antifungal ketoconazole. J Steroid Biochem. 1988;30(1-6):387–389. [DOI] [PubMed] [Google Scholar]
- 28. Weber MM, Will A, Adelmann B, Engelhardt D. Effect of ketoconazole on human ovarian C17,20-desmolase and aromatase. J Steroid Biochem Mol Biol. 1991;38(2):213–218. [DOI] [PubMed] [Google Scholar]
- 29. Veldhuis JD, Keenan DM, Liu PY, Takahashi PY. Kinetics of removal of intravenous testosterone pulses in normal men. Eur J Endocrinol. 2010;162(4):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roelfsema F, Veldhuis JD. Growth-hormone dynamics in healthy adults are related to age and gender, and strongly dependent on body mass index. Neuroendocrinology. 2016;103(3–4):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veldhuis JD, Keenan DM, Liu PY, Iranmanesh A, Takahashi PY, Nehra AX. The aging male hypothalamic-pituitary-gonadal axis: pulsatility and feedback. Mol Cell Endocrinol. 2009;299(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stearns EL, MacDonnell JA, Kaufman BJ, Padua R, Lucman TS, Winter JSD, Faiman C. Declining testicular function with age. Hormonal and clinical correlates. Am J Med. 1974;57(5):761–766. [DOI] [PubMed] [Google Scholar]
- 33. Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davidson JM, Chen JJ, Crapo L, Gray GD, Greenleaf WJ, Catania JA. Hormonal changes and sexual function in aging men. J Clin Endocrinol Metab. 1983;57(1):71–77. [DOI] [PubMed] [Google Scholar]
- 35. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293–311. [DOI] [PubMed] [Google Scholar]
- 36. Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13(4):353–363. [DOI] [PubMed] [Google Scholar]
- 37. Winters SJ, Takahashi J, Troen P. Secretion of testosterone and its delta4 precursor steroids into spermatic vein blood in men with varicocele-associated infertility. J Clin Endocrinol Metab. 1999;84(3):997–1001. [DOI] [PubMed] [Google Scholar]
- 38. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76(5):1140–1146. [DOI] [PubMed] [Google Scholar]
- 39. Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79(4):997–1000. [DOI] [PubMed] [Google Scholar]
- 40. Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003;52(9):1126–1128. [DOI] [PubMed] [Google Scholar]
- 41. Roelfsema F, Biermasz NR, Veldman RG, Veldhuis JD, Frölich M, Stokvis-Brantsma WH, Wit J-M. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab. 2001;86(6):2459–2464. [DOI] [PubMed] [Google Scholar]
- 42. Kalafatakis K, Russell GM, Harmer CJ, Munafo MR, Marchant N, Wilson A, Brooks JC, Durant C, Thakrar J, Murphy P, Thai NJ, Lightman SL. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proc Natl Acad Sci USA. 2018;115(17):E4091–E4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab. 1992;74(1):96–102. [DOI] [PubMed] [Google Scholar]
- 44. Ross IL, Levitt NS, Blom DJ, Haarburger D. Male and female hypogonadism are highly prevalent in South Africans with Addison’s disease. Horm Metab Res. 2014;46(10):691–696. [DOI] [PubMed] [Google Scholar]
- 45. Erichsen MM, Husebye ES, Michelsen TM, Dahl AA, Løvås K. Sexuality and fertility in women with Addison’s disease. J Clin Endocrinol Metab. 2010;95(9):4354–4360. [DOI] [PubMed] [Google Scholar]
- 46. Hangaard J, Andersen M, Grodum E, Koldkjaer O, Hagen C. Pulsatile luteinizing hormone secretion in patients with Addison’s disease. Impact of glucocorticoid substitution. J Clin Endocrinol Metab. 1998;83(3):736–743. [DOI] [PubMed] [Google Scholar]