Abstract
Mild neurocognitive impairments are common in people with human immunodeficiency virus (HIV) infection. HIV-encoded proteins, such as trans-activator of transcription (TAT), contribute to neuropathology and cognitive function in medicated subjects. The combination of TAT and comorbid methamphetamine use may further impair neurocognitive function in HIV-positive individuals by affecting dopaminergic systems in the brain. The current study examined the effects of TAT protein expression and methamphetamine exposure on cognitive function and dopamine systems in mice. Transgenic mice with inducible brain expression of the TAT protein were exposed to a binge methamphetamine regimen. TAT expression was induced via a doxycycline-containing diet during the final stage of the regimen and maintained throughout cognitive testing. Learning and executive function were assessed using an operant visual discrimination protocol, with a strategy switch and reversal. TAT expression and methamphetamine exposure improved visual discrimination learning. Combined TAT expression and methamphetamine exposure increased perseverative errors during reversal learning. TAT expression altered reversal learning by improving early stage, but impairing late stage, learning. TAT expression was also associated with an increase in dopamine transporter expression in the caudate putamen. These results highlight that TAT expression and methamphetamine exposure likely affect a range of selective cognitive processes, with some potentially improving function under certain conditions.
Keywords: Dopamine transporter, Methamphetamine binge, Reversal learning, Perseveration
1. Introduction
Mild neurocognitive impairments are common in people with human immunodeficiency virus (HIV; over 35%), even those undergoing combination antiretroviral therapy [1]. HIV-associated neurocognitive disorders (HAND) include impairments in learning, executive functions and working memory [2,3]. Underlying corticostriatal dysfunction, such HIV-induced decreases in caudate/basal ganglia volume [4,5] and dopamine levels [6], may be associated with poorer cognitive performance [5,7]. HIV-encoded proteins, such as trans-activator of transcription (TAT) and glycoprotein (gp) 120, contribute to neuronal dysfunction in the basal ganglia [8] and may explain the presence of cognitive impairments in subjects with low viral loads. For example, expression of gp120 in mice recapitulates learning deficits observed in HIV-positive humans [9].
Transgenic mice with inducible brain expression of the TAT protein [10] represent a powerful animal model to investigate the specific effects of TAT protein expression on cognitive function. Induction of the TAT protein results in neuropathology similar to that observed in HIV-infected humans [10] and leads to dysfunction in corticostriatal dopaminergic neurotransmission [11–17]. TAT exposure also induces a complex combination of cognitive dysfunction in rodents. For example, TAT impairs spatial learning [18,19] but improves spatial reversal learning [12]. TAT expression also affects aspects of sensory motor gating [20]. However, TAT expression does not recapitulate the cognitive consequences of HIV disease entirely. For example, TAT expression does not impair delay-dependent working memory [13], even though working memory deficits are common in HIV [2,3]. This combined evidence suggests that subcortical dopamine systems are particularly sensitive to neuropathology induced by HIV-associated proteins and this is associated with impairment of specific cognitive domains.
Corticostriatal systems are also highly susceptible to methamphetamine-induced neurotoxicity [21,22], which is a common comorbidity in HIV-infected subjects [23]. Comorbid methamphetamine use has been associated with increased neurocognitive impairments in HIV-infected individuals [24,25]. Furthermore, individuals who are both methamphetamine-dependent and HIV-infected show exacerbated neuronal injury [26] and cortical interneuron loss [27,28]. The susceptibility of corticostriatal dopaminergic systems to methamphetamine and the TAT protein, suggest their combined exposure may lead to further dysfunction. We have previously shown that TAT expression increases the sensitivity to methamphetamine reward [13] and enhances methamphetamine-induced sensitization [14]. However, their combined influence on cognitive function is understood less.
The aim of the present study was to determine the combined effects of TAT protein expression and methamphetamine exposure on cognitive function in mice. To effectively model methamphetamine use and TAT expression in human subjects, we combined the temporal control of TAT expression in inducible transgenic mice [10] with a binge regimen of methamphetamine exposure that closely resembles use in human subjects [22]. Subsequent to methamphetamine exposure, learning and executive function were assessed using an operant visual discrimination protocol. This protocol featured the acquisition of a visual discrimination rule as a measure of learning, followed by strategy switch and reversal stages to assess executive function. To assess the effects of TAT and methamphetamine on brain dopaminergic systems, dopamine transporter (DAT) levels were determined in mesolimbic and striatonigral brain areas.
2. Methods
2.1. Animals
A total of 48 male mice (9–14 months old at the beginning of the experiment) were provided by the Neuroscience and Animal Models Core of the Translational Methamphetamine AIDS Research Center (TMARC) and tested. Of these, 22 mice contained the GFAP promotor-controlled Tet-binding protein (TAT-) and 26 contained both the GFAP promotor-controlled Tet-binding protein and the TRE promotor-TAT protein transgene (TAT+). There were no significant differences in ages between the test groups (TAT-/saline, 12.6 ± 0.3 [SEM]; TAT+/saline, 12.4 ± 0.4; TAT-/methamphetamine, 12.6 ± 0.5; TAT+/methamphetamine, 12.9 ± 0.4). Inducible TAT transgenic mouse colonies with a C57BL/6 J background were obtained by generation of two separate transgenic lines Teton-GFAP mice and TRE-Tat86 mice, and then cross-breeding of these two transgenic mouse lines, as previously described [10]. The mice were housed 1–4 mice per cage in a humidity-and temperature-controlled animal facility on a 12 h/12 h reverse light/dark cycle (lights off at 7:00 a.m.). Mice had ad libitum access to food and water during exposure to methamphetamine binge (1–3 weeks). As doxycycline has been shown to induce behavioral changes when administered to control mice [13], all mice were given a doxycycline-containing diet (6 g/kg diet #F4096, Bioserv Flemington, NJ). Mice were provided with the diet on day 21 of methamphetamine exposure (i.e., two days prior to cycle 4 of administration) and continuing throughout the experiment (see Table 1). Inducing TAT expression via chronic exposure to a doxycycline-containing diet has been demonstrated to produce functional changes to synaptic physiology and alterations in behavior [29]. During behavioral training and testing, mice were food deprived to 85% free-feeding weight, but had ad libitum access to water. Behavioral testing was conducted 7 days/week during the dark phase of the light/dark cycle from 2 p.m to 7 p.m with mice from all groups being tested concurrently at any given time throughout the testing period. All of the experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and National Research Council’s Guide for the Care and Use of Laboratory Animals and approved by the University of California San Diego Institutional Animal Care and Use Committee.
Table 1.
Methamphetamine binge regimen.
| Cycle | 1 | 2 | 3 | 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 4 | 9 | 10 | 11 | 12 | 16 | 17 | 18 | 19 | 23 | 24 | 25 | 26 |
| 12:00 | 1 | 3 | 5 | 6 | 3 | 5 | 6 | 6 | 3 | 5 | 6 | 6 | 3 | 5 | 6 | 6 |
| 14:00 | 1 | 3 | 5 | 6 | 3 | 5 | 6 | 6 | 3 | 5 | 6 | 6 | 3 | 5 | 6 | 6 |
| 16:00 | 2 | 4 | 5 | 6 | 4 | 5 | 6 | 6 | 4 | 5 | 6 | 6 | 4 | 5 | 6 | 6 |
| 18:00 | 2 | 4 | 5 | 6 | 4 | 5 | 6 | 6 | 4 | 5 | 6 | 6 | 4 | 5 | 6 | 6 |
Methamphetamine doses are reported as base concentration (mg/kg).
2.2. Experimental design
Mice underwent the binge methamphetamine regimen and were placed on a doxycycline containing diet two days prior to the final cycle of the regimen. Mice remained on this diet for all subsequent behavioral testing. Visual discrimination training began two weeks after completion of the binge regimen. The advanced age of the mice tested led to approximately 30% of the mice failing to reach criteria independent of TAT expression and methamphetamine exposure. This may also be a consequence of doxycycline administration. We have previously observed behavioral changes as a direct result of doxycycline administration in TAT- mice [13], which is why doxycycline treated TAT- mice were used as controls in the present study. An equivalent control group fed a diet without doxycycline would be required to establish age-dependent versus doxycycline-dependent effects on task completion. Thus, final group numbers for behavioral data were: TAT-/saline = 7, TAT+/saline = 9, TAT-/methamphetamine = 9, and TAT+/methamphetamine = 7. After completion of behavioral testing, mice underwent anaesthetized magnetic resonance imaging (these data are not presented in this manuscript) and then brain samples from all mice (15–20 months old) were collected for immunohistochemistry analyses (TAT-/saline = 12, TAT+/saline = 13, TAT-/methamphetamine = 8, and TAT+/methamphetamine = 10).
2.3. Methamphetamine regimen
The methamphetamine binge regimen was previously developed in our laboratory to mimic human binge use parameters [22]. The binge consisted of subcutaneous injections of saline or methamphetamine (methamphetamine hydrochloride; Sigma, St. Louis, MO, USA; reported as base concentration) for four ‘cycles’ of 4 days (four injections/day) with 3 days between cycles (Table 1). The first cycle featured one week of dose-escalation followed by three repeated identical cycles of methamphetamine exposure. These 2–4 cycles consisted of a two-day dose-escalation (3–5 mg/kg) followed by two days of high dosing (6 mg/kg). Saline and methamphetamine were administered at a volume of 5 ml/kg.
2.4. Visual discrimination task
2.4.1. Discrimination training
Visual discrimination training and testing were conducted in twelve Plexiglas operant chambers (model ENV-307A, Med Associates Inc., St. Albans, VT, USA), each enclosed in a sound-attenuating cubicle. The task consisted of 2 continuous reinforcement (CRF) training stages followed by the visual discrimination test phase. During the first CRF stage, a single lever was presented (left or right, chosen at random). A single lever press resulted in the delivery of a single food reward (20 mg Sucrose pellet, TestDiet, IN, USA). The criterion to progress to the next stage was > 30 lever presses over three days. The second CRF stage was identical, except the lever retracted after each press. Following a nose poke in the reward receptacle (15 s hold), a 5 s delay was initiated before the lever was extended for the next trial. The criterion to progress to the next stage was > 40 lever presses with < 30% omissions in two of three days. For the visual discrimination task, both levers were extended and one of the stimulus lights above the lever was illuminated. The mouse was required to press the lever with the associated stimulus light illuminated. An incorrect response or failure to respond to either the sample or choice levers during the 20 s limited hold (i.e., an omission) resulted in a time out period (house lights on) of 5 s. Correct responses were calculated as a percentage of the total trials (not including omissions). The criterion to complete visual discrimination and progress to the strategy switch phase was > 70% correct trials and < 30% omissions over three days. Mice were given 60 trials per test session.
2.4.2. Strategy switch
Once criteria were met for visual discrimination stage, mice continued onto strategy switch testing. All the parameters for testing remained the same as for the visual discrimination test but the reward contingencies were changed. Rather than the stimulus light predicting the rewarded lever, the spatial location of the lever (left or right) was the relevant reward contingency. The criterion to complete testing for strategy shift and progress to reversal learning testing was > 70% correct trials and < 30% omissions over three days.
2.4.3. Spatial reversal learning
Once criteria were met for the strategy switch stage, mice continued onto reversal learning testing. All the parameters remained the same as for the strategy switch test but the reward contingencies were changed. For mice where the left lever had previously predicted the reward, the right lever was now the correct contingency, and vice versa where the left lever had previously predicted the reward. The criterion for completion of reversal learning testing was > 70% correct trials and < 30% omissions over three days.
2.4.4. Behavioral measures
Behavioral measures included the amount of trials (total, correct and error trials) to reach criterion. Latency measures including the amount of time (ms) the animals took to respond and to collect the food reward were also recorded. The specific strategies used were also recorded including win-stay and lose-shift. That is, following a rewarded trial, a win-stay event was recorded if a subject made a response at the same lever. Conversely, following an incorrect response, a lose-shift event was recorded if a subject made a response at the opposite lever.
Additional measures were obtained for reversal learning. Specifically, the number of perseverative errors, defined as the total number of responses on the previously rewarded lever until a correct response was recorded. Further, the number of trials was broken down into early and late learning phases [30]. To define early vs late phase learning, each session was split into blocks of 20 trials. Once mice reached > 50% correct responses in a given block, this and all subsequent blocks were considered late phase learning.
2.5. Dopamine transporter quantification
Briefly, as previously described [31], mouse hemibrain tissue sections were deparaffinized using xylene followed by rehydration in serial ethanol and water solutions. Next, tissue sections were treated for 30 min with 3% hydrogen peroxide/phosphate-buffered saline (PBS) and then incubated for 30 min with 2.5% normal serum, corresponding to the host species for the secondary antibody. Tissue sections were then incubated with anti-DAT (Santa Cruz Biotechnology, Dallas, TX; Cat# sc-32258; 1:100 in PBS) for 2 h at room temperature in a hydration box. Subsequently, tissue sections were washed with 0.1% Tween 20/PBS, before 30 min incubation with horse anti-rabbit IgG peroxidase-polymer secondary antibody (ImmPRESS, Vector Laboratories, Burlingame, CA, USA). After the tissues were washed with 0.1% Tween 20/PBS, the signals were developed with diaminobenzidine (ImmPACT DAB peroxidase substrate, Vector Laboratories) for 5 min. The immunostained sections were then dehydrated via serial ethanol and water solutions, de-waxed with xylene, and mounted using Cytoseal 60 (ThermoScientific). For the negative control, the primary antibody was omitted.
Subsequently, immunostained sections were scanned using a microscope slide scanner (Aperio ScanScope GL, Leica Biosystems, Buffalo Grove, IL, USA) equipped with a 20 × objective lens (yielding the resolution of 0.5 μm per pixel). Assessment of levels of DAT immunoreactivity was performed using the Aperio ImageScope software. For each case a total of three sections (5 images per section) were analyzed to estimate the average optical density of immunolabelled cells per unit area (mm2). Corrected optical density was calculated by subtracting the background optical density of the negative control (obtained from tissue sections immunostained in the absence of primary antibody) from the optical density of the immunostained sections.
2.6. Statistical analyses
All analyses were performed with IBM SPSS Statistics 20 (Armonk, NY, USA). Data were analyzed using analysis of variance (ANOVA), with TAT and Methamphetamine as the between-subject factors. Repeated-measures ANOVAs were used when within-subject factors were present. Data not meeting the assumption of homogeneity of variance were analyzed using Greenhouse-Geiser adjusted degrees of freedom. When appropriate, post hoc comparisons were performed using Least Significant Difference (LSD) analyses. Perseverative errors were analyzed non-parametrically using a Kruskal-Wallis ANOVA. Results are expressed as mean ± SEM. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Visual discrimination
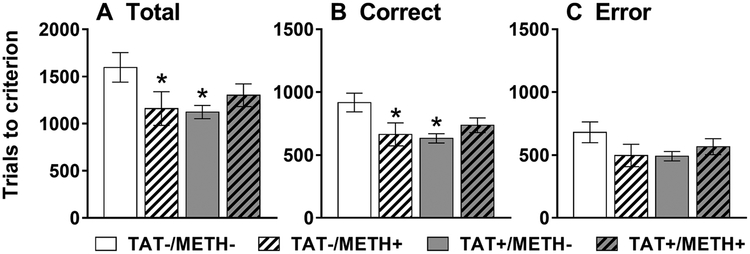
For the trials required to reach criterion, there were significant interactions of Methamphetamine × TAT for total trials (Fig. 1A; F1,28 = 4.6, p < 0.05) and correct trials (Fig. 1B; F1,28 = 6.1, p < 0.05). There was also a trend towards an interaction of Methamphetamine × TAT for error trials (Fig. 1C; F1,28 = 3.1, p < 0.1 NS). TAT expression and methamphetamine exposure alone, but not in combination, improved visual discrimination learning as demonstrated by a significant decrease in the total and correct trials to reach criterion. There was also a trend (p < 0.1) for combined TAT expression and methamphetamine exposure to decrease the number of correct trials to reach criterion (Fig. 1B).
Fig. 1.
Visual discrimination learning.
Total (A), correct (B) and error (C) trials to reach criterion on the visual discrimination task in TAT-expressing (TAT+) and/or methamphetamine-exposed (METH+) mice. Both TAT-expressing (TAT+/METH-) and methamphetamine-exposed (TAT-/METH+) mice required less trials to reach criterion than control (TAT-/METH-) mice. This effect was most evident in the total (A; Methamphetamine × TAT [F1,28 = 4.6, p < 0.05]) and correct (B; Methamphetamine × TAT [F1,28 = 6.1, p < 0.05]) trials required. A similar but non-significant trend was also observed for the number of errors (C; Methamphetamine × TAT [F1,28 = 3.1, p < 0.1 NS]). Data are expressed as mean ± SEM. *p < 0.05 compared to TAT-/METH-.
There were no significant effects or interactions of TAT or Methamphetamine on the response latency (Fig S1A–C) or win-stay/lose-shift strategies used during visual discrimination learning (Fig S2A).
3.2. Strategy shift
Neither TAT expression nor methamphetamine exposure significantly altered the total trials (Fig S3A), correct trials (Fig S3B) and error trials (Fig S3C) to reach criterion. There were also no significant effects or interactions of TAT or Methamphetamine on the response latency (Fig S1D–F) or win-stay/lose-shift strategies used during the strategy switch (Fig S2B).
3.3. Spatial reversal learning
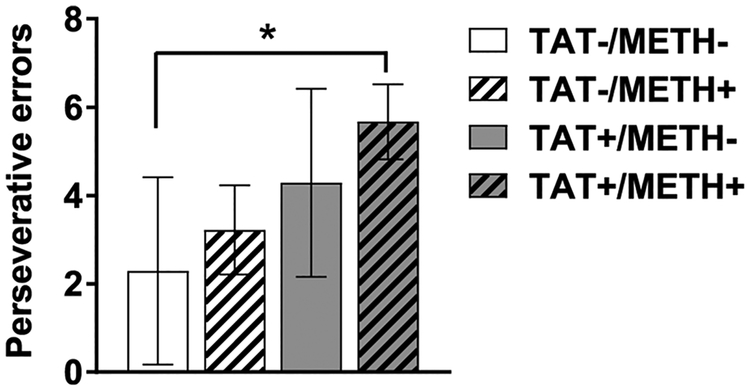
Neither TAT expression nor methamphetamine exposure significantly altered the total trials (Fig S3D), correct trials (Fig S3E) and error trials (Fig S3F) to reach criterion. There were no significant main effects or interactions of TAT or Methamphetamine on the response latency (Fig S1G–I) or win-stay/lose-shift strategies used during the strategy switch (Fig S2C). However, there was a significant difference between the groups on the number of perseverations (Fig. 2; H(3) = 8.5, p < 0.05). The control group showed the lowest level of perseverations, followed by the methamphetamine exposed group and the TAT-expressing group, with the greatest level of perseverative errors in the methamphetamine exposed, TAT-expressing group. Post hoc tests con-firmed that the methamphetamine exposed, TAT-expressing group made significantly more perseverative errors than control mice (p < 0.05).
Fig. 2.
Perseverative errors during reversal learning.
Perseverative errors at the initial reversal of reward contingencies in TAT-expressing (TAT+) and/or methamphetamine exposed (METH+) mice. TAT+/METH + mice made significantly more perseverative errors compared with the control (TAT-/METH-) group (H(3) = 8.5, p < 0.05). Data are expressed are mean ± SEM. *p < 0.05.
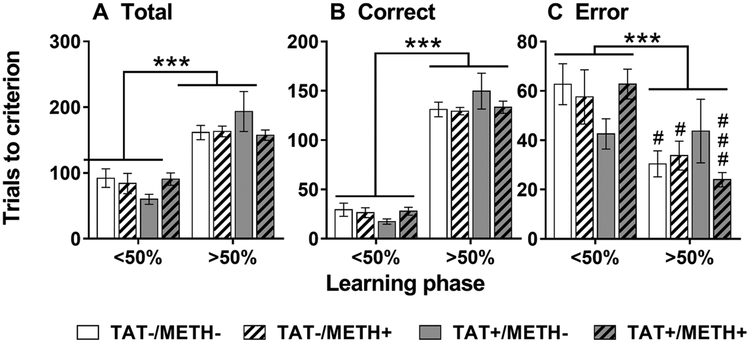
Reversal learning features two distinct phases of learning [30]: an early phase (< 50% correct responses) whereby the animal must learn to disassociate the prior reward contingencies, and a late phase (> 50% correct responses) whereby the animal must learn the new associations. The different phases can be independently altered after a variety of manipulations [30]. Compared with the late phase, the early phase featured less total (Fig. 3A; F1,28 = 71.8, p < 0.001) and correct trials (Fig. 3B; F1,28 = 383.6, p < 0.001) but more errors trials (Fig. 3C; F1,28 = 17.4, p < 0.001). Moreover, there was a significant interaction of Phase × Methamphetamine × TAT for error trials (F1,28 = 4.6, p < 0.05) and a similar trend for total trials (F1,28 = 3.4, p < 0.1 NS). TAT expression tended to decrease early phase errors and increase late phase errors (Fig. 3C) compared with controls, methamphetamine exposure or combined TAT expression and methamphetamine exposure. A similar pattern was observed for total trials during early and late phase learning (Fig. 3A). There were no significant effects or interactions of TAT or Methamphetamine during early or late phase learning for response latency (Fig S4A,B) or win-stay/lose-shift strategies (Fig S4C,D).
Fig. 3.
Early (< 50%) and late (> 50%) phases of reversal learning.
Total (A), correct (B) and error (C) trials to reach criterion during the early (< 50%) and late (> 50%) phases of reversal learning in TAT-expressing (TAT+) and/or methamphetamine exposed (METH+) mice. A significant decrease in error trials was observed during the late phase compared with the early phase of learning in all groups except for the TAT+/METH- mice (C; Phase × Methamphetamine × TAT [F1,28 = 4.6, p < 0.05]). TAT+/METH- mice tended to make less early phase errors and more late phase errors compared with the other groups. Data are expressed as mean ± SEM. *** p < 0.001. # p < 0.05, ### p < 0.001 when comparing < 50% with > 50% learning phases within group.
3.4. Dopamine transporter expression
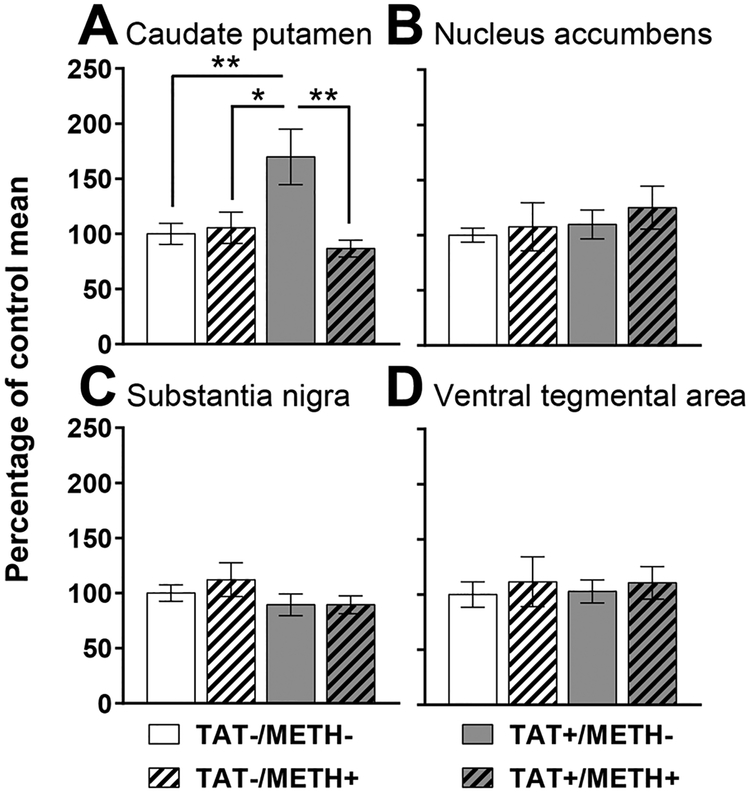
In the caudate putamen, there was a significant interaction of Methamphetamine × TAT for dopamine transporter expression (F1,35 = 5.2, p < 0.05). TAT expression significantly elevated DAT levels compared with controls, methamphetamine exposure and combined TAT expression and methamphetamine exposure (Fig. 4A). There were no significant effects or interactions of TAT or Methamphetamine on dopamine transporter expression in the nucleus accumbens (Fig. 4B), substantia nigra (Fig. 4C) or ventral tegmental area (Fig. 4D).
Fig. 4.
Dopamine transporter expression.
Relative dopamine transporter pixel intensity in the caudate putamen (A), nucleus accumbens (B), substantia nigra (C) and ventral tegmental area (D) in TAT-expressing (TAT+) and/or methamphetamine exposed (METH+) mice. TAT+/METH- mice had significantly greater dopamine transporter expression in the caudate putamen compared with all other groups (A; Methamphetamine × TAT [F1,35 = 5.2, p < 0.05]). Data are expressed as mean ± SEM. * p < 0.05, ** p < 0.01.
4. Discussion
The results show a complex pattern of outcomes after TAT expression and/or methamphetamine exposure. That is, TAT expression and methamphetamine exposure both independently improved visual discrimination learning. TAT expression improved early phase reversal learning, at the expense of late phase reversal learning. TAT expression was also associated with increased dopamine transporter levels in the caudate putamen. Surprisingly, the combination of TAT expression and methamphetamine exposure did not induce significant learning deficits, but did increase perseverations at the initiation of reversal learning suggesting slight impairments in executive function. Thus, TAT expression showed subtle effects on learning that may be due to alterations in dopaminergic function, but these effects are not summative or synergistic with those of methamphetamine.
Contrary to our expectations, TAT expression and methamphetamine exposure improved learning in a visual discrimination task, decreasing the total and correct trials to reach criterion. HIV-associated proteins such as gp120 [32], as well as methamphetamine exposure [33], have been shown to alter hypothalamic-pituitary-adrenal axis function, which may alter the motivation toward food rewards in operant behavioural tasks. However, this is unlikely as the improvement observed in the present study was not associated with differences in response latency or the strategies used during the task. Although a similar pattern was observed for error trials, the greater magnitude and larger effect on the correct trials required would suggest that this outcome may be attributed to an increased sensitivity to positive feedback. Rats previously exposed to methamphetamine show an increased sensitivity to, and reliance on, positive feedback during discrimination tasks [34]. There is also evidence that HIV-positive individuals direct more attention towards reward-associated information [35]. Thus, both TAT and methamphetamine exposure can increase the perceived relevance of positive feedback, which may explain the present findings. Reinforcement learning is heavily associated with dopamine function in mesolimbic projections [36]. We have shown that this regimen of methamphetamine exposure induces a persistent decrease in dopamine within the nucleus accumbens [22] and that TAT expression also tends to decrease dopamine in the nucleus accumbens [13]. Combined TAT expression and methamphetamine exposure tended to show the same pattern of improvement; however, this effect was not significant. The effects of dopamine levels on cognitive function has a well-established inverted-U-shaped relationship [37], with optimal levels of dopamine required for peak performance (i.e, too much or too little dopamine can lead to impaired performance). Thus, it is possible that combined effects of TAT expression and methamphetamine exposure on dopamine systems are too large, and performance gains are no longer evident.
TAT expression and methamphetamine exposure induced a complex pattern of effects on reversal learning in the present study. Specifically, combined TAT expression and methamphetamine exposure increased the level of perseverative responses at the initiation of reversal learning. The orbitofrontal cortex and caudate putamen are critical for reversal learning [38–40]. The methamphetamine regimen used in the current study led to persistently increased dopamine levels in the orbitofrontal cortex and decreased dopamine levels in the caudate putamen [22]. Alterations in the neurochemistry of these key regions, combined with TAT expression, could explain the increase in perseverative errors observed after combined TAT expression and methamphetamine exposure.
In contrast, TAT expression alone improved early phase reversal learning at the expense of late phase reversal learning. Given this effect was associated with selectively increased dopamine transporter levels in the caudate putamen of this group, it is tempting to conclude the two are associated. However, it is likely more complex as differing factors appear to mediate early and late phase reversal learning. For example, selective improvements in early phase reversal learning have been observed after increasing serotonin levels in the brain [30]. The selectivity of serotonin alterations to early phase reversal learning suggests that early and late phase reversal learning may be independent processes. TAT expression has been shown to increase serotonin levels in the nucleus accumbens [13] suggesting this may be a potential mechanism for the observed early phase improvements. However, whether cortical serotonin levels are altered is not known and how influential this may be in HIV infection remains to be determined. Most evidence suggests that serotonin level/function is decreased after HIV infection. For example, polymorphisms that reduce serotonin function impair cognitive performance in HIV-positive subjects [41] and Simian Immunodeficiency Virus infection in Macaques decreases serotonin levels [42]. This provides further evidence that the global effects of HIV disease are the result of multiple factors in combination and individual proteins/responses represent only one contributing factor in the symptom profile of HIV disease. In contrast to early phase learning, the caudate putamen is crucial for establishing and maintaining the new rule in late phase learning. For example, lesions of the medial caudate putamen impair reversal learning in rats by increasing the number of errors made when learning and maintaining the new rule [39]. Thus, alterations in dopamine signalling, due to increased dopamine transporter levels in the caudate putamen, may have selectively impaired late phase learning in TAT-expressing mice. This would be consistent with our prior work, whereby cessation of TAT protein expression was associated with recovery of dopamine indices and improvements in late phase reversal learning [12,13].
No significant effects of TAT expression and methamphetamine exposure were observed on the strategy-switch phase of testing. The prefrontal cortex is critical for shifting from one rule or strategy to another [38–40] so this would suggest that prefrontal cortex function is not impaired under the current experimental conditions.
In conclusion, both TAT expression and methamphetamine exposure may improve associative learning under certain experimental conditions by enhancing the response to positive feedback. However, an over-emphasis on positive feedback would not be beneficial in more challenging and less predictable real-world situations i.e., gambling. Furthermore, an increased drive for positive feedback due to TAT expression may make it more difficult for HIV-infected subjects, who are dependent, to abstain from using methamphetamine. These results highlight the complexity of modelling and understanding HIV disease and methamphetamine abuse. The HIV-associated TAT protein likely affects a range of factors, with some potentially improving function under certain conditions.
Supplementary Material
Acknowledgments
The authors were supported by the Translational Methamphetamine AIDS Research Center funded by the National Institute on Drug Abuse (P50DA26306). This work was also supported by an Advance Queensland Research Fellowship (AQRF04115–16RD1 to JPK). The funding source(s) had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The Translational Methamphetamine AIDS Research Center (TMARC) is supported by Center award P50DA026306 from the National Institute on Drug Abuse (NIDA) and is affiliated with the University of California, San Diego (UCSD), the Sanford-Burnham Medical Research Institute (SBMRI), and the University of California, Irvine (UCI). The TMARC is composed of: Director – Igor Grant, M.D.; Co-Directors – Ronald J. Ellis, M.D., Ph.D., Scott L. Letendre, M.D., and Cristian L. Achim, M.D., Ph.D.; Center Manager – Mariana Cherner, Ph.D.; Associate Center Manager – Erin E. Morgan, Ph.D.; Assistant Center Manager – Aaron M. Carr, B.A.; Behavioral Assessment and Medical (BAM) Core–Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, M.D. (Core Co-Director/NLU Chief), Ronald J. Ellis, M.D., Ph.D.; BAM Core–Neuropsychiatric Unit (NPU): Robert K. Heaton, Ph.D. (Core Co-Director/NPU Chief), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Erin E. Morgan, Ph.D., Matthew Dawson (NPU Manager); Neuroimaging (NI) Core: Gregory G. Brown, Ph.D. (Core Director), Thomas T. Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A., John R. Hesselink, M.D., Mary Jane Meloy, Ph.D., Craig E.L. Stark, Ph.D.; Neuroscience and Animal Models (NAM) Core: Cristian L. Achim, M.D., Ph.D. (Core Director), Marcus Kaul, Ph.D., Virawudh Soontornniyomkij, M.D.; Pilot and Developmental (PAD) Core: Mariana Cherner, Ph.D. (Core Director), Stuart A. Lipton, M.D., Ph.D.; Pilot Study Leaders: Jennifer E. Iudicello, Ph.D., Assawin Gongvatana, Ph.D., Rachel D. Schrier, Ph.D., Virawudh Soontornniyomkij, M.D., Marta Massanella, Ph.D.; Administrative Coordinating Core (ACC)–Data Management and Information Systems (DMIS) Unit: Anthony C. Gamst, Ph.D. (Unit Chief), Clint Cushman, B.A. (Unit Manager); ACC–Statistics Unit: Florin Vaida, Ph.D. (Unit Chief), Ian S. Abramson, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; ACC–Participant Unit: J. Hampton Atkinson, M.D. (Unit Chief), Jennifer Marquie-Beck, M.P.H. (Unit Manager); Project 1: Arpi Minassian, Ph.D. (Project Director), William Perry, Ph.D., Mark A. Geyer, Ph.D., Jared W. Young, Ph.D.; Project 2: AmandaB. Grethe, Ph.D. (Project Director), Susan F. Tapert, Ph.D., Assawin Gongvatana, Ph.D.; Project 3: Erin E. Morgan, Ph.D. (Project Director), Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (Project Director), Athina Markou, Ph.D., James Kesby, Ph.D.; Project 5: Marcus Kaul, Ph.D. (Project Director).
Abbreviations:
- HIV
human immunodeficiency virus
- TAT
trans-activator of transcription
- HAND
HIV-associated neurocognitive disorders
- DAT
dopamine transporter
- CRF
continuous reinforcement
- PBS
phosphate-buffered saline
- ANOVA
analysis of variance
- METH
methamphetamine
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbr.2018.04.046.
References
- [1].Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. , HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors, J. Neurovirol 17 (2011) 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. , The HNRC 500: neuropsychology of HIV infection at different disease stages, J. Int. Neuropsychol. Soc 1 (1995) 231–251. [DOI] [PubMed] [Google Scholar]
- [3].Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL, HIV-Associated neuro-cognitive disorder: pathogenesis and therapeutic opportunities, J. Neuroimmune Pharm 5 (2010) 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ,Barta PE, et al. , Reduced basal ganglia volume in HIV-1 associated dementia: results from quantitative neuroimaging, Neurology 43 (1993) 2099–2104. [DOI] [PubMed] [Google Scholar]
- [5].Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, et al. , Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection, Arch. Neurol 53 (1996) 155–158. [DOI] [PubMed] [Google Scholar]
- [6].Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, et al. , Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains, J. Neurovirol 15 (2009) 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hestad K, McArthur JH, Dalpan GJ, Selnes OA, Nancesproson TE, Aylward E, et al. , Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test-performance, Acta Neurol. Scand 88 (1993) 112–118. [DOI] [PubMed] [Google Scholar]
- [8].Purohit V, Rapaka R, Shurtleff D, Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia, Mol. Neurobiol 44 (2011) 102–110. [DOI] [PubMed] [Google Scholar]
- [9].Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, et al. , Methamphetamine exposure combined with HIV-1 disease or gp120 expression: comparison of learning and executive functions in humans and mice, Neuropsychopharmacology 40 (2015) 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim BO, Liu Y, Ruan YW, Xu ZC, Schantz L, He JJ, Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline, Am. J. Pathol 162 (2003) 1693–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM, In vivo microdialysis in awake, freely moving rats demonstrates HIV-1 tat-induced alterations in dopamine transmission, Synapse 63 (2009) 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kesby JP, Markou A, Semenova S, Effects of HIV/TAT protein expression and chronic selegiline treatment on spatial memory, reversal learning and neuro-transmitter levels in mice, Behav. Brain Res 311 (2016) 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kesby JP, Markou A, Semenova S, The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice, Neuropharmacology 109 (2016) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kesby JP, Najera JA, Romoli B, Fang Y, Basova L, Birmingham A, et al. , HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function, Brain Behav. Immun (2017) 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Midde NM, Gomez AM, Zhu J, HIV-1 tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes, J. Neuroimmune Pharm 7 (2012) 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Theodore S, Cass WA, Dwoskin LP, Maragos WF, HIV-1 protein Tat inhibits vesicular monoamine transporter-2 activity in rat striatum, Synapse 66 (2012) 755–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu J, Mactutus CF, Wallace DR, Booze RM, HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes, J. Pharmacol. Exp. Ther 329 (2009) 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Harricharan R, Thaver V, Russell VA, Daniels WM, Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats, Behav. Brain Functions BBF 11 (2015) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marks WD, Paris JJ, Schier CJ, Denton MD, Fitting S, McQuiston AR, et al. , HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron sub-populations, J. Neurovirol 22 (2016) 747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Paris JJ, Singh HD, Carey AN, McLaughlin JP, Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice, Behav. Brain Res 291 (2015) 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mark KA, Soghomonian JJ, Yamamoto BK, High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity, J. Neurosci 24 (2004) 11449–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kesby JP, Chang A, Markou A, Semenova S, Modeling human methamphetamine use patterns in mice: chronic and binge methamphetamine exposure, reward function and neurochemistry, Addict. Biol 23 (2018) 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD, Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006, AIDS Care 21 (2009) 575–582. [DOI] [PubMed] [Google Scholar]
- [24].Gupta S, Bousman CA, Chana G, Cherner M, Heaton RK, Deutsch R, et al. , Dopamine receptor D3 genetic polymorphism (rs6280TC) is associated with rates of cognitive impairment in methamphetamine-dependent men with HIV: preliminary findings, J. Neurovirol 17 (2011) 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. , Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons, J. Int. Neuropsychol. Soc 10 (2004) 1–14. [DOI] [PubMed] [Google Scholar]
- [26].Chang L, Ernst T, Speck O, Grob CS, Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities, Am. J. Psychiatry 162 (2005) 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, et al. , Cognitive deficits and degeneration of interneurons in HIV++ methamphetamine users, Neurology 67 (2006) 1486–1489. [DOI] [PubMed] [Google Scholar]
- [28].Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, et al. , Patterns of selective neuronal damage in methamphetamine-user AIDS patients, J. Acquir Immune Defic. Syndr 34 (2003) 467–474. [DOI] [PubMed] [Google Scholar]
- [29].Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH,Wise LE, et al. , Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice, Biol Psychiatry 73 (2013) 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, et al. , Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice, Cereb. cortex (New York, NY : 1991) 20 (2010) 1955–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Soontornniyomkij V, Kesby JP, Soontornniyomkij B, Kim JJ, Kisseleva T, Achim CL, et al. , Age and high-fat diet effects on glutamine synthetase immunoreactivity in liver and hippocampus and recognition memory in mice, Curr. Aging Sci 9 (2016) 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Costa A, Nappi RE, Polatti F, Poma A, Grossman AB, Nappi G, Stimulating effect of HIV-1 coat protein gp120 on corticotropin-releasing hormone and arginine vasopressin in the rat hypothalamus: involvement of nitric oxide, Exp. Neurol 166 (2000) 376–384. [DOI] [PubMed] [Google Scholar]
- [33].Zuloaga DG, Jacobskind JS, Raber J, Methamphetamine and the hypothalamic-pituitary-adrenal axis, Front. Neurosci 9 (2015) 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stolyarova A, O’Dell SJ, Marshall JF, Izquierdo A, Positive and negative feedback learning and associated dopamine and serotonin transporter binding after methamphetamine, Behav. Brain Res 271 (2014) 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anderson BA, Kronemer SI, Rilee JJ, Sacktor N, Marvel CL, Reward, attention, and HIV-related risk in HIV++ individuals, Neurobiol. Dis 92 (2016) 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH, Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens, Plos One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cools R, D’Esposito M, Inverted-U-shaped dopamine actions on human working memory and cognitive control, Biol. Psychiatry 69 (2011) e113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Izquierdo A, Jentsch JD, Reversal learning as a measure of impulsive and compulsive behavior in addictions, Psychopharmacology 219 (2012) 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ragozzino ME, The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility, Ann. N. Y. Acad. Sci 1121 (2007) 355–375. [DOI] [PubMed] [Google Scholar]
- [40].van Schouwenburg M, Aarts E, Cools R, Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia, Curr. Pharm. Des 16 (2010) 2026–2032. [DOI] [PubMed] [Google Scholar]
- [41].Villalba K, Devieux JG, Rosenberg R, Cadet JL, Serotonin-Related Gene polymorphisms and asymptomatic neurocognitive impairment in HIV-Infected alcohol abusers, Genet. Res. Int 2016 (2016) 7169172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Drewes JL, Meulendyke KA, Liao Z, Witwer KW, Gama L, Ubaida-Mohien C, et al. , Quinolinic acid/tryptophan ratios predict neurological disease in SIV-infected macaques and remain elevated in the brain under cART, J. Neurovirol 21 (2015) 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.