Abstract
Lysine acetyltransferases (KATs) play a critical role in the regulation of transcription and other genomic functions. However, a persistent challenge is the development of assays capable of defining KAT activity directly in living cells. Towards this goal, here we report the application of a previously reported dCas9-p300 fusion as a transcriptional reporter of KAT activity. First we benchmark the activity of dCas9-p300 relative to other dCas9-based transcriptional activators, and demonstrate its compatibility with second generation short guide RNA architectures. Next, we repurpose this technology to rapidly identify small molecule inhibitors of acetylation-dependent gene expression. These studies validate a recently reported p300 inhibitor chemotype, and reveal a role for p300’s bromodomain in dCas9-p300-mediated transcriptional activation. Comparison with other CRISPR-Cas9 transcriptional activators highlights the inherent ligand tuneable nature of dCas9-p300 fusions, suggesting new opportunities for orthogonal gene expression control. Overall, our studies highlight dCas9-p300 as a powerful tool for studying gene expression mechanisms in which acetylation plays a causal role, and provide a foundation for future applications requiring spatiotemporal control over acetylation at specific genomic loci.
Introduction
Lysine acetyltransferases (KATs) catalyze protein acetylation, a reversible posttranslational modification (PTM) that plays a critical role in many processes, including gene expression.1 Two of the most well-studied KATs are EP300 and its homolog CREBBP (commonly referred to jointly as p300/CBP). These two KATs possess a versatile substrate scope which includes histones, transcription factors, and members of the transcriptional regulatory apparatus itself.2 Accordingly, disruption of p300/CBP is associated with substantial changes in gene expression, and has been linked to several diseases.3–4 Besides its KAT domain, p300 and CBP additionally contain several non-catalytic modules including zinc fingers, acetylysine readers (bromodomain, BRD), methyllysine readers (PHD domain), and protein-protein interaction domains.2 Thus, a significant challenge in the study of p300/CBP lies in defining the specific role of the KAT domain in gene expression, as well as its targetable role in disease.
Considering methods to study cellular KAT activity, we were inspired by a recent report by Gersbach et al. which found that p300 could be delivered to specific genomic loci using the genomic-targeting methodology CRISPR-Cas9.5 Specifically, this study engineered a catalytically inactive variant of S. pyogenes Cas9 (dCas9) fused to truncated p300 module containing the BRD and KAT domains (dCas9-p300) (Figure 1). Expression of this fusion in combination with chimeric short guide RNAs (sgRNAs) targeted to proximal promoter and distal enhancer regions led to induction of several model genes. Critically, control studies found inducible gene expression to be absolutely dependent on p300 KAT activity, as it was abolished by KAT-inactivating mutations.5 In addition to expanding the CRISPR gene activation toolbox, a broader implication of this study was that KAT activity can play a causative role in activation of gene expression, an often accepted premise for which limited unambiguous evidence exists.6–7 Moreover, these studies suggested dCas9-p300 may represent a valuable tool for studying this phenomenon in cells.
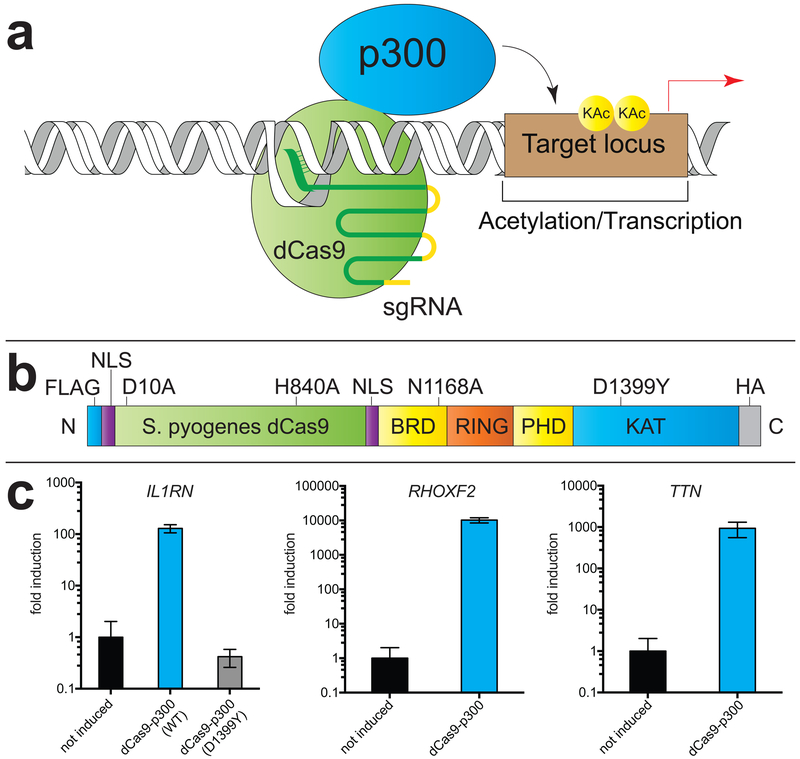
Figure 1.
A CRISPR-Cas9 transcriptional reporter of KAT activity. (a) Scheme for induction of acetylation/gene expression by dCas9-p300, first reported by Gersbach et al. (b) Domain architecture of dCas9-p300 fusion. (c) Activation of model inducible genes IL1RN, RHOXF2, and TTN by dCas9-p300 in HEK-293 cells. D1399Y refers to a dCas9-p300 construct harboring a mutation to the KAT catalytic domain.
Here we describe the application of dCas9-p300 as a cellular reporter of KAT-dependent transcription. Building on previous studies, we first recapitulate dCas9-p300-mediated transcriptional activation, and benchmark its activity relative to non-KAT dCas9 gene expression platforms. Next, we demonstrate p300’s transactivation potential can be targeted to a model gene (IL1RN) in a dCas9-dependent manner using diverse sgRNA and fusion protein architectures. Finally, we apply this platform to analyze small molecules for disruption of KAT-dependent gene expression, highlighting the unique ligandability of dCas9-p300 relative to other transcriptional activators. Overall, our studies showcase the utility of dCas9-p300 as a tool for studying KAT-dependent transcription, and provide a foundation for its use in screening and epigenome editing applications.
Results and Discussion
To apply dCas9-p300 as a transcriptional reporter of KAT activity, we first assessed its utility in cellular experiments. Consistent with previous reports,5 transient overexpression of dCas9-p300 in combination with sgRNAs tiling the IL1RN promoter strongly activated expression of this model gene in HEK-293T cells without modifying bulk acetylation (Figures 1, S1). Induced transcription of IL1RN was time-dependent, and continued to increase up to 72 h post-transfection (Figure S1). We found several other genes (RHOXF2, TTN) could also be induced by dCas9-p300, albeit to varying extents (Figure 1c). No induction of IL1RN was observed when an identical experiment was performed using dCas9-p300 D1399Y, a construct in which a key aspartate involved in the binding of acetyl-CoA to the p300 catalytic domain is mutated (Figure 1c). Of note, analysis of genomics data reveals that D1399Y is the most commonly observed missense mutations in p300 in human cancer, indicative of its ability to disrupt KAT-dependent p300 tumor suppression.8 This demonstrates the ability of dCas9-p300 induced transcription of IL1RN to be applied as a cellular reporter of KAT activity.
Next we sought to benchmark dCas9-p300’s transcriptional activation relative to other recently reported dCas9-based methods.9–12 The goal of these studies was two-fold: (i) to directly compare genomic recruitment of enzyme catalysis (dCas9-p300) versus recruitment of protein-protein interaction motif (dCas9-VP64) as transactivation mechanisms, and (ii) to obtain insights into the ability of dCas9-p300 to utilize alternative sgRNA architectures, an area that has not been previously explored. To accomplish this, we compared the ability of dCas9-p300 to activate IL1RN relative to components of the “Synergistic Activation Mediator” (SAM) platform, a method that has been powerfully applied in genome-wide screens.10 SAM-mediated gene expression is based on co-targeting of dCas9-VP64 and two MS2-HSF-p65 fusion proteins to genomic loci via an MS2 aptamer-containing short guide RNA (referred to as a sgRNA 2.0; Figure 2/S2). To compare these two approaches, we transiently transfected HEK-293 cells with components of the SAM platform or dCas9-p300 using sgRNA 2.0’s targeting the IL1RN promoter. The strength of IL1RN induction followed the order dCas9-p300 ≥ SAM >> dCas9-VP64 (Figure S2). This rank order is consistent with previous studies of dCas9-p300 at the highly inducible IL1RN locus.5 However, it is important to note that these comparisons may be dependent on genomic context, and a recent study found SAM to induce higher levels of gene expression at the ASCL1 and NEUROD1 promoters.13 Interestingly, we did not observe a large effect upon replacement of dCas9-VP64 with dCas9 using the SAM platform, suggesting recruitment of MS2-HSF-p65 is sufficient to induce transcription when targeted to the IL1RN promoter. Importantly, dCas9-p300 promoted strong expression in these experiments, indicating its compatibility with second generation short guide RNA architectures.
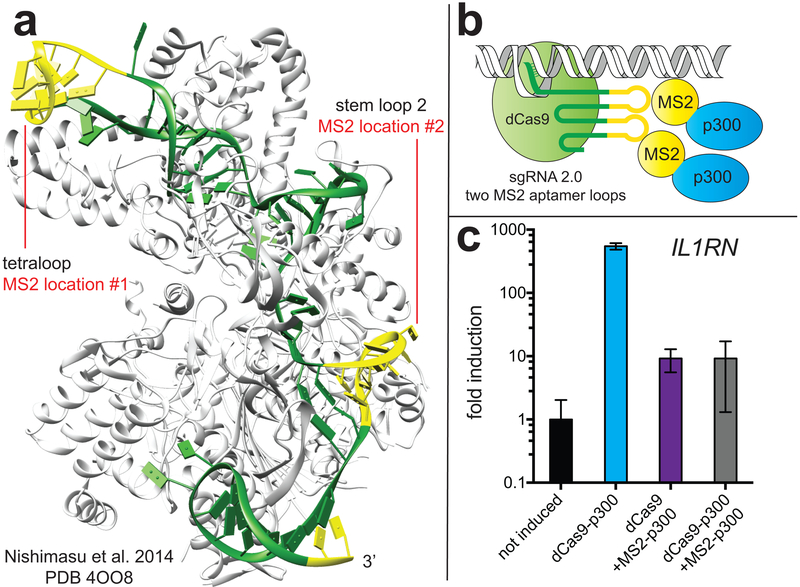
Figure 2.
KAT-dependent gene expression can be stimulated using diverse genomic recruitment strategies. (a) Crystal structure of Cas9-sgRNA complex (PDB 4OO8) illustrating sites for short guide RNA engineering. (b) Cartoon schematic of dCas9-dependent recruitment of MS2-p300 fusions using sgRNA 2.0. (c) Comparison of KAT-dependent expression of IL1RN using dCas9-p300 and MS2-p300 with sgRNA 2.0 architectures.
Motivated by this finding, we next explored the ability of dCas9-sgRNA 2.0 complexes to mediate multivalent recruitment of p300 KATs to the IL1RN locus (Figure 2). In contrast to dCas9, which binds to genomic loci as a monomer, MS2 coat proteins bind their cognate aptamer as dimers,14 allowing a single sgRNA 2.0 to potentially recruit four copies of p300 catalytic activity. Thus, we designed a construct in which a CMV promoter drives constitutive expression of an MS2 coat protein fused at its C-terminus to the p300 core (Figure 2b). The p300 core used in these studies consists of the BRD, RING, and KAT domains, and is identical to that found in the dCas9-p300 construct. Employing this construct in combination with dCas9 and sgRNA 2.0s targeting the IL1RN promoter resulted in modest induction of gene expression after 72 hrs (Figure 2c). The less efficient transactivation of gene expression by MS2-p300 suggests it may be less efficiently recruited (potentially due to acetylation of the MS2 RNA-binding domain), less active, or improperly oriented at the IL1RN promoter relative to dCas9-p300. Considering the latter hypothesis, recent studies employing a dCas9-Suntag construct to recruit the methylcytidine dioxygenase TET1 found the linker length between the GCN4 repeats on the dCas9-Suntag module to be essential for efficient recruitment of TET1 catalysis at specific genomic loci,15 suggesting a rationale for inefficient transactivation by MS2-p300. Indeed, in contrast to the SAM system, we found that coincident expression and recruitment of dCas9-p300 and MS2-p300 fusions squelched the induction of gene expression promoted by either component alone, consistent with counterproductive effects (Figure 2c). These studies provide insights into the ability of alternative sgRNA and fusion protein constructs to target KAT activity to specific genomic loci.
Next, we sought to apply dCas9-p300 as a tool to define the effect of small molecules on KAT-dependent transcription (Figure 3). We envisioned such an application of dCas9-p300 could fulfill two purposes. First, molecules directly inhibiting p300 KAT domain would be expected to decrease IL1RN induction, providing a cellular readout of p300 activity and rapid assessment of p300 target occupancy, which has been difficult to assess using other approaches.16–17 Second, this approach should be amenable to the identification of molecules interacting with the transcriptional regulatory apparatus downstream of dCas9-p300, and thus have the potential to illuminate biological processes specifically necessary for KAT-dependent transcription. To explore this strategy, we screened a panel of small molecules for modulation of dCas9-p300 induced gene expression (Figure 3). Included in this initial panel were molecules interacting directly with acetylation-dependent signaling such as KAT, bromodomain, and histone deacetylase (HDAC) inhibitors, as well as ligands we hypothesized may target downstream biological processes necessary for dCas9-p300 transactivation, such as RNA Polymerase II (RNA Pol II) C-terminal domain phosphorylation and Polycomb Repressive Complex methyltransferase activity.18–21 To maximize dynamic range, treatments were carried out 48–72 h post-transfection, over which time IL1RN-induced gene expression increased ~10-fold (Figure S1/S3). Using this approach, we observed the majority of molecules tested had only modest effects on dCas9-p300 induction of IL1RN. This may stem partly from the choice of concentrations used in our single point screen, as we purposely assessed inhibition of dCas9-p300 at concentrations determined to be non-toxic in HEK-293T in order to minimize off-target effects (Figure S3b). It is important to note that both the unicate nature, as well as the concentrations chosen for this pilot study, may lead to false negatives in this assay format. Three molecules in the panel demonstrated the strongest inhibitory effects: NCGC00496795 (“Patent KATi”), (+)-JQ1 (JQ1), and SGC-CBP30 (CBP30).19–20, 22 NCGC00496795 (“Patent KATi”) is a racemic member of a family of spirocyclic p300 inhibitors that were recently reported in the patent literature (Figure 3b, S4a).22 Follow-up studies validated its ability to inhibit both p300 biochemical activity (Figure S4b) as well as dCas9-p300 driven gene expression in a dose-dependent fashion (Figure 3c). This molecule did not inhibit SAM-dependent gene activation, also consistent with specific dCas9-p300 inhibition (Figure S4c). Although we defer a full characterization of this compound class to future publications,23 the potent inhibition of dCas9-p300-induced IL1RN expression are consistent with the reported ability of this chemotype to target KAT domains, and validate our assay’s ability to identify p300 inhibitors.
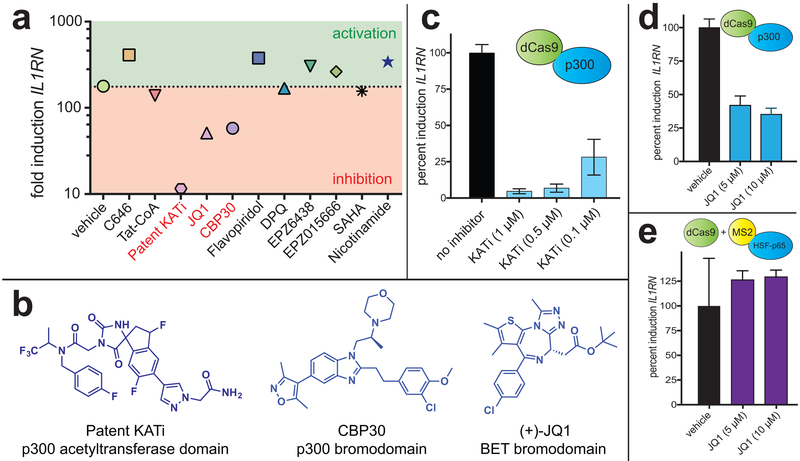
Figure 3.
Influence of small molecule stimuli on CRISPR-Cas9 acetyltransferase. (a) Single point screening data. Single point concentrations are provided in the Supporting Information. Patent KATi = NCGC00496795. (b) Structures of bromodomain-targeting small molecules identified as inhibitors of dCas9-p300-dependent gene expression. (c) Dose-dependent inhibition of KAT-dependent gene expression by NCGC00496795. (d) Dose-dependent inhibition of KAT-dependent gene expression by JQ1. (e) JQ1 alters dCas9-p300-dependent gene expression but not SAM-dependent gene expression.
Notably, the other two molecules identified as dCas9-p300 inhibitors target bromodomains, small protein motifs that specifically bind acetylated lysine residues (Figure 3b).24 JQ1 is a chemical probe of the BET bromodomain family, which includes BRD2, BRD3, BRD4, and BRDT.19 Acetylation-dependent recruitment of BRD4 to genes is associated with RNA Pol II phosphorylation and transcript elongation, common features of activated gene expression. To build on the results of our pilot screen, we first confirmed dose-dependent inhibition of dCas9-p300-mediated gene expression by JQ1 (Figure 3d). We observed JQ1 concentrations as low as 5 μM were sufficient to inhibit IL1RN expression (~30%). Controls verified this was not due to inhibition of dCas9-p300 expression, which we found to be slightly upregulated by JQ1 treatment (Figure S5a). Next, we performed experiments to assess whether BET bromodomain binding constitutes a specific or general requirement for dCas9-mediated transactivation. To accomplish this, we compared JQ1’s ability to inhibit IL1RN gene expression driven by either i) dCas9-p300 or ii) components of the SAM system (dCas9/MS2-HSF-p65). Interestingly, while JQ1 antagonized dCas9-p300, it had no effect on SAM-driven IL1RN expression (Figure 3e). As a control we found that gene activation by both dCas9-p300 and SAM was inhibited by flavopiridol, a general inhibitor of transcriptional elongation (Figure S5d). The distinct effects of JQ1 are consistent with the view that transcriptional activation programs may exhibit unique cofactor dependencies.25–26 These studies indicate dCas9-p300 is uniquely dependent on acetylation-dependent protein-protein interactions relative to other dCas9-based transcriptional activators.
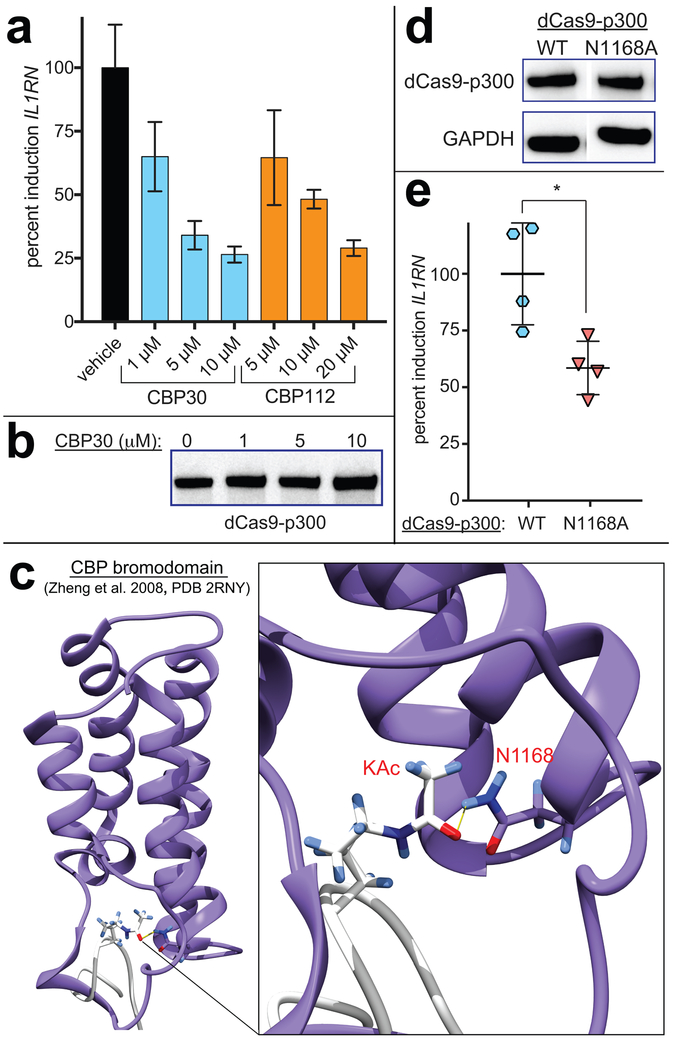
Next we turned our attention to understanding the effects of CBP30, a recently reported selective probe of the p300/CBP bromodomain.20 CBP30 binds the p300/CBP bromodomain with ~40-fold selectivity relative to BET family members and has demonstrated phenotypic effects on p53 and IRF4-dependent transcription in cells.20, 27 Although it also targets an acetylation-dependent protein-protein interaction, CBP30 is unique relative to JQ1 in that it has the potential to directly interact with the BRD-containing dCas9-p300 construct. Again, we initially validated the effects of CBP30 on dCas9-p300 driven gene expression. CBP30 demonstrated dose-dependent inhibition of Il1RN transcription at concentrations similar to JQ1 (Figure 4a). Western blot analysis indicated this inhibition was not due to changes in ectopic expression of dCas9-p300 (Figure 4b). The structurally distinct p300/CBP bromodomain inhibitor SGC-CBP112 (CBP112) exhibited similar inhibition (Figure 4a).28 One caveat to these findings was the use of CBP30 and CBP112 at higher concentrations than have been required for cellular activity in previous studies.20, 27–28 This may reflect the fact that the target of these molecules (p300 BRD) is being constitutively overexpressed, limiting their potency, or alternatively indicate engagement of an off-target. Therefore, to more conclusively examine the role of the BRD in dCas9-p300-mediated gene expression we prepared a construct harboring a bromodomain mutation (CBP, N1168A; p300 N1132A) known to abrogate acetyllysine binding (Figure 4c).29 Consistent with the effects of CBP30, the dCas9-p300 bromodomain mutant showed diminished transactivation potential (Figure 4d-e). Also consistent with an on-target effect, the residual activity of the dCas9-p300 bromodomain mutant was not inhibited by CBP30 (Figure S6). Of note, previous studies have suggested the bromodomain of p300 may alter the in cis catalytic activity of the acetyltransferase domain,30–31 providing a rationale for the observed effects. However, it is important to note that our studies do not definitively rule other contributions of the p300 bromodomain to dCas9-p300 mediated transcription, including effects on processive acetylation or genome-wide localization.32 These studies establish a role for the bromodomain in fine-tuning KAT-dependent transcriptional activation at the IL1RN locus.
Figure 4.
Impact of p300 bromodomain on KAT-dependent gene expression. (a) Dose-dependent inhibition of KAT-dependent gene expression by CBP30 and CBP112. (b) CBP30 does not affect dCas9-p300 overexpression. (c) Crystal structure of CBP bromodomain (PDB 2RNY) highlighting role of N1168 residue (N1132 in full length p300, N1516 in dCas9-p300) in acetyllysine recognition. (d) dCas9-p300 bromodomain mutant (N1168A) mutant is expressed at similar levels to wild-type dCas9-p300. Uncropped full blot data is provided in Supporting Information. (e) Mutations known to disrupt the p300 bromodomain-acetyllysine interaction reduce transcriptional activation by dCas9-p300. Significance analyzed by two-tailed Student’s t test (* = P < 0.05).
In summary, we have described the application of a CRISPR-Cas9 acetyltransferase as a cellular reporter of KAT-dependent transcription. Our studies further establish the scope of this technology and its compatibility with diverse sgRNA architectures. In addition, we apply dCas9-p300 to understand how small molecule stimuli affect acetylation-dependent gene expression programs, providing one of the first examples in which a dCas9-effector fusion has been applied to study the effector protein itself. Performing a model screen, we found that dCas9-p300 capably identified ligands interacting directly with p300 (NCGC00496795, CBP30), allowing us to study the role of the bromodomain in p300-dependent transcription, and also providing one of the first independent validations of the potency and cellular activity of a recently reported spirocyclic p300 inhibitor chemotype. In addition, our small molecule studies also revealed that dCas9-p300-dependent transcription is susceptible to inhibition by ligands targeting downstream acetylation-dependent protein-protein interactions (JQ1).19–20, 23 This latter finding is both intuitive and striking, as it implies dCas9-p300 establishes an acetylation-dependent “two-hybrid-like” interaction at specific regulatory elements between endogenous cellular components (histones/bromodomains). Based on these results, we anticipate dCas9-p300 should be immediately useful for KAT and BRD inhibitor validation, and potentially amenable to high-throughput discovery applications upon optimization of assay stability and reporter readout.33 In addition to identifying new inhibitors, such studies may also provide new insights into KAT-dependent transcription. For example, our results indicate the p300 bromodomain is required for maximal KAT-dependent gene activation at the IL1RN locus. Further mutational analysis of dCas9-p300 may aid in the identification of novel determinants of cellular p300 activity. Finally, our studies have implications for small molecule control of dCas9 gene activation. Specifically, we find that dCas9-p300 is inherently ligandable, and also displays distinct dependencies on downstream cofactors (i.e. BRD4) at the IL1RN locus, some of which are not shared by the SAM system. In the future this knowledge could facilitate the development of chemical genetic methods in which tailored inhibitors are used to orthogonally control multiple gene expression programs,34–35 and provide an additional strategy for chemical control of dCas9 function.36–39 Overall, our studies highlight dCas9-p300 as a powerful tool for studying gene expression mechanisms in which acetylation plays a causal role, and provide a foundation for its future applications in inhibitor validation and biological discovery.
Supplementary Material
Acknowledgements
The authors thank S. Kales (NCATS) and D. Esponsito (Protein Expression Laboratory, Leidos Biomedical Research) for helpful discussions. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (ZIA BC011488–04) and National Center for Advancing Translational Sciences, National Institutes of Health. In addition, this project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number HHSN261200800001E.
Footnotes
Supporting Information
Figures S1-S6, Supplementary Tables S1-S4, and supporting materials and methods. This material is available free of charge via the Internet at http://pubs.acs.org.
References:
- 1.Verdin E; Ott M, 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol 2015, 16, 258–264. [DOI] [PubMed] [Google Scholar]
- 2.Dancy BM; Cole PA, Protein lysine acetylation by p300/CBP. Chem. Rev 2015, 115, 2419–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roelfsema JH; Peters DJ, Rubinstein-Taybi syndrome: clinical and molecular overview. Expert Rev. Mol. Med 2007, 9, 1–16. [DOI] [PubMed] [Google Scholar]
- 4.Farria A; Li W; Dent SY, KATs in cancer: functions and therapies. Oncogene 2015, 34, 4901–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton IB; D’Ippolito AM; Vockley CM; Thakore PI; Crawford GE; Reddy TE; Gersbach CA, Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol 2015, 33, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedford DC; Brindle PK, Is histone acetylation the most important physiological function for CBP and p300? Aging (Albany NY) 2012, 4, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rando OJ, Combinatorial complexity in chromatin structure and function: revisiting the histone code. Curr. Opin. Genet. Dev 2012, 22, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C; Yuan YA, Structural insights into histone H3 lysine 56 acetylation by Rtt109. Structure 2008, 16, 1503–1510. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Pinera P; Kocak DD; Vockley CM; Adler AF; Kabadi AM; Polstein LR; Thakore PI; Glass KA; Ousterout DG; Leong KW; Guilak F; Crawford GE; Reddy TE; Gersbach CA, RNA-guided gene activation by CRISPR-Cas9-based transcription factors. Nat. Methods 2013, 10, 973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konermann S; Brigham MD; Trevino AE; Joung J; Abudayyeh OO; Barcena C; Hsu PD; Habib N; Gootenberg JS; Nishimasu H; Nureki O; Zhang F, Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 2015, 517, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeder ML; Linder SJ; Cascio VM; Fu Y; Ho QH; Joung JK, CRISPR RNA-guided activation of endogenous human genes. Nat. Methods 2013, 10, 977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert LA; Larson MH; Morsut L; Liu Z; Brar GA; Torres SE; Stern-Ginossar N; Brandman O; Whitehead EH; Doudna JA; Lim WA; Weissman JS; Qi LS, CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez A; Tuttle M; Pruitt BW; Ewen-Campen B; Chari R; Ter-Ovanesyan D; Haque SJ; Cecchi RJ; Kowal EJK; Buchthal J; Housden BE; Perrimon N; Collins JJ; Church G, Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valegard K; Murray JB; Stockley PG; Stonehouse NJ; Liljas L, Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature 1994, 371, 623–626. [DOI] [PubMed] [Google Scholar]
- 15.Morita S; Noguchi H; Horii T; Nakabayashi K; Kimura M; Okamura K; Sakai A; Nakashima H; Hata K; Nakashima K; Hatada I, Targeted DNA demethylation in vivo using dCas9-peptide repeat and scFv-TET1 catalytic domain fusions. Nat. Biotechnol 2016, 34, 1060–1065. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery DC; Sorum AW; Meier JL, Chemoproteomic profiling of lysine acetyltransferases highlights an expanded landscape of catalytic acetylation. J. Am. Chem. Soc 2014, 136, 8669–8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery DC; Garlick JM; Kulkarni RA; Kennedy S; Allali-Hassani A; Kuo YM; Andrews AJ; Wu H; Vedadi M; Meier JL, Global Profiling of Acetyltransferase Feedback Regulation. J. Am. Chem. Soc 2016, 138, 6388–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowers EM; Yan G; Mukherjee C; Orry A; Wang L; Holbert MA; Crump NT; Hazzalin CA; Liszczak G; Yuan H; Larocca C; Saldanha SA; Abagyan R; Sun Y; Meyers DJ; Marmorstein R; Mahadevan LC; Alani RM; Cole PA, Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem. Biol 2010, 17, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippakopoulos P; Qi J; Picaud S; Shen Y; Smith WB; Fedorov O; Morse EM; Keates T; Hickman TT; Felletar I; Philpott M; Munro S; McKeown MR; Wang Y; Christie AL; West N; Cameron MJ; Schwartz B; Heightman TD; La Thangue N; French CA; Wiest O; Kung AL; Knapp S; Bradner JE, Selective inhibition of BET bromodomains. Nature 2010, 468, 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hay DA; Fedorov O; Martin S; Singleton DC; Tallant C; Wells C; Picaud S; Philpott M; Monteiro OP; Rogers CM; Conway SJ; Rooney TP; Tumber A; Yapp C; Filippakopoulos P; Bunnage ME; Muller S; Knapp S; Schofield CJ; Brennan PE, Discovery and optimization of small-molecule ligands for the CBP/p300 bromodomains. J. Am. Chem. Soc 2014, 136, 9308–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arrowsmith CH; Bountra C; Fish PV; Lee K; Schapira M, Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discov 2012, 11, 384–400. [DOI] [PubMed] [Google Scholar]
- 22.Michaelides M; Hansen T; Dai Y; Zhu G; Frey R; Gong J; Penning T; Curtin M; McClellan W; Clark R; Torrent M; Mastracchio A; Kesicki EA; Kluge AF; Patane MA; Van Drie J; Ji Z; Lai CC; Wang C Spirocyclic hat inhibitors and methods for their use. US20160235716 A1, 2015. [Google Scholar]
- 23.Lasko LM; Jakob CG; Edalji RP; Qiu W; Montgomery D; Digiammarino E; Hansen TM; Risl R; Frey R; Manaves V; Shaw B; Algire M; Hessler P; Lam L; Uziel T; Faivre E; Ferguson D; Buchanan F; Martin RL; Torrent M; Chiang GG; Karukurichi K; Langston JW; de Vries P; Van Drie JH; McElligott D; Kesicki E; Marmorstein R; Sun C; Cole PA; Rosenberg SH; Michaelides MR; Lai A; Bromberg KD, Discovery of a Potent Catalytic p300/CBP Inhibitor that Targets Lineage-Specific Tumors. Nature 2017, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippakopoulos P; Picaud S; Mangos M; Keates T; Lambert JP; Barsyte-Lovejoy D; Felletar I; Volkmer R; Muller S; Pawson T; Gingras AC; Arrowsmith CH; Knapp S, Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 2012, 149, 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conaway RC; Sato S; Tomomori-Sato C; Yao T; Conaway JW, The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem. Sci 2005, 30, 250–255. [DOI] [PubMed] [Google Scholar]
- 26.Dugan A; Majmudar CY; Pricer R; Niessen S; Lancia JK; Fung HY; Cravatt BF; Mapp AK, Discovery of Enzymatic Targets of Transcriptional Activators via in Vivo Covalent Chemical Capture. J. Am. Chem. Soc 2016, 138, 12629–12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conery AR; Centore RC; Neiss A; Keller PJ; Joshi S; Spillane KL; Sandy P; Hatton C; Pardo E; Zawadzke L; Bommi-Reddy A; Gascoigne KE; Bryant BM; Mertz JA; Sims RJ, Bromodomain inhibition of the transcriptional coactivators CBP/EP300 as a therapeutic strategy to target the IRF4 network in multiple myeloma. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picaud S; Fedorov O; Thanasopoulou A; Leonards K; Jones K; Meier J; Olzscha H; Monteiro O; Martin S; Philpott M; Tumber A; Filippakopoulos P; Yapp C; Wells C; Che KH; Bannister A; Robson S; Kumar U; Parr N; Lee K; Lugo D; Jeffrey P; Taylor S; Vecellio ML; Bountra C; Brennan PE; O’Mahony A; Velichko S; Muller S; Hay D; Daniels DL; Urh M; La Thangue NB; Kouzarides T; Prinjha R; Schwaller J; Knapp S, Generation of a Selective Small Molecule Inhibitor of the CBP/p300 Bromodomain for Leukemia Therapy. Cancer Res 2015, 75, 5106–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng L; Zhang Q; Gerona-Navarro G; Moshkina N; Zhou MM, Structural basis of site-specific histone recognition by the bromodomains of human coactivators PCAF and CBP/p300. Structure 2008, 16, 643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dancy BM; Crump NT; Peterson DJ; Mukherjee C; Bowers EM; Ahn YH; Yoshida M; Zhang J; Mahadevan LC; Meyers DJ; Boeke JD; Cole PA, Live-cell studies of p300/CBP histone acetyltransferase activity and inhibition. Chembiochem 2012, 13, 2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zucconi BE; Luef B; Xu W; Henry RA; Nodelman IM; Bowman GD; Andrews AJ; Cole PA, Modulation of p300/CBP Acetylation of Nucleosomes by Bromodomain Ligand I-CBP112. Biochemistry 2016, 55, 3727–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringel AE; Cieniewicz AM; Taverna SD; Wolberger C, Nucleosome competition reveals processive acetylation by the SAGA HAT module. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E5461–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klann TS; Black JB; Chellappan M; Safi A; Song L; Hilton IB; Crawford GE; Reddy TE; Gersbach CA, CRISPR-Cas9 epigenome editing enables high-throughput screening for functional regulatory elements in the human genome. Nat. Biotechnol 2017, 35, 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalatan JG; Lee ME; Almeida R; Gilbert LA; Whitehead EH; La Russa M; Tsai JC; Weissman JS; Dueber JE; Qi LS; Lim WA, Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell 2015, 160, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y; Xiong X; Wong S; Charles EJ; Lim WA; Qi LS, Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat. Methods 2016, 13, 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maji B; Moore CL; Zetsche B; Volz SE; Zhang F; Shoulders MD; Choudhary A, Multidimensional chemical control of CRISPR-Cas9. Nat. Chem. Biol 2017, 13, 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y; Zhan Y; Chen Z; He A; Li J; Wu H; Liu L; Zhuang C; Lin J; Guo X; Zhang Q; Huang W; Cai Z, Directing cellular information flow via CRISPR signal conductors. Nat. Methods 2016, 13, 938–944. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen DP; Miyaoka Y; Gilbert LA; Mayerl SJ; Lee BH; Weissman JS; Conklin BR; Wells JA, Ligand-binding domains of nuclear receptors facilitate tight control of split CRISPR activity. Nat. Commun 2016, 7, 12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zetsche B; Volz SE; Zhang F, A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat. Biotechnol 2015, 33, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.