Abstract
Modulation of pain may result from engagement of opioid receptors in multiple brain regions. Whether sensory and affective qualities of pain are differentially affected by brain opioid receptor circuits remains unclear. We previously reported that opioid actions within the rostral anterior cingulate cortex (rACC) produces selective modulation of affective qualities of neuropathic pain in rodents but whether such effects may occur in other areas of the anterior cingulate cortex (ACC) is not known. Here, morphine was microinjected in three regions of the ACC or in the rostral ventromedial medulla (RVM) and pain behaviors in naïve, sham or spinal nerve ligated (SNL) rats were evaluated. In naïve animals, the tail-flick response was inhibited by RVM, but not ACC, morphine. ACC morphine did not affect tactile allodynia (von Frey test) or mechanical (Randall-Sellito) or thermal (Hargreaves) hyperalgesia in SNL rats. In contrary, RVM morphine reduced tactile allodynia and produced both antihyperalgesic and analgesic effects against mechanical and thermal stimuli as well as conditioned place preference selectively in nerve injured rats. Within the RVM, opioids inhibit nociceptive transmission reflected in both withdrawal thresholds and affective pain behaviors. Activation of mu opioid receptors within specific rACC circuits, however, selectively modulates affective dimensions of ongoing pain without altering withdrawal behaviors. These data suggest that RVM and ACC opioid circuits differentially modulate sensory and affective qualities of pain, allowing optimal behaviors that promote escape and survival. Targeting specific ACC opioid circuits may allow for treatment of chronic pain while preserving the physiological function of acute pain.
Introduction
One of the major challenges in the development of therapies for the unmet medical need presented by chronic pain is the effective relief of ongoing pain while preserving the vital protective function of acute pain. Correlational imaging analyses suggest that different brain regions contribute to the perception, or modulation of sensory and affective aspects of pain [41]. While these dimensions of pain are integrated to form the human pain experience, psychophysical and neuroimaging observations suggest that sensory and affective aspects may be partially separable and subject to preferential modulation by medications acting in specific circuits. In this regard, clinically used opioids are known to predominantly influence affective dimensions of pain while largely leaving responses to noxious stimuli unchanged [6; 35].
Substantial evidence from human and animal studies implicates the anterior cingulate cortex (ACC) in processing of affective aspects of pain. For example, following hypnotic suggestions [40] or during positive emotional states [51], the engagement of ACC circuits has been shown to be associated with modulation of the affective component without change in pain intensity. PET studies using opioid radiotracers found increased release of endogenous opioids in this region during acute experimental pain in healthy subjects [48; 50], and during a migraine attack in migraneurs [9], supporting the role of endogenous opioid neurotransmission in modulation of pain affect. Notably, reduced MOR availability in the ACC was found in patients with fibromyalgia [47] and post-stroke pain [56] suggesting deficiencies in pain modulatory mechanisms. We have previously reported in rats, that release of endogenous opioids in the rostral ACC (rACC) is necessary for relief of the aversiveness of ongoing pain [34]. Thus, blockade of rACC MORs in rats with spinal nerve ligation (SNL) prevented conditioned place preference (CPP) to different non-opioid pain-relieving treatments. Additionally, direct microinjection of morphine in the rACC promoted CPP in injured rats but had no effect on tactile withdrawal responses.
The periaqueductal gray area (PAG) and the rostral ventromedial medulla (RVM) are structures that contribute to an opioid-sensitive descending bidirectional modulation of nociceptive signaling [37]. Electrophysiological studies in rodents have identified pain OFF and ON cells in the RVM, that respectively inhibit and facilitate nociception [14; 19] and likely modulate the resulting pain experience based on factors including, for example, context, past memories and others allowing for determination of optimal behavioral choices. Functional connectivity between the ACC and brainstem regions comprising the descending pain pathways have been observed in a number of pain neuroimaging studies in humans [5] but whether and how opioid activity in this cortical region participates in descending modulation is not well understood. While neuroimaging studies reveal the network of brain regions that may be associated with modulation of pain, investigations of causal opioid effects in a single brain area are not readily achievable in humans. The goal of the present study was to determine whether microinjections of morphine in the RVM, or in three separate subdivisions of the ACC, may selectively modulate affective and sensory aspects of acute and ongoing neuropathic pain in rats.
Materials and methods
2.1. Animals
Male, Sprague-Dawley rats, 250–300g were obtained from Harlan Laboratories, Indianapolis, IN. Animals were group housed on a 12 h/12 h light/dark cycle. Food and water were available ad libitum. All described procedures received approval from the Institutional Animal Care and Use Committee (IACUC) of the University of Arizona. Animals were monitored throughout the duration of the study to reduce unnecessary stress and/or pain and the number of animals was used in accordance with the International Association for the Study of Pain ethical guidelines. Investigators for all behavioral experiments were blinded to the treatment groups.
2.2. Surgery
2.2.1. Spinal nerve ligation surgery
Spinal nerve ligation (SNL) surgery was performed as described previously [24]. Rats were maintained under 2% v/v isoflurane anesthesia delivered in a 3:2 ratio of nitrous oxide and oxygen. A paraspinal incision was made and the left tail muscle excised. Part of the L5 transverse process was removed to expose the L5 and L6 spinal nerves, which were then isolated and ligated with a non-absorbable 6–0 braided silk thread proximal to the formation of the sciatic nerve. The surrounding skin and muscle were closed with absorbable 3–0 sutures. Sham surgery was performed in an identical manner omitting the ligation step. All rats were monitored for normal behaviors (grooming and mobility) and for general health and weight gain post-surgery.
2.2.2. Intracranial ACC and RVM cannulation
Stereotaxic cannulation surgeries were performed in animals anesthetized with a ketamine/xylazine combination (80/12 mg/kg, i.p.; Western Medical Supply/Sigma). Bilateral cannulation of the ACC was performed as previously described [21; 34]. A pair of 26-guage stainless steel guide cannulas cut 4 mm below the pedestal (Plastics One Inc., Roanoke, VA) were directed toward the following ACC injection sites: ACC site 1 (anteroposterior (AP): +4.0 mm from bregma; medial-lateral (ML) ± 0.6 mm; dorsoventral (DV): −2.0 mm from skull), ACC site 2 (AP: +2.6 mm from bregma; ML: ± 0.6 mm; DV: −1.8 mm from skull) or ACC site 3 (AP: +0.2 mm from bregma; ML: ± 0.6 mm; DV: −1.8 mm from skull) [38]. Stainless steel guide cannulas (26-guage) cut 11 mm below the pedestal were directed toward the RVM injection site (AP: −2.1 mm from interaural line; ML ± 0.6 mm; DV: −8.5 mm from dura) [38]. Guide cannulas were cemented in place and secured to the skull by small stainless-steel machine screws. Stainless steel dummy cannulas were inserted into each guide to keep the guide free of debris. Rats then received a subcutaneous gentamycin (1 mg/ml) injection, were housed individually, and were allowed to recover for 7–10 days. Animals were monitored and assessed daily for overall health.
2.3. Behavioral testing
2.3.1. Von Frey testing
On the day of the experiment, animals were placed in suspended chambers with wire mesh floors for 30 minutes to habituate prior to testing. A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) in logarithmically spaced increments ranging from 0.41 to 15 g (4–150 N) were applied perpendicular to the plantar surface of the ipsilateral hind paw until the filament buckled. Withdrawal threshold was determined by sequentially increasing and decreasing the stimulus strength (“up and down” method), analyzed using a Dixon nonparametric test, and expressed as the mean withdrawal threshold [11].
2.3.2. Hargreaves assay
Nociceptive paw-withdrawal latencies to noxious radiant heat were performed according to the Hargreaves method [18]. Rats were allowed to acclimate in Plexiglas enclosures with raised glass floors for 2 days at 30 min/day and for 15 minutes prior to testing. After acclimation, a radiant heat source (115V, UGO Basile Biological Research Apparatus Model 7371, Comerio VA, Italy) was positioned under the glass floor directly beneath the hind paw. The radiant heat source was activated with a reaction time counter (timer) and paw-withdrawal latency was determined by a motion-sensitive detector source that halted both the timer and the heat source when the hindpaw was withdrawn. The withdrawal latency to the nearest 0.1 s was automatically recorded. Baseline latencies were established at 17–25 s to allow a sufficient window for the detection of possible hyperalgesia. A maximal cutoff of 33 s was used to prevent tissue damage.
2.3.3. Tail-flick assay
The tail-flick assay was performed to evaluate thermal nociception. Animals were gently held around the trunk, and the distal half of the tail was immersed in a 52°C circulating warm-water bath (Neslab Instruments, Inc., Newington, N.H.). The latency to tail flick was measured in the number of seconds it took for the rat to withdraw its tail. A maximal cut off of 13 s was used to avoid tissue damage.
2.3.4. Randall-Selitto assay
Nociceptive withdrawal thresholds were assessed using the Randall-Selitto algesimeter (UGO Basile Biological Research Apparatus Model 7200, Comerio VA, Italy). On the day of experimentation, rats were allowed to acclimate for 15–20 minutes prior to testing. Following acclimation, animals were placed onto a soft cotton cloth and the hind paw was placed onto a stable base of the device. Controlled, increasing mechanical pressure was applied with a blunt point until a withdrawal response or vocalization resulted. The maximum force applied was 500 g to avoid tissue damage.
2.3.5. Open field assay
The open field test was performed to measure locomotion, grooming, and rearing. Animals were placed into a large, Plexiglas cubic box, measuring 47 cm3. The top of the cube was left uncovered. The bottom of the box was divided into nine even squares. The rat was injected with either saline or morphine into the ACC or RVM and, after 20 min, placed into the center of the cubic box and left to freely explore for five minutes while it’s movement, grooming, and rearing behavior was recorded by a video camera (Logitech C270 HD Webcam) positioned directly above the box.
2.3.6. Conditioned place preference (CPP)
A single trial conditioning protocol was used for CPP as previously described [25; 33]. Rats underwent daily handling by the experimenter before the preconditioning phase. On preconditioning day, rats were placed into the CPP boxes with free access to all chambers. To verify whether a preexisting chamber bias existed, automated software (Photobeam Activity System 2.0.7) was used to determine the time spent in each chamber across 15 minutes. Animals spending more than 80% (720 s) or less than 20% (180 s) of the total time in either chamber were eliminated from further testing (23/112 animals). On conditioning day, rats with ACC or RVM cannulas received a saline injection into the ACC or RVM and were placed into a conditioning chamber for 30 min. Four hours later, rats received morphine into the ACC or RVM, were placed into the opposite chamber for 30 min. On test day, rats were placed into the middle CPP chamber and were allowed to explore all chambers for 15 minutes; the time in chambers was automatically recorded using the Photobeam Activity System 2.0.7 to determine chamber preference. Difference scores were calculated as test time minus preconditioning time spent in the morphine-paired chamber.
2.4. Drug administration:
2.4.1. Intra-brain injections
Unilateral injection cannulae extending 1 mm beyond the end of the guide cannulae were connected to a 2 μL Hamilton syringe and driven by a syringe pump (Stoelting, Quintessential Stereotaxic Injector). Microinjections of morphine sulfate (National Institute of Drug Abuse Drug Supply Program) or vehicle (saline) were administered bilaterally into one of three ACC sites referred to as ACC sites 1, 2, or 3 (see below) at a dose of 20 μg/0.5 μL/side or into the RVM at a dose of 10 μg/0.5 μL/side. A lower dose (10 μg/0.5 μL/side) was used in the CPP experiment with ACC site 2 as a follow up to our previously published data with 20 μg/0.5 μL/side [33]. Post-experiment, animals were euthanized with CO2 overdose and 0.5 μL of Black India Ink was injected into the ACC or the RVM to verify cannula placement. Data from animals with misplaced cannulas were removed from analyses.
2.5. Statistical analysis:
Statistical analyses were calculated using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). Evoked pain time-course experiments were analyzed using a 2-way repeated measures ANOVA with time as a within-subject factor and treatment as a between-subject factor. Where significance was observed, a Sidak’s multiple comparison post hoc test was performed for tail flick experiments (2 groups) and a Tukey’s post hoc test was performed for von Frey, Hargreaves and Randall-Sillito experiments (4 groups). For CPP experiments, data are presented as difference scores (i.e., the difference between the time spent in the drug-paired chamber on testing day and on baseline day). Previous experiments confirmed that the employed CPP procedure is unbiased. Thus, a positive CPP score represents place preference, a negative score indicates aversion and zero indicates no preference [27; 31]. To evaluate whether the animals show significant preference or aversion, differences from a hypothetical value of 0 (i.e., no preference) were determined for each group’s difference score using a one-sample t-test. Subsequently, to compare between the two treatment groups, an unpaired t-test was used. All results were expressed as mean ± SEM. Significance was set at p<0.05.
Results
This study investigated the role of opioid activation in the ACC and the RVM in modulation of sensory and affective components of acute and chronic pain in rats. Three separate sites in the ACC referred to as: ACC site 1 (AP: +4.0 mm), ACC site 2 (equivalent to previously reported rostral ACC; AP: +2.6 mm [22]) and ACC site 3 (equivalent to previously published caudal ACC; AP: +0.2 mm [22]) were investigated. Multiple modalities of sensory stimuli were employed including normally innocuous tactile stimulation and noxious thermal and mechanical stimulation applied to either the hindpaw or the tail.
1. Morphine microinjection in the RVM, but not in the ACC, inhibits tail flick responses in naïve rats.
To determine if opioid activation in the ACC or the RVM modulates spinal reflexes in naïve rats, we used the 52°C hot water tail immersion test. Bilateral microinjection of morphine (20 μg/0.5 μl) or saline (vehicle) into any of the three studied ACC sites had no effect on tail withdrawal latencies (Fig. 1A-C). In contrast, microinjection of morphine (10 μg/0.5 μl) directly into the RVM produced a robust anti-nociceptive effect, demonstrated by increased tail flick latency (Fig. 1D). Peak anti-nociceptive effects occurred at 40 min following injection and was significantly different from that of saline injected animals (2-way ANOVA; F (4, 56) = 8.07; P<0.0001; Sidak’s post hoc test).
Figure 1. Morphine administration in the RVM but not the ACC produces analgesia in uninjured rats.

Tail withdrawal latencies to thermal heat (52°C water bath) were measured at baseline and during a 90 min time course after brain microinjections. (A) Morphine (20 μg/0.5 μl) (N=8) or saline (N=8) administration into the ACC site 1 had no effect. (B) Morphine (20 μg/0.5 μl) (N=8) or saline (N=9) into the ACC site 2 had no effect. (C) Morphine (20 μg/0.5 μl) (N=7) or saline (N=7) into the ACC site 3 had no effect. (D) Morphine (10 μg/0.5 μl) microinjection into the RVM (N=9) significantly increased tail withdrawal latency to thermal heat compared to saline-injected controls (N=7). *P<0.05 (2-way ANOVA with Sidak’s post hoc test). Data are means ± SEM.
2. Morphine microinjection in the RVM but not in the ACC reverses mechanical allodynia in rats with neuropathic pain.
Rats with SNL surgery developed tactile allodynia, as indicated by significant reductions in hindpaw withdrawal thresholds to probing with von Frey filaments when compared with pre-SNL baseline levels (Fig. 2; P<0.0001, unpaired t-test). Sham surgery had no significant effect on tactile responses. Bilateral microinjection of saline into the ACC or the RVM of SNL and sham animals did not elicit any changes in paw-withdrawal. Administration of morphine (20 μg/0.5 μl) into any of the three ACC locations had no effect on paw withdrawal thresholds (Fig. 2A-C). However, tactile allodynia was significantly reversed 20 minutes after morphine (10 μg/0.5 μl) microinjection into the RVM (P < 0.05). The maximal anti-allodynic effect of morphine was observed 60 minutes after microinjection into the RVM and was significantly different from saline treated rats for the 90 min duration of the experiment (2-way ANOVA; F (15, 125) = 12.42; P < 0.0001; Tukey’s post hoc test) (Fig. 2D).
Figure 2. Morphine administration in the RVM but not the ACC reverses tactile allodynia in SNL rats.
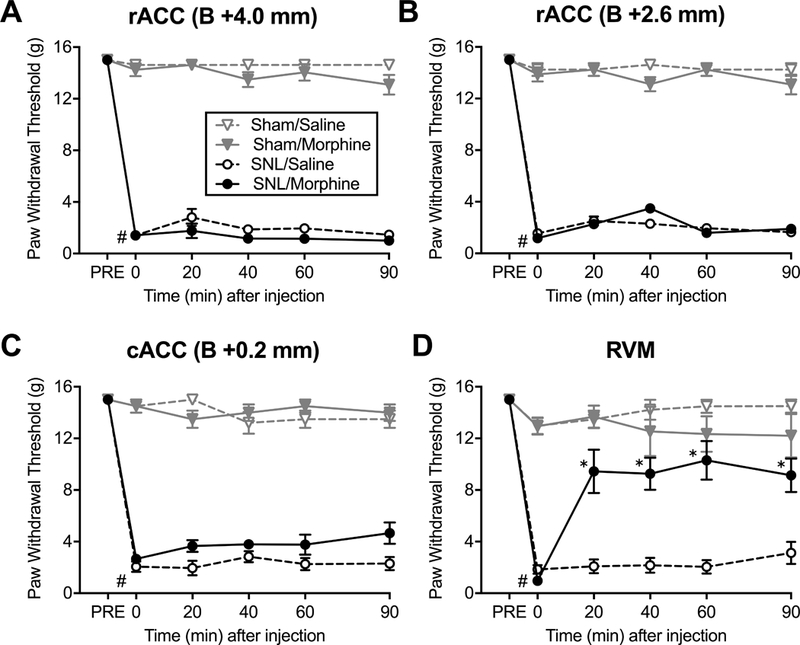
Tactile withdrawal thresholds of the ipsilateral hindpaws were measured using von Frey filaments before surgery (PRE), post-surgery at baseline (Time=0) and during a 90 min time course after brain microinjections. All SNL groups developed tactile allodynia #P<0.0001 (unpaired t-test). (A) Morphine (20 μg/0.5 μl) or saline administration into the ACC site 1 of SNL and sham rats had no effect (N=7–8). (B) Morphine (20 μg/0.5 μl) or saline into the ACC site 2 had no effect (N=7–8). (C) Morphine (20 μg/0.5 μl) or saline into the ACC site 3 had no effect (N=6). (D) Microinjection of morphine (10 μg/0.5 μl) into the RVM significantly increased paw withdrawal thresholds in SNL rats compared to saline-injected animals (N=6–9). *P<0.05 (2-way ANOVA with Tukey’s post hoc test). Data are means ± SEM.
3. Morphine microinjection in the RVM, but not in the ACC, produces antihyperalgesic and analgesic effects to mechanical stimulus.
SNL rats developed mechanical hyperalgesia, as measured using Randall-Selitto paw pressure test. Compared to pre-surgery baselines, SNL rats showed significantly reduced paw withdrawal thresholds (Fig. 3; P<0.0001, unpaired t-test). Sham surgery had no significant effect on tactile responses. Saline microinjections had no effect in any group of rats. Morphine (20 μg/0.5 μl) administration into any of the three ACC locations did not change paw withdrawal thresholds (Fig. 3A-C). In contrast, RVM morphine (10 μg/0.5 μl) increased paw withdrawal thresholds above the baseline in both sham and SNL rats, demonstrating anti-hyperalgesic and analgesic effects (2-way ANOVA; F (15, 140) = 152.8; P < 0.0001) (Fig. 3D). Compared to saline treated groups, the effects of RVM morphine were significantly different in SNL as well as in sham rats during the entire 90 min time course (Tukey’s post hoc test).
Figure 3. Morphine administration in the RVM but not the ACC reverses mechanical hyperalgesia in SNL rats.
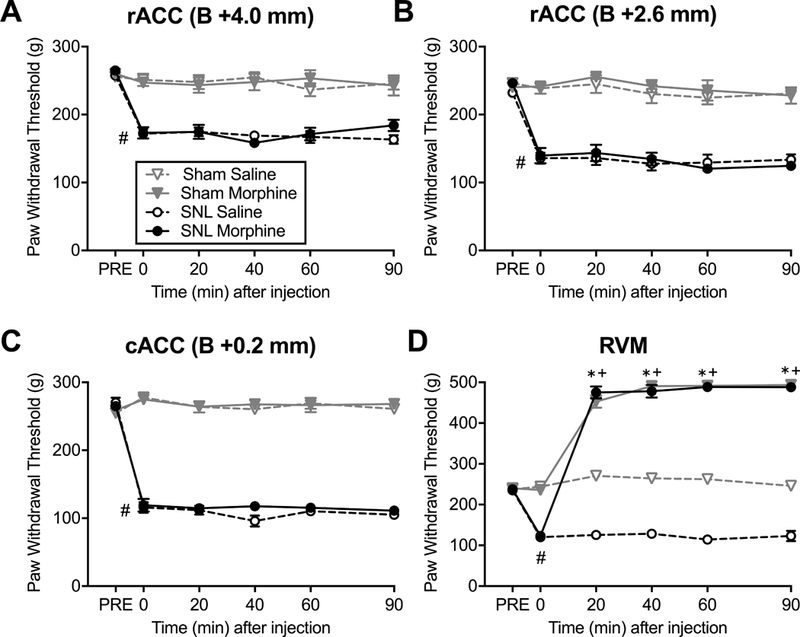
Noxious mechanical withdrawal thresholds of the ipsilateral hindpaws were measured using the Randall-Selitto test before surgery (PRE), post-surgery at baseline (Time=0) and during a 90 min time course after brain microinjections. All SNL groups developed mechanical hyperalgesia #P<0.0001 (unpaired t-test). (A) Morphine (20 μg/0.5 μl) or saline into the ACC site 1 of SNL and sham rats had no effect (N=8–13) on paw withdrawal thresholds. (B) Morphine (20 μg/0.5 μl) or saline into the ACC site 2 had no effect (N=9–18). (C) Morphine (20 μg/0.5 μl) or saline into the ACC site 3 had no effect (N=7–12). (D) Compared to saline, microinjection of morphine (10 μg/0.5 μl) into the RVM significantly increased paw withdrawal thresholds in both SNL (N=8; *P<0.05) and sham (N=8; +P<0.05) rats. (2-way ANOVA with Tukey’s post hoc test). Data are means ± SEM.
4. Morphine microinjection in the RVM but not in the ACC reverses thermal hyperalgesia in rats with neuropathic pain.
Rats with SNL surgery developed thermal hyperalgesia, demonstrated by decreased latency to withdraw the injured paw relative to pre-surgery baselines (Fig. 4; P<0.0001, unpaired t-test). Sham-operated rats maintained similar withdrawal latencies to their pre-surgery baseline measurements. Saline injections had no effect on withdrawal latencies. Likewise, microinjection of morphine (20 μg/0.5 μl) into any of the three ACC locations did not change paw withdrawal latencies (Fig. 4A-C). However, microinjection of morphine (10 μg/0.5 μl) into the RVM increased paw withdrawal latencies above baseline in both SNL and sham groups, demonstrating anti-hyperalgesic and analgesic effects (2-way ANOVA; F (15, 160) = 39.27; P < 0.0001) (Fig. 4D). Compared to saline treated animals, the effects of RVM morphine were significantly different in SNL as well as in sham rats during the entire 90 min time course (Tukey’s post hoc test).
Figure 4. Morphine administration in the RVM but not the ACC reverses thermal hyperalgesia in SNL rats.

Thermal withdrawal latencies of the ipsilateral hindpaws were measured using the Hargreaves test before surgery (PRE), post-surgery at baseline (Time=0) and during a 90 min time course after brain microinjections. All SNL groups developed thermal hyperalgesia #P<0.0001 (unpaired t-test). (A) Morphine (20 μg/0.5 μl) or saline into the ACC site 1 of SNL and sham rats had no effect (N=8–12). (B) Morphine (20 μg/0.5 μl) or saline into the ACC site 2 had no effect (N=6–9). (C) Morphine (20 μg/0.5 μl) or saline into the ACC site 3 had no effect (N=6–10). (D) Compared to saline, microinjection of morphine (10 μg/0.5 μl) into the RVM significantly increased paw withdrawal latency in both SNL (N=8–9; *P<0.05) and sham (N=9–10; +P<0.05) rats. (2-way ANOVA with Tukey’s post hoc test). Data are means ± SEM.
5. Morphine microinjection in the ACC site 1 and 3 does not relieve the aversiveness of chronic neuropathic pain.
The conditioned place preference (CPP) paradigm was used to assess whether opioid activity in a selected brain circuit can relieve the aversiveness of ongoing neuropathic pain. We have shown previously that bilateral microinjection of morphine (20 μg/0.5 μl) into the rostral ACC (site 2) produced robust CPP in animals with neuropathic pain [34]. Here, we extended our previous findings using a lower dose of morphine (Fig. 5B). Bilateral administration of 10 μg/0.5 μl morphine produced significant CPP only in SNL rats (P = 0.003; one sample t-test) and the difference between SNL and sham groups was significant (P = 0.035; unpaired t-test). Furthermore, we investigated two additional ACC sites. When morphine was microinjected bilaterally into site 1 or site 3 of the ACC, even at the higher dose of 20 μg/0.5 μl, SNL animals did not show significant preference for the morphine-paired chamber and were comparable to sham-operated animals (Fig. 5A, 5C). Microinjection of morphine (10 μg/0.5 μl) into the RVM produced significant preference for morphine-paired chamber in SNL rats (P = 0.013; one sample t-test). In contrast, sham-operated control rats demonstrated small, non-significant aversion to morphine-paired chamber (P = 0.181; one sample t-test) (Fig. 5D). The effect of morphine was significantly different between SNL and sham groups (P = 0.0038; unpaired t-test). The locations of morphine microinjections in the three ACC sites and in the RVM are shown schematically in Supplementary Figure S1.
Figure 5. Morphine administration into the RVM and the rACC (site 2), but not ACC site 1 or 3, elicits CPP in SNL rats.
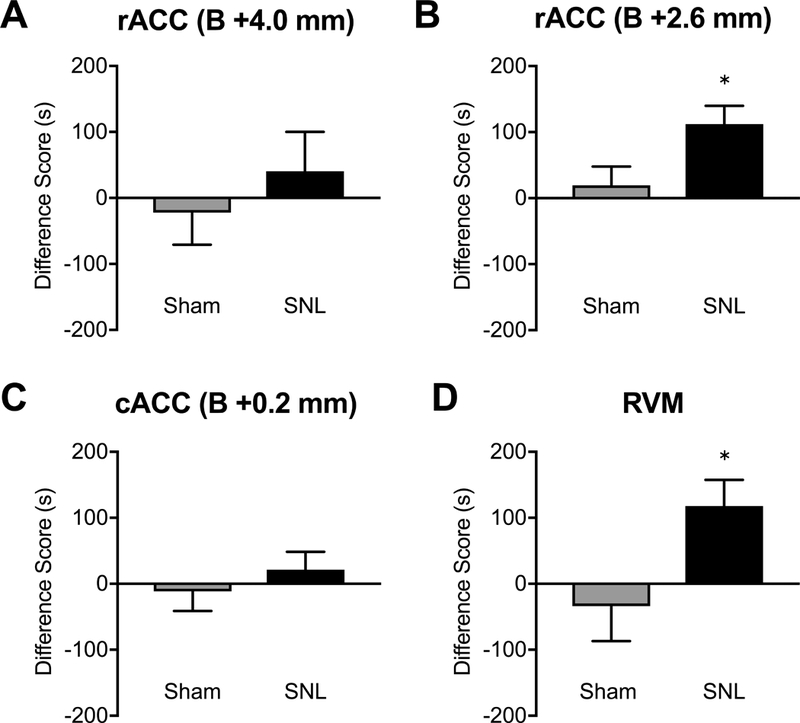
(A) Administration of morphine (20 μg/0.5 μl) into the ACC site 1 did not elicit CPP in either SNL (N=16) or sham (N=18) rats. (B) Morphine (10 μg/0.5 μl) into the ACC site 2 produced CPP in SNL (N=10) but not sham (N=8) rats. (C) Morphine (20 μg/0.5 μl) into the ACC site 3 did not produce CPP in SNL (N=18) or sham (N=12) rats. (D) Microinjection of morphine (10 μg/0.5 μl) into the RVM produced CPP in SNL (N=9) but not sham (N=10) rats. *P<0.05, unpaired t-test. Data are means ± SEM.
6. ACC morphine does not produce motor impairments.
To determine if morphine administration in the ACC could produce motor effects that would interfere with the ability of the animals to withdraw from noxious stimulation, we measured the number of crossings and the number of rearing in the open field test in sham and SNL rats following microinjection of morphine or saline into the ACC site 1, 2, or 3. Figure 6 demonstrates that SNL surgery had no effect on rats’ exploratory and rearing behavior. Moreover, the number of crossings and the number of rearing was not different between rats receiving saline and morphine microinjections into any of the three ACC sites.
Figure 6. Morphine administration into the ACC has no effect on motor activity.
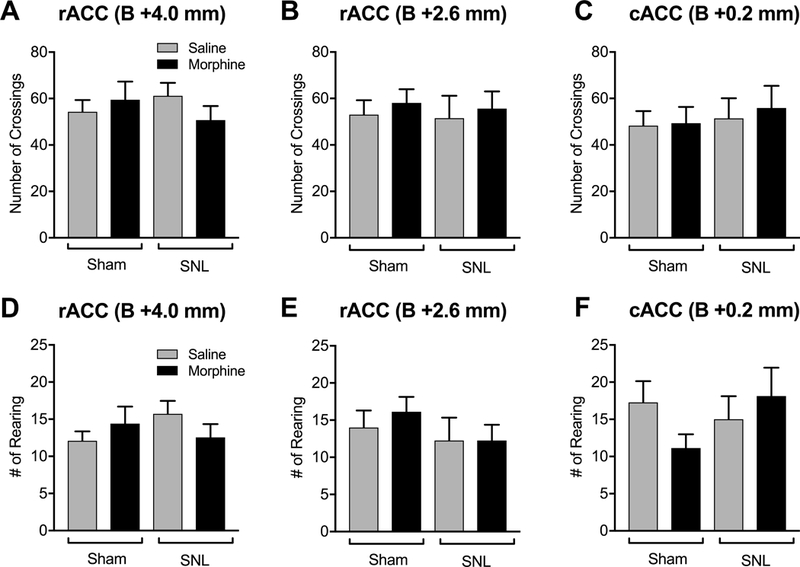
SNL surgery had no effect on the number of crossings (A, B, C) and rearing (D, E, F) in the open field test. Administration of morphine (20 μg/0.5 μl) into the ACC site 1 (A) site 2 (B) or site 3 (C) did not influence the number of crossings in either SNL or sham rats. Morphine in the ACC site 1 (D) site 2 (E) or site 3 (F) did not influence rearing in either SNL or sham rats. Data are means ± SEM.
Discussion
The present study investigated the role of opioid signaling in three sub-regions of the ACC and in the RVM on sensory and affective pain responses in naïve, sham and SNL rats. Using intracranial microinjections of morphine, our studies reveal that activation of opioid receptors in the RVM (a) produces analgesic responses to noxious thermal and mechanical stimulation in uninjured rats, (b) reverses nerve injury-induced hypersensitivity and elicits analgesia in injured rats and (c) inhibits motivated behavior resulting from injury-induced aversive qualities of ongoing pain. In contrast, morphine microinjection into three different ACC sites had no effect on thermal or mechanical responses in both uninjured and injured rats and modulated injury-induced ongoing pain only in one of the three ACC sites evaluated. These data differentiate opioid actions on acute and ongoing pain within cortical and brainstem circuits and are consistent with clinical observations that systemically-given opioids preferentially act in the ACC, and other affect-related regions, to alleviate affective qualities of pain without altering nociceptive thresholds. [6; 34]. Opioids have been reported to modulate thresholds in humans with experimental hyperalgesia [36]. Thus, in addition to the ACC, systemic opioids may act via synergistic effects at multiple levels of the neuraxis [26; 43; 44], including the RVM, to modulate sensory aspects of normally innocuous stimuli.
The ACC has been implicated in cognitive, evaluative and motivational functions [32; 57; 58] and plays a major role in processing the affective qualities of pain [52]. Glutamatergic activation of the ACC has been shown to elicit aversive pain-like behaviors in naive rats [21], while ACC lesion can selectively inhibit affective, but not sensory, qualities of neuropathic pain [22; 39]. Yalcin and colleagues reported that optogenetic activation of the ACC in the absence of injury produced pain-related depressive-like behaviors without influencing tactile thresholds [2] and that optogenetic inhibition of the ACC was sufficient to alleviate the aversive consequences of neuropathic pain [49]. Other preclinical reports, however, have shown modulation of injury-related hypersensitivity using different pharmacological or optogenetic manipulations in the ACC [17; 23; 30; 42; 45; 46].
It has long been recognized that nociception produces variable pain responses [4] based on interpretation of threat due to context, memories and other factors (reviewed in [8; 55]). Modulation of nociceptive signals can occur within spinal circuits as well as from supraspinal sites via descending pathways [3]. A number of cortical and subcortical regions including prefrontal, somatosensory and insular cortices participate in descending pain modulation through projections to the PAG-RVM pathway. Outputs from the RVM comprise a well-characterized, opioid-sensitive bidirectional modulatory pathway that ultimately influences nociception at the spinal level [15]. Release of endogenous opioids in the brainstem has been suggested in human studies to account for the analgesic effects of placebo during acute noxious stimulation [5; 29; 60]. However, how brain circuits above the brainstem, participate in descending modulation of sensory or affective qualities of pain remains poorly understood.
Opioids are known to preferentially modulate affective, rather than sensory, qualities of pain in humans [35]. Consistent with this, preclinical studies from Fuchs [28] and our [34] laboratories investigating opioid actions within the rat ACC have similarly concluded that opioids selectively modulate affective, but not sensory features of neuropathic pain. Additionally, opioid receptors within the ACC were required for the relief of affective qualities of ongoing pain with non-opioid pain relief [34]. The ACC has anatomical and functional connections to the PAG and human neuroimaging investigations support a role of this cortical area in naloxone-sensitive top-down modulation of nociceptive transmission in the spinal cord [13]. Preclinical studies of cortical modulation of acute or neuropathic pain, however, have generally targeted a limited subdomain termed the rostral ACC (+2.6 mm from bregma in rats) [22]. In contrast, the perigenual regions of the ACC have been suggested to be required for visceral pain [12; 59].
We evaluated possible modulation of different modalities of acute or chronic pain within three ACC subdomains to determine contributions to affective and sensory qualities of pain. We compared morphine effects in the ACC with actions within the RVM. Morphine administration at a dose of 10 μg in the RVM of naïve or sham-operated rats, produced a time-related antinociceptive effect following noxious thermal stimuli applied to the tail or to the hindpaw (hot water tail flick test, Hargreaves test) and following noxious mechanical hindpaw stimulation (Randall Selitto test). In rats with SNL injury, RVM morphine injection reversed tactile allodynia and thermal and mechanical hyperalgesia and produced an analgesic response reflected by increased response thresholds above baseline. These findings are consistent with previous work that has shown that MOR agonists directly inhibit the activity of RVM ON cells and disinhibit OFF cells, resulting in the inhibition of nociceptive transmission at the dorsal horn of the spinal cord in uninjured animals as well as in animals with nerve injury [14; 19]. Additionally, we demonstrated that RVM morphine elicited CPP in SNL, but not sham, rats. These findings correspond to our previous observation that inactivation of the RVM with lidocaine produced CPP in animals with nerve injury [39] and in animals with inflammatory mediators applied to the dura mater to model headache pain [10]. It therefore seems likely that opioid actions within the RVM have significant effects to modulate affective qualities of ongoing pain by inhibiting injury-induced nociceptive input through descending projections to the spinal or medullary dorsal horn.
In contrast to the results observed within the RVM, we were unable to detect effects of ACC morphine administration on tactile, thermal or mechanical stimulation in uninjured or SNL rats. No significant effects were observed following morphine microinjection in three separate sites in the ACC (site 1: +4.0 mm from bregma; site 2: +2.6 mm from bregma; site 3: +0.2 mm from bregma) in spite of administration of a 20 μg dose, 2-fold higher than that shown to be effective in the RVM. This dose of morphine did not produce effects on generalized behavior following injection into any of the three ACC sites studied as quantified by the number of open field crossing and rearing. Thus, opioids in these ACC locations do not appear to modulate evoked mechanical and thermal pain responses in uninjured rats or in animals with neuropathic injury. These findings are also consistent with previous reports from multiple laboratories of lack of opioid actions on evoked behaviors using site 2 co-ordinates for microinjection of morphine [28; 34]. Together, these findings suggest that opioid circuits within the ACC do not appear to engage the PAG-RVM-spinal cord pain pathways to modulate nociception. Although there was no effect of opioid activation in the ACC on reflexive pain responses, we confirmed and extended our previous finding that administration of morphine in the rACC (site 2) elicited CPP in SNL rats [34], demonstrating that opioid analgesics may act at this subdivision of the ACC to modulate affective/motivational aspects of ongoing pain. Fuchs and colleagues reported similar selective actions of ACC morphine using an assay that captures the aversive qualities of evoked stimuli [16]. In the present study, morphine administration into two neighboring sites in the ACC did not promote CPP, suggesting that affective features of pain are localized to some, but not all, ACC sub-regions. In support of this, Johanson et al. similarly found that lesion of the rACC (equivalent to site 2) but not caudal ACC (equivalent to site 3) blocked pain-induced conditioned place avoidance [22].
It should be noted that our observations on morphine-induced activation of ACC or RVM opioid circuits may not necessarily be generalizable to other neurotransmitter systems. Nevian and colleagues reported that ACC serotonin may be important in modulation of nerve-injury related evoked responses [45; 46]. Zhou and colleagues have reported that an adenylyl cyclase AC1 inhibitor was effective in nerve-injured animals [54]. Notably, this study was performed in mice using comparably larger injection volumes that likely encompass additional brain regions. It is not clear how the coordinates employed may translate to rat studies as the size of the ACC differs between these species [53]. Additionally, in spite of investigating three ACC opioid sites, it is possible that other ACC regions may be important in descending modulation of nociception. It should also be noted that other clinically used therapeutics may similarly show separation between modulation of affective and sensory qualities of pain through actions in the ACC. Neuroimaging studies in humans with experimental sensitization showed gabapentin-induced cortical deactivation [20] including within the ACC. We have previously reported that ACC microinjection of gabapentin elicited CPP in nerve-injured rats without modulation of evoked hypersensitivity [1]. The separation of modulation of sensory and affective qualities of pain within the ACC are also supported by human cingulotomy evaluations that have demonstrated relief of intractable pain with minimal, or no, sensory disturbances [7]. These findings suggest that selective modulation of affective qualities of pain and pain-related depressive effects may be achievable by targeting specific neuronal pathways within the ACC [2; 22; 39]. Characterization of ACC neurons that modulate pain affect through emerging strategies including cell-specific manipulations, may therefore be exploited to identify new non-opioid therapies.
Supplementary Material
Acknowledgements:
This work was supported by a grant from the National Institutes on Drug Abuse (R01DA041809 to FP) and by the Wellcome Trust Pain Consortium (the London Pain Consortium) (KB, AHD).
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References:
- [1].Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017;158(12):2386–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry 2015;77(3):236–245. [DOI] [PubMed] [Google Scholar]
- [3].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beecher HK. Pain in Men Wounded in Battle. Ann Surg 1946;123(1):96–105. [PMC free article] [PubMed] [Google Scholar]
- [5].Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 2006;120(1–2):8–15. [DOI] [PubMed] [Google Scholar]
- [6].Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Science translational medicine 2011;3(70):70ra14. [DOI] [PubMed] [Google Scholar]
- [7].Brotis AG, Kapsalaki EZ, Paterakis K, Smith JR, Fountas KN. Historic evolution of open cingulectomy and stereotactic cingulotomy in the management of medically intractable psychiatric disorders, pain and drug addiction. Stereotactic and functional neurosurgery 2009;87(5):271–291. [DOI] [PubMed] [Google Scholar]
- [8].Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews Neuroscience 2013;14(7):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].DaSilva AF, Nascimento TD, DosSantos MF, Zubieta JK. Migraine and the Mu-opioidergic system-Can we directly modulate it? Evidence from neuroimaging studies. Curr Pain Headache Rep 2014;18(7):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Annals of neurology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–462. [DOI] [PubMed] [Google Scholar]
- [12].Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience 2005;133(2):533–542. [DOI] [PubMed] [Google Scholar]
- [13].Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science 2009;326(5951):404. [DOI] [PubMed] [Google Scholar]
- [14].Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 2000;122:245–253. [DOI] [PubMed] [Google Scholar]
- [15].Fields HL. Understanding how opioids contribute to reward and analgesia. Regional anesthesia and pain medicine 2007;32(3):242–246. [DOI] [PubMed] [Google Scholar]
- [16].Fuchs PN, McNabb CT. The place escape/avoidance paradigm: a novel method to assess nociceptive processing. Journal of integrative neuroscience 2012;11(1):61–72. [DOI] [PubMed] [Google Scholar]
- [17].Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim YT, Fuchs PN, Mohanty SK. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One 2015;10(2):e0117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988;32(1):77–88. [DOI] [PubMed] [Google Scholar]
- [19].Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience 1994;63(1):279–288. [DOI] [PubMed] [Google Scholar]
- [20].Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, Vennart W, Tracey I. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A 2005;102(50):18195–18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nature neuroscience 2004;7(4):398–403. [DOI] [PubMed] [Google Scholar]
- [22].Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America 2001;98(14):8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kang SJ, Kwak C, Lee J, Sim SE, Shim J, Choi T, Collingridge GL, Zhuo M, Kaang BK. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol Brain 2015;8(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992;50(3):355–363. [DOI] [PubMed] [Google Scholar]
- [25].King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nature neuroscience 2009;12(11):1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kovelowski CJ, Bian D, Hruby VJ, Lai J, Ossipov MH, Porreca F. Selective opioid delta agonists elicit antinociceptive supraspinal/spinal synergy in the rat. Brain Res 1999;843(1–2):12–17. [DOI] [PubMed] [Google Scholar]
- [27].Kuo CC, Yen CT. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. Journal of neurophysiology 2005;94(3):1825–1836. [DOI] [PubMed] [Google Scholar]
- [28].LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Experimental neurology 2006;197(1):22–30. [DOI] [PubMed] [Google Scholar]
- [29].Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2(8091):654–657. [DOI] [PubMed] [Google Scholar]
- [30].Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010;330(6009):1400–1404. [DOI] [PubMed] [Google Scholar]
- [31].Mitchell JM, Margolis EB, Coker AR, Allen DC, Fields HL. Intra-VTA deltorphin, but not DPDPE, induces place preference in ethanol-drinking rats: distinct DOR-1 and DOR-2 mechanisms control ethanol consumption and reward. Alcoholism, clinical and experimental research 2014;38(1):195–203. [DOI] [PubMed] [Google Scholar]
- [32].Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nature neuroscience 2014;17(10):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Navratilova E, Xie J, King T, Porreca F. Evaluation of reward from pain relief. Annals of the New York Academy of Sciences 2013;1282:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35(18):7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Oertel BG, Preibisch C, Wallenhorst T, Hummel T, Geisslinger G, Lanfermann H, Lotsch J. Differential opioid action on sensory and affective cerebral pain processing. Clinical pharmacology and therapeutics 2008;83(4):577–588. [DOI] [PubMed] [Google Scholar]
- [36].Olesen AE, Staahl C, Arendt-Nielsen L, Drewes AM. Different effects of morphine and oxycodone in experimentally evoked hyperalgesia: a human translational study. Br J Clin Pharmacol 2010;70(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paxinos GW C The Rat Brain in Stereotaxic Coordinates. Amsterdam,: Academic Press, 2007. [Google Scholar]
- [39].Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 2011;152(7):1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 1999;82(2):159–171. [DOI] [PubMed] [Google Scholar]
- [41].Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277(5328):968–971. [DOI] [PubMed] [Google Scholar]
- [42].Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, Chen J. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 2008;153(1):268–278. [DOI] [PubMed] [Google Scholar]
- [43].Rossi GC, Pasternak GW, Bodnar RJ. Synergistic brainstem interactions for morphine analgesia. Brain Res 1993;624(1–2):171–180. [DOI] [PubMed] [Google Scholar]
- [44].Rossi GC, Pasternak GW, Bodnar RJ. Mu and delta opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res 1994;665(1):85–93. [DOI] [PubMed] [Google Scholar]
- [45].Santello M, Bisco A, Nevian NE, Lacivita E, Leopoldo M, Nevian T. The brain-penetrant 5-HT7 receptor agonist LP-211 reduces the sensory and affective components of neuropathic pain. Neurobiol Dis 2017;106:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Santello M, Nevian T. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 2015;86(1):233–246. [DOI] [PubMed] [Google Scholar]
- [47].Schrepf A, Harper DE, Harte SE, Wang H, Ichesco E, Hampson JP, Zubieta JK, Clauw DJ, Harris RE. Endogenous opioidergic dysregulation of pain in fibromyalgia: a PET and fMRI study. Pain 2016;157(10):2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scott DJ, Stohler CS, Koeppe RA, Zubieta JK. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse 2007;61(9):707–714. [DOI] [PubMed] [Google Scholar]
- [49].Sellmeijer J, Mathis V, Hugel S, Li XH, Song Q, Chen QY, Barthas F, Lutz PE, Karatas M, Luthi A, Veinante P, Aertsen A, Barrot M, Zhuo M, Yalcin I. Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J Neurosci 2018;38(12):3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, Wagner KJ, Wester HJ, Tolle TR. Opioidergic activation in the medial pain system after heat pain. Pain 2006;122(1–2):63–67. [DOI] [PubMed] [Google Scholar]
- [51].Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29(3):705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature reviews Neuroscience 2005;6(7):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vogt BA, Paxinos G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain structure & function 2014;219(1):185–192. [DOI] [PubMed] [Google Scholar]
- [54].Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 2011;3(65):65ra63. [DOI] [PubMed] [Google Scholar]
- [55].Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front Neurosci 2013;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tolle TR. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain 2004;108(3):213–220. [DOI] [PubMed] [Google Scholar]
- [57].Woo CW, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol 2015;13(1):e1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Woo CW, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, Atlas LY, Wager TD. Quantifying cerebral contributions to pain beyond nociception. Nat Commun 2017;8:14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yan N, Cao B, Xu J, Hao C, Zhang X, Li Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiology of learning and memory 2012;97(1):156–164. [DOI] [PubMed] [Google Scholar]
- [60].Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Annals of the New York Academy of Sciences 2009;1156:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


