Abstract
The increased use of split liver transplantation (SLT) represents one strategy to increase the supply of organs. Although outcomes after SLT and whole liver transplantation (WLT) are similar on average among pediatric recipients, we hypothesized that the relationship between graft type and outcomes may vary depending on patient, donor, and surgical characteristics. We evaluated graft survival among pediatric (<18 years), deceased-donor, liver-only transplant recipients from March, 2002, until December, 2015, using data from the the Scientific Registry of Transplant Recipients. Graft survival was assessed in a Cox proportional hazards model, with and without effect modification between graft type and donor, recipient, and surgical characteristics, to identify conditions where the risk of graft loss for SLT and WLT were similar. In a traditional multivariable model, characteristics associated with graft loss included donor age >50 years, recipient weight <10 kg, acute hepatic necrosis, autoimmune diseases, tumor, public insurance, and cold ischemia time (CIT) >8 hours. In an analysis that explored whether these characteristics modified the relationship between graft type and graft loss, many characteristics associated with loss actually had similar outcomes irrespective of graft type including weight <10 kg, acute hepatic necrosis, autoimmune diseases, and tumor. In contrast, several subgroups had worse outcomes when SLT was used, including recipient weight 10–35 kg, non-BA cholestasis, and metabolic disease. Allocation score, share type, or CIT did not modify risk of graft type and graft failure. Although one might anticipate that individuals with higher rates of graft loss would be worse candidates for SLT, data suggest that these patients actually have similar rates of graft loss. These findings can guide surgical decision-making and may support policy changes that promote the increased use of SLT for specific pediatric recipients.
Keywords: pediatric, liver, transplant, split, effect modification
INTRODUCTION
Pediatric liver transplantation provides life-saving therapy for children with end-stage liver disease and other metabolic conditions but continues to be hindered by a scarcity of available organs.(1) Waitlisted children typically receive fewer offers for deceased donor organs than adults, suggesting that they are especially vulnerable to an imbalance in need and availability.(2) Consequently, neonates have the highest rate of waitlist mortality for any age group, with nearly one-third of waitlisted neonates dying before receiving a suitable offer.(3)
The use of split liver transplantation (SLT) represents one opportunity to increase the supply of organs and has the potential to shorten waitlist times and decrease pre-transplant morbidity and mortality, particularly for children. Recent evidence suggests that outcomes following SLT are now likely comparable to whole liver transplantation (WLT) for both pediatric and adult recipients.(4–6) At the same time, the benefit of SLT may vary among patients with different donor, recipient, or surgical characteristics. For example, a study of adult transplant recipients concluded that graft failure following SLT and WLT was equivalent on average, but that status 1 recipients had poorer outcomes when receiving SLT compared to WLT.(7) Analysis of recipient, donor, and surgical characteristics can serve to identify optimal individuals for higher-risk organs that are not at increased risk of graft loss as well as suboptimal recipients for whom whole livers would yield better outcomes.(8)
In pediatric liver transplantation, several donor, recipient, and surgical characteristics have been shown to influence outcomes, such as donor age, cause of death, fulminant disease in the recipient, and prolonged cold ischemia time (CIT).(9,10) As with adults, these characteristics may yield subgroups of pediatric candidates whose outcomes are worse when receiving SLT, and other groups for whom outcomes are not affected by graft type. Better understanding of which characteristics are modified by graft type would serve to better inform surgical decision-making about which recipients are appropriate for SLT and could potentially inform policy to promote SLT. In this study, we used a large national registry to explore which characteristics modify the association between graft type and graft failure among pediatric deceased organ recipients in order to better understand the opportunities to expand the organ supply through further use of SLT.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.(11) The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors.
Study Population
We identified 5,345 pediatric liver-only, first-time transplant recipients of a deceased WLT or SLT who received an organ between March 1, 2002 (i.e., after implementation of the PELD/MELD system), and December 31, 2015; patients with missing weight (n = 1) or missing CIT (n = 284) were excluded. We compared donor, recipient, and surgical characteristics between recipients of WLT and SLT using χ2 tests.
Graft Type
All individuals were defined as having an SLT if they received a portion of a deceased donor graft. Sensitivity analyses exploring the impact of organs used by one recipient (i.e., “cut down”) versus two recipients, and in vivo versus ex vivo splits showed no difference in graft failure, a finding consistent with other studies demonstrating similar occurrence of graft failure, biliary strictures, and vascular thromboses.(7,12)
Graft Survival
Graft failure was identified as any reported graft failure or death (i.e., “all cause graft loss”). This means that:(1) all deaths are attributed to a graft failure, but not all graft failure leads to death; and (2) risk of graft failure is always greater than risk of death. The functional form for recipient, donor, and surgical characteristics were explored using Kaplan-Meier curves and log-rank tests. We used Cox proportional hazards models to characterize the association between allograft type and graft survival after adjustment for the following variables: donor age and race, cause of death, recipient weight at transplant, recipient sex, recipient race/ethnicity, underlying disease, laboratory PELD/MELD at transplant, status 1 designation, insurance type, CIT, and share type (i.e., local, regional or national). The decision to include these specific variables in the final model for multivariable regression was derived from associations between covariates with risk factors and the outcome in both the published literature as well as statistical tests within this cohort.(9) Recipients were censored upon re-transplantation or multi-organ transplantation (e.g., liver-kidney).
Effect Modification
To identify specific recipient, donor, and surgical characteristics that modify the effect of allograft type on graft survival, we performed Cox regression analysis with effect modification between allograft type with additional covariates for which an a priori hypothesis existed that the association between the covariate and graft failure may vary by allograft type. Test for effect modification was assessed in an unadjusted analysis as well as a parsimonious model that adjusted for donor cause of death, recipient weight, recipient diagnosis, allocation score, share type, and CIT as previously described.(8) Donor age was excluded from effect modification analysis given the small number of individuals receiving a split from a donor >50 years (n < 100). Donor race, recipient race, and recipient insurance were excluded due to there being no a priori reason to consider that the association between allograft type and graft failure would vary by these characteristics. From this model, the relative impact of SLT versus WLT could be evaluated, with factors that exacerbated the impact of SLT versus WLT being defined as “optimal,” whereas those that had no impact (or attenuated the impact) on SLT versus WLT were defined as “suboptimal.”
Potential for Increased SLT
In order to further quantify opportunities to increase the use of SLT among pediatric liver transplant candidates, we identified the number of waitlisted individuals who died, or were delisted due to medical unsuitability or declining health, having been on the waitlist for at least 7 days in the period from March 1, 2002 to December 31, 2016 (n = 1,160); since it can be reasonably assumed that individuals would only be listed if they were appropriate candidates for transplant, individuals delisted 7 days later due to death or medical unsuitability represent instances where an offer through SLT would have benefited the candidates. The number of individuals with optimal characteristics that had been listed but then delisted served to provide an estimate of the potential reduction in waitlist mortality that may have occurred if greater use of SLT had occurred.
Statistical Analysis
All statistical tests used a two-sided α of 0.05. Confidence intervals are reported using the method of Louis and Zeger, as previously reported.(13) All analyses were performed using STATA 14.0 (College Station, TX, USA). This study was approved by the Institutional Review Board of Johns Hopkins University School of Medicine. No organs were used from executed prisoners.
RESULTS
Patient Characteristics
Among 5,345 pediatric recipients in our study, 1,694 (31.7%) received an SLT and 3,651 (68.3%) received a WLT (Table 1). SLT recipients were less likely than WLT recipients to have a donor that was <18 years (59.4% for SLT and 84.3% for WLT) and more likely to have a donor between 18 and 50 years (38.7% vs 13.6%; P < 0.001). SLT recipients were less likely to have a donor with anoxia (19.5% vs 35.1%) and more likely that the donor had head trauma (63.1% vs 49.8%; P < 0.001). A larger percentage of SLT recipients were <10 kg (51.7% vs 32.9%; P < 0.001), had biliary atresia (BA) (44.2% vs 36.0%; P < 0.001), and were status 1 (37.7% vs 31.2%; P < 0.001). SLT recipients were less likely to receive the organ from a national share (4.7% vs 23.5%; P < 0.001), but CIT did not vary between the two graft types.
Table 1:
Characteristics of 5,345 pediatric deceased donor liver transplants performed in the United States in the PELD/MELD era by graft type
| Characteristic | WLT | SLT | P | ||
|---|---|---|---|---|---|
| Total (N, %) | 3,651 (68.3) | 1,694 (31.7) | |||
| Donor | |||||
| Age (years) | |||||
| <18 | 3,079 (84.3) | 1,008 (59.5) | <0.001 | ||
| 18-50 | 497 (13.6) | 656 (38.7) | |||
| ≥50 | 75 (2.1) | 30 (1.8) | |||
| Female | 1,553 (42.5) | 644 (38.0) | 0.002 | ||
| Ethnicity/race | |||||
| Caucasian, non-Hispanic | 2,015 (55.2) | 1,050 (62.0) | <0.001 | ||
| African American | 765 (21.0) | 260 (15.4) | |||
| Hispanic | 736 (20.2) | 331 (19.5) | |||
| Asian | 72 (2.0) | 25 (1.5) | |||
| Mixed/other | 63 (1.7) | 28 (1.7) | |||
| Cause of death | |||||
| Anoxia | 1,281 (35.1) | 331 (19.5) | <0.001 | ||
| Stroke | 382 (10.5) | 225 (13.3) | |||
| Head trauma | 1,816 (49.8) | 1,069 (63.1) | |||
| unknown/other | 172 (4.7) | 69 (4.1) | |||
| Recipient | |||||
| Weight (kg) | |||||
| <10 | 1,201 (32.9) | 875 (51.7) | <0.001 | ||
| 10-35 | 1,367 (37.4) | 710 (41.9) | |||
| ≥35 | 1,083 (29.7) | 109 (6.4) | |||
| Female | 1,887 (51.7) | 847 (50.0) | |||
| Ethnicity/race | |||||
| Caucasian, non-Hispanic | 1,919 (52.6) | 820 (48.4) | <0.001 | ||
| African American | 639 (17.5) | 270 (15.9) | |||
| Hispanic | 772 (21.2) | 450 (26.6) | |||
| Asian | 199 (5.5) | 102 (6.0) | |||
| Mixed/other | 122 (3.3) | 52 (3.1) | |||
| Diagnosis | |||||
| Biliary atresia (BA) | 1,315 (36.0) | 748 (44.2) | <0.001 | ||
| Acute hepatic necrosis | 471 (12.9) | 222 (13.1) | |||
| Autoimmune | 229 (6.3) | 28 (1.7) | |||
| Congenital cholestasis (non-BA) | 148 (4.1) | 91 (5.4) | |||
| Metabolic | 561 (15.4) | 219 (12.9) | |||
| Tumor | 346 (9.5) | 170 (10.0) | |||
| Other | 581 (15.9) | 216 (12.8) | |||
| Allocation score at transplant | |||||
| aPELD/MELD <30, non-status 1 | 1,657 (45.4) | 618 (36.5) | <0.001 | ||
| aPELD/MELD ≥30, non-status 1 | 854 (23.4) | 438 (25.9) | |||
| aPELD/MELD ≥30, status 1 | 1,140 (31.2) | 638 (37.7) | |||
| Insurance | |||||
| Private | 1,731 (47.4) | 715 (42.2) | <0.001 | ||
| Public | 1,791 (49.1) | 935 (55.2) | |||
| Other/missing | 129 (3.5) | 44 (2.6) | |||
| Surgery | |||||
| Share type | |||||
| Local | 1,285 (35.2) | 744 (43.9) | <0.001 | ||
| Regional | 1,509 (41.3) | 871 (51.4) | |||
| National | 856 (23.5) | 79 (4.7) | |||
| Cold ischemia time (hours) | |||||
| <8 | 2,190 (60.0) | 1,009 (59.6) | 0.09 | ||
| 8-12 | 1,054 (28.9) | 525 (31.0) | |||
| ≥12 | 406 (11.1) | 160 (9.5) | |||
WLT: whole liver transplant; SLT: split liver transplant
Graft Failure
Although SLT was associated with increased graft failure in an unadjusted model (HR: 1.031.171.32), there was no evidence of increased risk after adjustment for confounding donor, recipient, and surgical characteristics, on average among the entire study population (aHR: 0.911.071.24; Table 2). Characteristics associated with graft failure in a traditional multivariable model included donor age 18−50 years (aHR: 1.051.251.48) and ≥50 years (aHR: 1.922.663.68), recipient weight <10 kg (aHR: 1.241.441.69), recipients with acute hepatic necrosis (aHR: 1.231.571.99), autoimmune diseases (aHR: 1.552.052.71), tumor (aHR: 1.571.972.47), and other diseases (aHR: 1.191.451.76), but status 1 was not associated with greater graft failure (aHR: 0.750.891.06 ). CIT was also associated with graft failure at 8−12 hours (aHR: 1.061.221.39) and ≥12 hours (aHR: 1.201.451.74). Increased graft failure was also seen in unadjusted model for stroke (HR: 1.221.481.80), recipient weight ≥35 kg (HR: 1.181.391.62), and an allocation score ≥30 or status 1 (HR 1.211.381.58), but these were not independently significant after adjusting for other confounders.
Table 2:
Characteristics associated with graft failure in pediatric recipients
| Characteristic | Unadjusted | Multivariable | |||
|---|---|---|---|---|---|
| HR | P | aHR | P | ||
| Split liver transplant | 1.031.171.33 | 0.02 | 0.911.071.24 | 0.4 | |
| Donor | |||||
| Age (years) | |||||
| <18 | -- | -- | -- | -- | |
| 18-50 | 1.221.401.61 | <0.001 | 1.051.241.48 | 0.01 | |
| ≥50 | 2.453.234.26 | <0.001 | 1.912.643.65 | <0.001 | |
| Race/ethnicity | |||||
| Caucasian | -- | -- | -- | -- | |
| African American | 0.931.091.28 | 0.3 | 0.991.161.36 | 0.1 | |
| Hispanic | 0.800.941.10 | 0.4 | 0.830.971.14 | 0.7 | |
| Asian | 0.721.121.73 | 0.6 | 0.620.971.50 | 0.9 | |
| Mixed/other | 0.580.941.52 | 0.8 | 0.610.991.61 | 1.0 | |
| Cause of death | |||||
| Anoxia | -- | -- | -- | -- | |
| Stroke | 1.221.481.80 | <0.001 | 0.891.101.37 | 0.4 | |
| Head Trauma | 0.961.111.28 | 0.2 | 0.901.041.21 | 0.6 | |
| Other/unknown | 0.720.991.36 | 0.9 | 0.680.941.29 | 0.7 | |
| Recipient | |||||
| Weight (kg) | |||||
| <10 | 1.071.241.42 | 0.003 | 1.241.441.69 | <0.001 | |
| 10-35 | -- | -- | -- | -- | |
| ≥35 | 1.181.391.62 | <0.001 | 0.901.091.32 | 0.4 | |
| Female | 0.931.051.18 | 0.5 | 0.951.071.21 | 0.3 | |
| Race/ethnicity | |||||
| Caucasian | -- | -- | -- | -- | |
| African American | 1.051.231.44 | 0.01 | 0.951.111.32 | 0.2 | |
| Hispanic | 0.901.051.22 | 0.5 | 0.800.951.11 | 0.5 | |
| Asian | 0.550.751.02 | 0.1 | 0.580.791.07 | 0.1 | |
| Mixed/other | 0.761.071.51 | 0.7 | 0.680.961.37 | 0.8 | |
| Diagnosis | |||||
| Biliary atresia (BA) | -- | -- | -- | -- | |
| Acute hepatic necrosis | 1.351.621.94 | <0.001 | 1.241.582.02 | <0.001 | |
| Autoimmune | 1.411.812.31 | <0.001 | 1.552.062.72 | <0.001 | |
| Congenital cholestasis (non-BA) | 0.710.991.39 | 0.9 | 0.771.071.51 | 0.7 | |
| Metabolic | 0.790.981.20 | 0.8 | 0.911.141.44 | 0.3 | |
| Tumor | 1.451.772.16 | <0.001 | 1.581.992.50 | <0.001 | |
| Other | 1.151.381.65 | <0.001 | 1.191.461.78 | <0.001 | |
| Allocation PELD/MELD | |||||
| <30 | -- | -- | -- | -- | |
| ≥30, non-status 1 | 0.810.951.12 | 0.6 | 0.750.891.06 | 0.2 | |
| ≥30 and status 1 | 1.201.381.58 | <0.001 | 0.891.071.28 | 0.5 | |
| Insurance status | |||||
| Private | -- | -- | -- | -- | |
| Public | 1.041.171.33 | 0.01 | 1.061.211.38 | 0.006 | |
| Other/missing | 0.440.681.04 | 0.1 | 0.480.741.15 | 0.2 | |
| Surgery | |||||
| Share | |||||
| Local | -- | -- | -- | -- | |
| Regional | 0.830.941.07 | 0.4 | 0.830.951.09 | 0.4 | |
| National | 0.710.851.01 | 0.1 | 0.700.861.06 | 0.1 | |
| Cold ischemia time (hours) | |||||
| <8 | -- | -- | -- | -- | |
| 8-12 | 1.031.181.35 | 0.02 | 1.061.211.39 | 0.005 | |
| ≥12 | 1.151.381.65 | <0.001 | 1.201.451.74 | <0.001 | |
Effect Modification
To determine if specific characteristics modified the effect between graft type and graft failure, several variables were tested in unadjusted and adjusted models. In these models, a coefficient of 1 (or a non-significant coefficient at the 95% confidence level) indicates that, for individuals within that subcohort, the risk of graft failure did not vary between individuals receiving an SLT or WLT (Figure). Alternatively, a coefficient of 1.4 means that, among individuals with that specific characteristic (e.g., a specific weight category), individuals who received a SLT had 1.4 times the risk of graft failure than individuals with a WLT. In general, subcohorts with the highest overall graft failure were not further negatively impacted by having an SLT vs WLT, and therefore would be optimal candidates for SLT, whereas recipients with overall favorable outcomes had higher rates of graft failure with SLT compared to WLT, and would be suboptimal candidates. For example, recipient weight <10 kg was associated with increased graft failure in general (Table 2, aHR: 1.241.441.69), but this risk was the same irrespective of graft type (Figure, aHR: 0.821.001.22) suggesting this is an optimal characteristic for SLT. In contrast, recipients with weight between 10 and 35 kg had overall lower risk of graft failure but actually had 1.46 times higher rate of graft failure following SLT compared to WLT (aHR: 1.051.462.02). A similar pattern was evident when considering indication for transplant, where an equivalent risk of graft failure in SLT and WLT existed for recipients that had the highest overall risk─acute hepatic necrosis (aHR for SLT vs WLT: 0.801.111.53), autoimmune disease (aHR SLT vs WLT: 0.921.713.17), and tumor (aHR SLT vs WLT: 0.721.031.48)─whereas SLT was associated with higher graft failure, and suboptimal, for individuals that generally had favorable overall risk such as non-BA congenital cholestasis (aHR for SLT vs WLT: 1.132.144.07) and metabolic disorders (aHR SLT vs WLT: 1.081.572.28). Notably, BA had generally low risk of graft failure, and was not adversely impacted by use of SLT (aHR SLT vs WLT: 0.881.091.36). Graft type did not modify the association between allocation score and graft failure, with similar risk for SLT versus WLT in individuals with PELD/MELD <30 (aHR: 0.951.181.47), ≥30 without status 1 (aHR: 0.981.301.72), and ≥30 with status 1 (aHR: 0.941.151.41).
Figure:
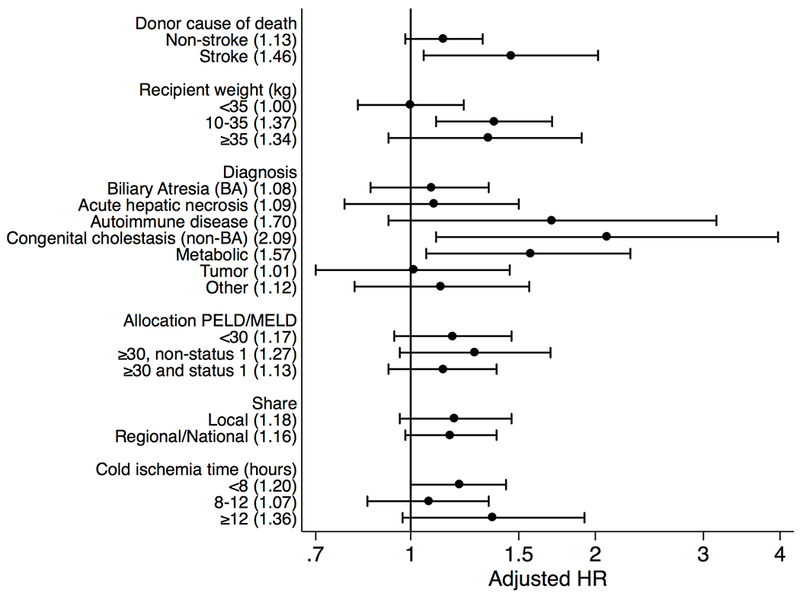
Forest plot of adjusted hazard ratios for relative risk of graft failure in individuals receiving a split liver transplant vs whole liver transplant based on specific donor, recipient and surgical characteristics.
Although higher risk of graft failure was seen with CIT >8 hours, this association was not modified by graft type in recipients with CIT 8–12 hrs (aHR SLT vs WLT: 0.851.071.34) or CIT ≥12 hrs (aHR SLT vs WLT: 0.971.361.92). Among individuals with CIT <8 hours, there was a trend toward increased graft failure in individuals when receiving SLT compared to WLT (aHR SLT vs WLT: 1.001.201.43). Graft type did not modify risk of graft failure in individuals who received a local share (aHR SLT vs WLT: 0.961.181.46) or regional/national share (aHR SLT vs WLT: 0.981.161.38). While donor stroke was not generally associated with graft failure, recipients of SLT from donors with stroke had increased graft failure compared to WLT from donor with stroke (aHR SLT vs WLT: 1.051.462.02).
Potential for Increased SLT
For those characteristics in which some subcohorts were optimal (i.e., weight <10 kg; all recipient diagnoses except non-BA cholestasis and metabolic disorder), we identified the number of recipients that were waitlisted for at least 7 days but ultimately died or were delisted due to medical unsuitability while waiting for an offer over the study period (i.e., 178 months; n = 1,160). Among waitlist deaths, 451 pediatric candidates were <10 kg and 348 (78%) were on the waitlist for at least 7 days before death or delisting due to poor health indicating that as many as approximately 23 candidates per year may likely have benefitted from increased availability of SLT. Furthermore, within this group of 348 pediatric candidates, 320 (92%) had optimal underlying conditions and 28 had suboptimal conditions (i.e., 13 with non-BA neonatal cholestasis and 15 with metabolic disorders) indicating that as many as 22 children per year with optimal weight and underlying disease died after waiting at least 7 days for an organ and would likely have benefited from increased use of SLT.
DISCUSSION
Increasing evidence suggests that outcomes following SLT and WLT are similar.(4,6,7,14) However, this assessment should not be taken to indicate that risk of graft failure is equivalent for all subgroups of patients. While there may be a tendency by healthcare providers to anticipate that the sickest children with the highest rates of pre-transplant mortality (e.g., PELD/MELD ≥30, or status 1) would be relatively poorer candidates for SLT and have worse outcomes, our findings from this national study of 5,345 pediatric recipients indicate that the opposite is true. Specifically, characteristics associated with overall higher rates of graft failure (e.g., recipient weight <10 kg; recipient diagnosis of acute hepatic necrosis, autoimmune disorders, or tumor; and CIT ≥ 8 hours) had equivalent risk of graft failure among SLT recipients when compared to WLT. At the same time, pediatric recipients with the lowest overall risk of graft failure (e.g., recipient weight 10–35 kg; non-BA congenital cholestasis, metabolic disorders; CIT <8 hours) fared worse when they received a SLT compared to WLT. These findings were independent of pre-transplant mortality risk as determined by PELD/MELD.
Our findings that graft failure among children less than 10 kg and those with status 1 are not adversely impacted by the type of deceased donor allograft type dovetails with the fact that patients with these characteristics are also recipients with highest rates of death while awaiting liver transplantation.(1,15) Consequently, our findings strengthen the argument that there should be broader use of SLT for these fragile subgroups of children and that such a practice, and policy, would likely translate to important reductions in waitlist death, the stated goal of our current allocation system.(16) Notably, broader geographic sharing, including national sharing, was not associated with worse outcomes for children. Our analysis also attempted to quantify the degree to which increased use of SLT could potentially reduce waitlist mortality and determined that most children that die on the waitlist were waiting for at least a week, and approximately half these children meet criteria for being an optimal recipient for SLT, with a predicted graft survival that would be similar for SLT and WLT. These findings should be considered in the context of work by Hsu et al. that showed nearly half of the children who died on the waitlist never received a single offer of a liver.(2) Furthermore, recent research by Perito et al. showed that approximately half of the most “split-able” livers, by strict criteria, were not utilized for SLT.(17)
While our findings show the potential for a modest, but meaningful, opportunity to reduce waitlist mortality following greater use of SLT for children in select groups (e.g., recipient weight <10 kg), there are likely several other downstream benefits as well. First, broader use of SLT would likely mean that many children could be transplanted at lower allocation scores, corresponding both to lower pre-transplant morbidity and cost. Second, broader use of SLT for select groups of children would then allow for greater number of whole organs to be available for other groups such as slightly larger children. At the same time, one limitation of our study is that we did not formally incorporate an analysis that combined pre-transplant and post-transplant mortality (i.e., survival benefit). However, given that we analyzed all cause graft loss and there was no increase in graft failure for select recipients, it can be inferred that there is no increase in post-transplant mortality as well.
One important limitation of our study is that, through SRTR, we do not have information about other meaningful outcomes such as biliary strictures and vascular thromboses. Similarly, we do not have specific information as to why some subgroups had higher rates of graft failure. The SPLIT (Studies in Pediatric Liver Transplantation) Consortium published outcomes from 1995–2006 and identified both higher rates of graft failure as well as biliary strictures and vascular complications in children receiving technical variant grafts.(12) However, it is not clear if these complications currently exist at higher rates in SLT, especially given more recent reports that graft failure in SLT, both immediate and long-term, is currently equivalent to WLT.(4) Reports from adult literature have been conflicting with some studies showing similar rates of biliary stricture and vascular thromboses, whereas other studies still showing higher rates of these surgical complications.(6,18–20) Nonetheless, one likely explanation for higher rates of graft failure among certain groups is that they have higher rates of these well-established complications leading to graft failure.
A second limitation of our study is that it is derived from observational, as opposed to experimental, data; randomized trials would be impractical so observational studies represent the best opportunity to identify the benefit of different types of allografts. In this instance, it is possible that favorable outcomes seen in SLT are due to careful candidate selection on the part of healthcare teams. However, this potential for bias is not likely to impact our analysis since we have adjusted for many known characteristics associated with disease severity including both PELD/MELD, and therefore are making comparisons among people with similar health status. Nonetheless, residual confounding of disease severity may occur. A final limitation is that the sample size was very small for certain subcohorts (e.g., weight >35 kg) making it hard to obtain a precise estimate for the relative impact of SLT versus WLT in these groups.
Although many considerations go into the decision to use SLT for a specific patient, there is clear agreement in the transplant community that demand for organs exceed the supply, and that minimization of pre-transplant mortality risk should be the highest priority. In the context of demonstrably equivalent outcomes for adult recipients of SLT, our findings further support that greater use of SLT in the majority of recipients can address the problem of organ scarcity such that fewer children would die while awaiting an offer and still have acceptable outcomes after transplantation.
ACKNOWLEDGEMENTS
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of, or interpretation by, the SRTR or the U.S. Government.
GRANTS AND FINANCIAL SUPPORT
Dr. Mogul is supported by grant number 5K08HS023876-02 from the Agency for Healthcare Research and Quality. Dr. Segev is supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Massie is supported by grant number K01DK101677 from the National Institute of Diabetes and Digestive and Kidney Diseases.
ABBREVIATIONS
- CIT
Cold Ischemia Time
- PELD
Pediatric End-stage Liver Disease
- MELD
Model for End-stage Liver Disease
- SLT
Split Liver Transplant
- WLT
Whole Liver Transplant
Footnotes
CONFLICTS OF INTEREST AND DISCLOSURES
Dorry Segev has financial relationships from Novartis and Sanofi. Kathy Schwarz has financial relationships with Gilead, Bristol Meyers Squibb, Roche, and Up to Date. There are no conflicts of interest.
REFERENCES
- 1.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant. 2017;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 2.Hsu EK, Shaffer ML, Gao L, Sonnenday C, Volk ML, Bucuvalas J, et al. Analysis of Liver Offers to Pediatric Candidates on the Transplant Wait List. Gastroenterology. 2017. October;153(4):988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: A path toward improving outcomes and eliminating wait-list mortality. Liver Transplant. 2017. January;23(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogul DB, Luo X, Bowring MG, Chow EK, Massie AB, Schwarz KB, et al. Fifteen-Year Trends in Pediatric Liver Transplants: Split, Whole Deceased, and Living Donor Grafts. J Pediatr. 2018. May;196:148–153.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, et al. Deceased-donor split-liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg. 2013. October;217(4):672–684.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle MBM, Maynard E, Lin Y, Vachharajani N, Shenoy S, Anderson C, et al. Outcomes with split liver transplantation are equivalent to those with whole organ transplantation. J Am Coll Surg. 2013. July;217(1):102–12; discussion 113–114. [DOI] [PubMed] [Google Scholar]
- 7.Cauley RP, Vakili K, Fullington N, Potanos K, Graham DA, Finkelstein JA, et al. Deceased-donor split-liver transplantation in adult recipients: is the learning curve over? J Am Coll Surg. 2013. October;217(4):672–684.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segev DL, Maley WR, Simpkins CE, Locke JE, Nguyen GC, Montgomery RA, et al. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatol. 2007. December;46(6):1907–18. [DOI] [PubMed] [Google Scholar]
- 9.McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011. July;254(1):145–54. [DOI] [PubMed] [Google Scholar]
- 10.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006. April;6(4):783–90. [DOI] [PubMed] [Google Scholar]
- 11.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014. August;14(8):1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, et al. Impact of graft type on outcome in pediatric liver transplantation: a report From Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg. 2007. August;246(2):301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostat Oxf Engl. 2009. January;10(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker NS, Barshes NR, Aloia TA, Nguyen T, Rojo J, Rodriguez JA, et al. Analysis of recent pediatric orthotopic liver transplantation outcomes indicates that allograft type is no longer a predictor of survivals. Liver Transplant. 2008. August;14(8):1125–32. [DOI] [PubMed] [Google Scholar]
- 15.McDiarmid SV, Anand R, Lindblad AS, Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002. July 27;74(2):173–81. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine, Committee on Organ Procurement and Transplantation Policy, Division of Health Sciences Policy. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule [Internet]. National Academies Press; DC; 1999. Available from: 10.17226/9628 [DOI] [PubMed] [Google Scholar]
- 17.Perito ER, Roll G, Dodge JL, Rhee S, Roberts JP. Split liver transplantation and pediatric waitlist mortality in the United States: potential for improvement. Transplantation. 2018. April 21; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cescon M, Grazi GL, Ravaioli M, Ercolani G, Del Gaudio M, Vivarelli M, et al. Conventional split liver transplantation for two adult recipients: a recent experience in a single European center. Transplantation. 2009. November 15;88(9):1117–22. [DOI] [PubMed] [Google Scholar]
- 19.Mabrouk Mourad M, Liossis C, Kumar S, Gunson BK, Mergental H, Isaac J, et al. Vasculobiliary complications following adult right lobe split liver transplantation from the perspective of reconstruction techniques. Liver Transplant. 2015. January;21(1):63–71. [DOI] [PubMed] [Google Scholar]
- 20.Mallik M, Callaghan CJ, Hope M, Gibbs P, Davies S, Gimson AE, et al. Comparison of liver transplantation outcomes from adult split liver and circulatory death donors. Br J Surg. 2012. June;99(6):839–47. [DOI] [PubMed] [Google Scholar]


