Abstract
Background:
Uptake of riboflavin (RF) by intestinal epithelial cells occurs via a specific carrier-mediated process that involves the apically localized RF transporter-3 (RFVT3). Previous studies have shown that sodium butyrate (NaB) affects intestinal uptake of other substrates and expression of their membrane transporters, but its effect on intestinal uptake of RF and expression of RFVT3 has not been examined.
Aims:
To investigate the effect of NaB on intestinal RF uptake process and expression of the RFVT3.
Methods:
Two experimental models were used in this study: Human-derived intestinal epithelial Caco-2 cells and ex vivo mouse colonoids. 3H-RF uptake assay, western blot, RT-qPCR and chromatin immunoprecipitation (ChIP) assay were performed.
Results:
Treating Caco-2 cells with NaB led to a significant increase in carrier-mediated RF uptake. This increase was associated with a significant induction in the level of expression of the hRFVT3 protein, mRNA and heterogenous nuclear RNA (hnRNA) Similarly, treating mouse colonoids with NaB led to a marked increase in the level of expression of the mRFVT3 protein, mRNA and hnRNA. NaB did not affect hRFVT3 mRNA stability, rather it caused significant epigenetic changes (histone modifications) in the SLC52A3 gene where an increase in H3Ac and a reduction in H3K27me3 levels were observed in the NaB treated Caco-2 cells compared to untreated controls.
Conclusion:
These findings demonstrate that NaB up-regulates intestinal RF uptake and that the effect appears to be mediated, at least in part, at the level of transcription of the SLC52A3 gene and may involve epigenetic mechanism(s).
Keywords: Riboflavin, RFVT3, intestine, sodium butyrate, colonoids
Introduction
Riboflavin (RF), a member of the water-soluble family of vitamins, is essential for normal human health. In its coenzyme forms [flavin mononucleotide (FMN) and flavin dinucleotide (FAD)], RF plays key metabolic roles in biological oxidation-reduction reactions [1]; it also plays a role in protein folding [2] and has both anti-oxidant and anti-inflammatory properties [3, 4]. A role for RF (and for the microbiota-generated metabolites used in its biosynthesis) in normal immune function has also been demonstrated [5–7]. Deficiency of RF occurs in conditions like inflammatory bowel diseases (IBD) [8], chronic alcoholism [9], and diabetes mellitus [10]; it also occurs in patients with infantile Brown-Vialetto-Van Laere Syndrome (BVVLS), a rare neurological disorder linked to genetic mutations in RF transporters, where supplementation with pharmacological doses of RF brings about a significant improvement in the clinical symptoms of the affected subjects [11–14].
Mammals, including humans, obtain RF from exogenous sources through intestinal absorption as they lack of ability to synthesize the vitamin endogenously. The intestine encounters RF from two sources: a dietary source, which is absorbed in the small intestine, and a bacterial (gut microbiota) source, which is absorbed in the large intestine [15–18]. Studies have shown that the small and large intestinal RF uptake processes are similar and both occur via a specific carrier-mediated mechanism [17, 19–21]. While all the three recently cloned RF transporters (RFVT-1, −2 and −3, products of the SLC52A1, SLC52A2, and SLC52A3 genes, respectively) were found to be expressed in the gut, expression of RFVT3 has been shown to be the highest [22–26]. At the cellular level, expression of the RFVT3 was also found to be exclusively restricted at the apical membrane domain of the absorptive cells [26]. An essential role for RFVT3 in intestinal RF uptake has been demonstrated in studies using in vitro knockdown (siRNA) and in vivo intestinal-specific knockout approaches [26, 27]. Finally, several genetic mutations in the RFVT3 transporter have been identified in humans and shown to lead to clinical manifestations [12, 28].
Butyrate is one of the important short chain fatty acids (SCFAs) produced in the large intestinal lumen by bacterial fermentation of dietary carbohydrates, specifically resistant starch and dietary fibers [29, 30]. The normal colonic lumen contains approximately 100–150 mM total SCFAs, which consists of acetate, propionate and butyrate in the ratio of 6:2:2, respectively [30–33]. Of these three SCFAs, butyrate is the preferred fuel for the colonic epithelial cells and is important for the maintenance of their health [34]. Recent studies have shown that the microbiota generated butyrate exerts a significant effect on the intestinal absorptive function [35–39], but its effect on intestinal absorption of RF and on expression of RFVT3 has not been examined. Here we tested the effect of NaB on intestinal RF uptake using in vitro and ex vivo models. The results showed that NaB stimulates intestinal RF uptake and that the effect is in part mediated at the level of transcription of the SLC52A3 gene and may involve epigenetic mechanism(s).
Materials and Methods
Materials:
Human-derived intestinal epithelial Caco-2 were obtained from American Type Culture Collection (ATCC, Manassas, VA). 3H-RF (specific activity 12.5 Ci/mmole; radiochemical purity > 97%) was purchased from Moravek Biochemicals (Brea, CA). All other cell culture and molecular biology reagents were of analytical grade and were obtained from commercial vendors. The primers used to perform RT-qPCR analysis were purchased from Sigma Genosys (Woodlands, TX).
Cell culture and RF uptake:
Caco-2 cells were grown in EMEM medium supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin at 37°C in 5% CO2-95% air environment. Confluent Caco-2 cells grown in 12 wells plates were serum starved in EMEM containing 0.5% FBS for 24 hrs and treated with different concentrations of NaB (1, 2, 5, and 10 mM), or with sodium acetate (1 mM) and sodium propionate (1mM) for 24 hrs and used to determine the initial-rate (3 min) of RF uptake. Briefly, Caco-2 cells were incubated in KR buffer (133 mM NaCl, 4.93 mM KCl, 1.23 mM MgSO4, 0.85 mM CaCl2, 5 mM glucose, 5 mM glutamine, 10 mM HEPES, and 10 mM MES, pH 7.4) at 37°C in the presence of 3H-RF (25 nM). After incubation, cells were subjected for radioactivity measurement in a liquid scintillation counter (Beckman Coulter) as described before [20, 40].
Mouse colonoids preparation:
For ex vivo studies, we used 8–10-weeks old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) to generate colonoids as described before [41–43]. Mice were euthanized, the colonic tissue was removed and prepared colonic crypts as described before [41–43]. The generated colonoids were treated with 1mM NaB for 24 hrs and used for mRFVT3 protein, mRNA and hnRNA expression studies. These animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Veteran Administration Medical Center (VAMC), Long Beach, CA.
RNA extraction and RT-qPCR:
To determine the RFVT3 mRNA and hnRNA expression levels, total RNA from Caco-2 cells and mouse colonoids treated with NaB and untreated controls was extracted as described [26, 40]. Total RNA (2 μg) was reverse transcribed to cDNA using i-Script reverse transcriptase (RT) kit (Bio-Rad, CA). RT-qPCR was performed using gene specific primers for determining the mRNA (hRFVT3 forward 5ꞌ-CCTTTCCGAAGTGCCCATC-3ꞌ and reverse 5ꞌ- AGAAGGTGGTGAGGTAGTAGG-3ꞌ; mRFVT3 forward 5’-GGATCAGTGGAAGCCAGTG-3’ and reverse 5’GACCTGTTAGGCAGGAAGATG-3’; hβ-actin forward 5ꞌ-AGCCAGACCGTCTCCTTGTA-3’ and reverse 5ꞌ-TAGAGAGGGCCCACCACAC-3ꞌ; mβ-actin forward 5’ATCCTCTTCCTCCCTGGA-3’ and reverse 5’-TTCATGGATGCCACAGGA-3’) and hnRNA (hRFVT3 forward 5ꞌ- TCTCAGCACTTGGCTTTATC −3ꞌ and reverse 5ꞌ - CTCCCATGCGTATGTATGTA −3ꞌ; mRFVT3 forward 5’-GCCTAGGAAAACCTTGCCCAGT-3’ and reverse 5’-CAGAGGGACTGGTGGAGGACA-3’; hβ-actin forward 5ꞌ TTCCTGGGTGAGTGGAG-3’ and reverse 5ꞌ- GGACTCCATGCCTGAGAG −3ꞌ; mβ-actin forward 5’-AGATGACCCAGGTCAGTATC-3’ and reverse 5’-GAGCAGAAACTGCAAAGAT-3’) expression levels in a CFX96 real-time icycler (Bio-Rad). After initial denaturation at 95°C for 5 min, the amplification program was repeated 45 times (95°C with a 30-s hold, 55°C with 15-s hold followed by 72°C with 30-s hold for extension and fluorescence measurement). The mRNA and hnRNA expression levels of RFVT3 were normalized relative to β-actin and the expression level was calculated following relative relationship method as described before [44].
hRFVT3 mRNA stability assay:
Caco-2 cells were pretreated with 5 μg/ml actinomycin D (AcD; a potent transcription inhibitor) for 1 hr to stop the transcription and incubated with 1 mM NaB for specified time points (0, 4, 8, 12 and 24 hrs). Total RNA (2 μg) was extracted and hRFVT3 mRNA expression levels were determined by RT-qPCR using hRFVT3 specific primers and relative relationship method as mentioned above.
Western blot analysis:
Caco-2 cells and mouse colonoids treated with NaB and controls were lysed in 1X protease cocktail inhibitor mixture (Roche, Indianapolis, IN) containing RIPA buffer (Sigma) and the total protein was prepared as described [45, 46]. Sixty micrograms of total protein was loaded on 4–12% premade mini gels (Invitrogen) and protein was transferred to polyvinylidene difluoride (PVDF) membranes. After transfer the membranes were blocked in Odyssey blocking buffer (LI-COR) and then probed either with hRFVT3 (1:200 dilution) (custom antibody generated by Alpha diagnostic, San Antonio, TX) or mRFVT3 (1:500 dilution) (Biosis Antibodies, Woburn, MA) and β-actin (Santa Cruz Biotechnology) polyclonal antibodies. The specificity of both the antibodies were established before using variety of cell lysates [27, 45, 46]. The membranes were washed then probed with corresponding secondary antibodies (LICOR Biosciences) in 1:30,000 dilutions for 1 hr at room temperature and the specific bands were quantified using Odyssey application software supplied in an Odyssey Infrared imaging system (LI-COR Biosciences).
Chromatin immunoprecipitation (ChIP) assay and qPCR:
NaB-treated and untreated Caco-2 cells were subjected for ChIP analysis as described before [40, 47]. The cross-linked chromatin was incubated overnight with 1–2 μg of the specific antibody [H3, H3Ac, H3K27me3 and IgG (Millipore, Billerica, MA)] and the immunoprecipitated samples were subjected to DNA purification and analyzed by qPCR using SLC52A3 promoter specific primers (forward 5ꞌGGGTTCGCTCAGTGAAGGTA-3ꞌ and reverse 5ꞌ-AGCAGGAGTGTGTCCGTTGTG-3ꞌ) to amplify the region −199 to +8 relative to transcriptional start site (relative to TSS as +1) using the PCR conditions as described before [40].
Statistical analyses:
Carrier-mediated RF uptake data presented in this study are means ± SE of multiple independent experimental determinations. RF uptake by the carrier-mediated process was determined by subtracting uptake of 3H-RF in the presence of 1 mM unlabeled RF from total uptake (i.e., from uptake in the absence of unlabeled RF). Statistical significance was determined using the student’s t-test/ Mann-Whitney Ranksum test with statistical significance set at P < 0.05. Western blot, RT-qPCR and ChIP-qPCR analyses are expressed as means ± SE of at least from three to six independent experiments.
Results
Effect of NaB on RF uptake by Caco-2 cells:
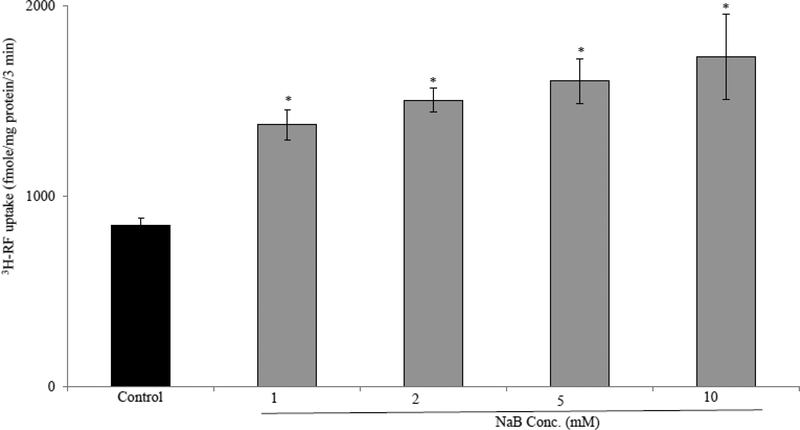
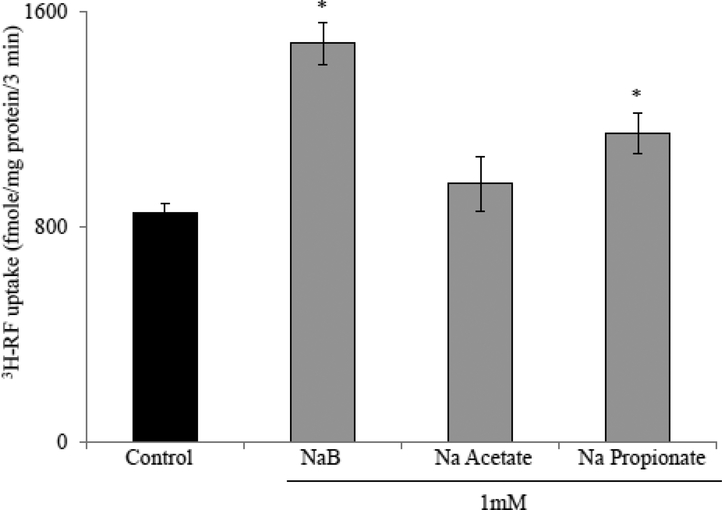
To determine the effect of NaB treatment on intestinal RF uptake, we examined the initial-rate of carrier-mediated 3H-RF (25nM) uptake in confluent monolayers of Caco-2 cells treated with 1mM NaB for 24 hrs. The results showed that such a treatment leads to a significant (P < 0.01) induction in RF uptake. When higher concentrations of NaB were used (2, 5, and 10 mM), a slight-dose dependent increase in RF uptake was observed above what was seen with 1mM NaB (Fig. 1). We used 1 mM NaB as our working concentration in all subsequent studies. In another investigation, we examined and compared the effect of the other SCFAs that exist in significant amount in the colonic lumen (i. e., acetate and propionate) on 3H-RF uptake by Caco2 cells and found that NaB to be the most potent inducer of the vitamin uptake (Fig. 2).
Figure 1. Effect of NaB on RF uptake by Caco-2 cells.
Caco-2 cells were treated with increased concentration (1, 2, 5 and 10 mM) of NaB for 24 hrs and RF uptake was assayed. Data are means ± SE of multiple determinations from three (n=3) independent experiments * P < 0.01.
Figure 2. Effect of different short chain fatty acids (SCFAs) on intestinal RF uptake.
Confluent Caco-2 monolayers were treated with different SCFAs (1mM) for 24 hrs followed by determination of RF uptake. Data are means ± SE of multiple determinations from three (n=3) independent experiments * P < 0.01.
Effect of NaB on the level of expression of RFVT3 protein, mRNA and hnRNA in Caco-2 cells (in vitro) and mouse colonoids (ex vivo):
To investigate the basis of the observed induction in carrier-mediated RF uptake after NaB treatment, expression levels of RFVT3 protein was examined. After 24 hrs of NaB treatment, Caco-2 cells were harvested and total cell homogenate was subjected to western blot analysis to determine the level of hRFVT3 protein expression. The results showed that NaB treatment leads to a significant (P < 0.01) induction in hRFVT3 protein expression compared to untreated cells (Fig. 3A). Similar finding was observed when ex vivo mouse colonoids were exposed to NaB (1mM NaB; 24 hrs) in that a significant (P < 0.05) induction in mRFVT3 protein expression was observed in colonoids treated with NaB compared to untreated controls.
Figure 3. Effect of NaB on level of expression of the RFVT3 protein in Caco-2 cells and in mouse colonoids.
Western blot analysis was performed to determine the expression of hRFVT3 (A) and mRFVT3 (B) protein levels in Caco-2 cells (n=6) and mouse colonoids (n=5), respectively, after NaB treatment as described in “Materials & Methods”. The images are representative of multiple independent experiments with similar results. Data are means ± SE of at least from five independent experiments **P < 0.05, * P < 0.01.
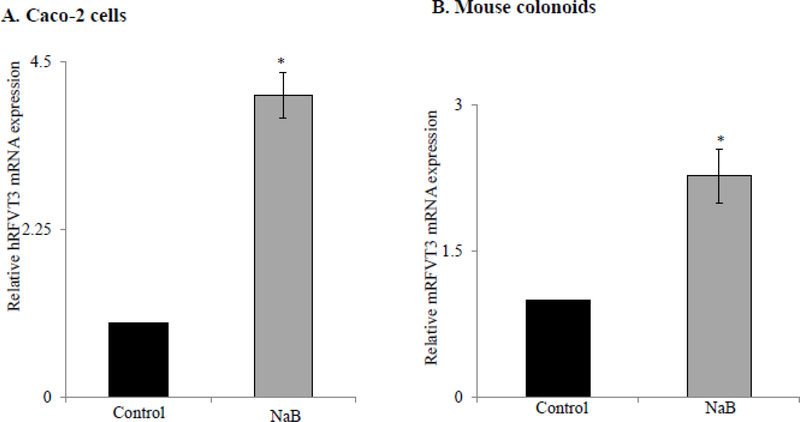
To further investigate the mechanism responsible for the observed NaB-dependent induction of RF uptake, we also examined the effect of NaB on hRFVT3 and mRFVT3 mRNA expression levels in Caco-2 cells and mouse colonoids, respectively by RT-qPCR. The results showed a significant (P < 0.01) induction in the levels of expression of hRFVT3 and mRFVT3 mRNA in both Caco-2 cells and mouse colonoids after NaB treatment compared to their respective untreated controls (Fig. 4A & B).
Figure 4. Effect of NaB on level of expression of RFVT3 mRNA in Caco-2 cells and in mouse colonoids.
RT-qPCR was performed to determine the hRFVT3 (A) and mRFVT3 (B) mRNA expression in NaB treated and untreated Caco-2 cells (n=3) and colonoids (n=4) using gene specific primers as described in “Materials & Methods”. Data are means ± SE of at least from three independent experiments * P < 0.01.
Changes in mRNA level could occur via changes in RNA stability and/or changes in transcription rate of the relevant gene (SLC52A3 in this case). To investigate if changes in RNA stability caused the observed increase in intestinal hRFVT3 mRNA expression levels during NaB treatment, we performed RNA stability assay as described by us and others before [35, 48]. Briefly, Caco-2 cells were pre-incubated with 5μg/ml actinomycin D (AcD), a potent RNA synthesis inhibitor, and then treated with 1mM NaB for different time points (0, 4, 8, 12, and 24 hrs). The results showed that hRFVT3 mRNA expression level in cells treated with AcD alone or with AcD plus NaB to be similar (Fig. 5). This suggests that NaB does not affect hRFVT3 mRNA stability rather the effect is transcriptionally mediated. To investigate the rate of transcription of SLC52A3 gene after NaB treatment, the expression level of RFVT3 (SLC52A3) heterogenous RNA (hnRNA) (the first product of gene transcription and whose level of expression reflects the rate of transcription of a given gene) was determined by RT-qPCR. The results showed a significant (P < 0.01 for Caco-2 cells, and P < 0.05 for mouse colonoids) induction of both the hRFVT3 and mRFVT3 hnRNA expression in NaB-treated groups compared to their respective untreated controls (Fig. 6A & B).
Figure 5. Effect of NaB on hRFVT3 mRNA stability in Caco-2 cells.
Caco-2 cells were pre-incubated with 5μg/ml actinomycin D (AcD) for 1hr, then treated with NaB (1mM) for 24 hrs. hRFVT3 mRNA expression levels were quantified by RT-qPCR. Data are means ± SE of six independent experiments.
Figure 6. Effect of NaB on level of expression of RFVT3 hnRNA in Caco-2 cells and in mouse colonoids.
RT-qPCR was performed to determine the hRFVT3 (A) and mRFVT3 (B) hnRNA expression levels in NaB treated and untreated Caco-2 cells (n=3) and colonoids (n=4) using gene specific primers as described in “Materials & Methods”. Data are means ± SE of at least from three independent experiments * P < 0.01; ** P < 0.05.
Involvement of epigenetic mechanisms in the regulation of SLC52A3 transcriptional activity by NaB:
As described above, NaB stimulated intestinal RF uptake by inducing the expression of RFVT3 and that this effect is mediated, at least in part, via transcriptional mechanism(s) involving the SLC52A3 gene. Since NaB is known to exert epigenetics effects (via acting as histone deacetylase inhibitor and its suppression of histone de-acetylation lead to accumulation of multi-acetylated forms of histone, which in turn alters the compactness of chromatin therefore affecting DNA folding and gene expression [49, 50]), we examined possible involvement of epigenetic mechanisms (e.g., histone modifications) in the effect of NaB on expression of the RFVT3. We also examined possible involvement of DNA methylation (specifically trimethylation of histone 3 lysine 27; H3K27me3) since this histone modification also influence gene expression [40, 51, 52]. For this, we performed a ChIP-qPCR analysis and focused on the SLC52A3 promoter region (−199 to +8) since it is essential for driving basal activity of the promoter and is involved the regulation of the RF uptake process in intestinal epithelial cells under other conditions [40, 53]. The results showed that treating Caco-2 cells with NaB (1 mM; 24 hrs) to be associated with a significant (P < 0.01) increase in the histone acetylation marker (H3Ac) at SLC52A3 promoter region compared to untreated control cells (Fig. 7A). We also observed a significant (P < 0.05) decrease in the level of repressor marker H3K27me3 in SLC52A3 promoter region in cells treated with NaB compared to untreated cells (Fig.7B). Together, these findings suggest that the up-regulation of hRFVT3 expression in the presence of NaB may involve induction in histone hyper-acetylation and selective histone methylation at the SLC52A3 gene promoter region.
Figure 7. ChIP-qPCR analysis of H3Ac and H3K27me3 in immunoprecipitated DNA fragments on SLC52A3 promoter in Caco-2 cells treated with NaB.
ChIP-qPCR data showing the ratio of H3Ac (A) or H3K27me3 (B) relative to total H3 levels in Caco-2 cells treated with NaB and untreated control cells. Values are normalized to total input DNA and expressed as means ± SE of three (n=3) independent experiments from different sample preparations. * P < 0.01; **P < 0.05.
Discussion
As mentioned earlier, humans and other mammals obtain RF from dietary and microbiota sources via absorption in the small and large intestine, respectively. This occurs via a specific carrier-mediated process that involves the RFVT3, a transporter that is exclusively localized at the apical membrane domain of the polarized intestinal epithelial cells [20, 22, 26]. It has also been known for some time that NaB causes induction in intestinal uptake of certain substrates and in the level of expression of the uptake systems involved in these processes [35–39]. Little is known about the effect of NaB on intestinal/colonic uptake of water-soluble vitamins including RF. Thus, our aim in this investigation was to address this issue using the in vitro human-derived intestinal epithelial Caco-2 cells and ex vivo mouse colonoids.
Our results showed that treatment of Caco-2 cells with NaB to lead to a significant induction in RF uptake and this up-regulation was associated with the marked increase of hRFVT3 protein and mRNA expression. Similarly, treating mouse colonoids with NaB was found to lead to an induction in mRFVT3 protein and mRNA expression. As it is known, the increase in the level of expression of a given mRNA could be due to cause by changes in level of its stability, and/or could be an indication for the involvement of transcriptional mechanism(s). To test the first possibility, we performed mRNA stability assay and found no change in hRFVT3 mRNA stability in cells treated with NaB compared to untreated controls. To test the second possibility, we determine the level of expression of the SLC52A3 hnRNA in Caco-2 cells and mouse colonoids treated with 1mM NaB. The results indeed showed a significant increase in the level of expression of the RFVT3 hnRNA in cells treated with NaB compared to untreated controls. This clearly suggest the involvement of transcriptional mechanism in mediating the effect of NaB on intestinal RF uptake and on expression of the uptake system involved.
Since epigenetic mechanisms play an important role in the regulation of gene expression, and that NaB is known to induce epigenetic alterations in the genes that it affects [35, 40, 47, 50–52], we also searched for possible epigenetic alterations in the SLC52A3 promoter after NaB treatment. We focused on histone modifications since it plays important role in regulating transcriptional activities. Our findings showed that a significant increase in the activity of H3Ac in cells treated with NaB compared to untreated controls. In contrast the activity of the heterochromatin (repressor) H3K27me3 was found to be significantly reduced in NaB treated cells. Together, these findings suggest that possible involvement of epigenetic mechanism(s) in the SLC52A3 promoter activity by NaB.
In summary, our investigation shows that the NaB stimulates intestinal RF uptake process and that this occurs via induction in the level of expression of the RFVT3 in the intestine. In addition, these results suggest that this effect occurring at the level of transcription of the SLC52A3 gene and may involve epigenetic mechanism(s).
Acknowledgements
This study was supported by grants from the Department of Veterans Affairs and the NIH [grants DK107474 to VSS, and DK58057, DK56057 and AA018071 to HMS].
Footnotes
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Reference
- 1.Cooperman JM, Lopez R. Riboflavin In: Handbook of Vitamins: Nutritional, Biochemical and Clinical Aspects. New York: Dekker; p. 299–327, 1984. [Google Scholar]
- 2.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science. 2000; 290: 1571–1574. [DOI] [PubMed] [Google Scholar]
- 3.Sanches SC, Ramalho LN, Mendes-Bras M, et al. (vitamin B-2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem Toxicol. 2014; 67:65–71. [DOI] [PubMed] [Google Scholar]
- 4.Liu D, Zempleni J. Low activity of LSD1 elicits a pro-inflammatory gene expression profile in riboflavin-deficient human T Lymphoma Jurkat cells. Genes Nutr. 2014; 9: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazur-Bialy AI, Buchala B, Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity - a study on the RAW 264.7 cell line. Br J Nutr. 2013;110: 509–514. [DOI] [PubMed] [Google Scholar]
- 7.Schramm M, Wiegmann K, Schramm S, et al. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol. 2014; 44: 728–741. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989; 84: 744–748. [PubMed] [Google Scholar]
- 9.Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am J Clin Nutr. 1973; 26: 858–860. [DOI] [PubMed] [Google Scholar]
- 10.Kodentsova VM, Vrzhesinskaia OA, Trofimenko EV, et al. The vitamin status of children with diabetes mellitus. Vopr Med Khim. 1994; 40: 45–48. [PubMed] [Google Scholar]
- 11.Bosch AM, Abeling NG, Ijlst L, et al. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis. 2011; 34:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green P, Wiseman M, Crow YJ, et al. Brown-Vialetto-Van Laere Syndrome, a Ponto-Bulbar Palsy with Deafness, Is Caused by Mutations in C20orf54. Am J Hum Genet. 2010; 86:485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho G, Yonezawa A, Masuda S, et al. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric gciduria type 2, 1s caused by a microdeletion in the riboflavin transporter gene GPR172B. Hum Mut. 2011; 32: E1976–E1984. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JO, Gibbs JR, Megarbane A, et al. Exome sequencing reveals riboflavin transporter mutations as a cause of motor neuron disease. Brain. 2012; 185: 2875–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iinuma S Synthesis of riboflavin by intestinal bacteria. J Vitaminol. (Kyoto) 1955; 1: 6–13. [DOI] [PubMed] [Google Scholar]
- 16.Kasper H Vitamin absorption in the colon. Am J Protocol. 1970; 21: 341–345. [PubMed] [Google Scholar]
- 17.Said HM, Arianas P. Transport of riboflavin in human intestinal brush border membrane vesicles. Gastroenterology. 1991; 100: 82–88. [DOI] [PubMed] [Google Scholar]
- 18.Sorrell MF, Frank O, Thompson AD, Aquino A, Baker H. Absorption of vitamins from the large intestine. Nutr Rep Int. 1971; 3: 143–148. [Google Scholar]
- 19.Middleton HM. Uptake of riboflavin by rat intestinal mucosa in vitro. J Nutr. 1985; 120: 588–593. [DOI] [PubMed] [Google Scholar]
- 20.Said HM, Ma TY. Regulation of riboflavine intestinal uptake by Caco-2 cells. Am J Physiol. 1994; 266: G15–G21. [DOI] [PubMed] [Google Scholar]
- 21.Said HM, Mohammadkhani R. Uptake of riboflavin across the brush border membrane of rat intestine: regulation by dietary vitamin levels. Gastroenterology.1993; 105: 1294–1298. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Inoue K, Ohta KY, et al. Identification and functional characterization of rat riboflavin transporter 2. J Biochem. 2009; 145: 437–443. [DOI] [PubMed] [Google Scholar]
- 23.Yao Y, Yonezawa A, Yoshimatsu H, et al. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr. 2010; 140: 1220–1226. [DOI] [PubMed] [Google Scholar]
- 24.Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol. 2008; 295: C632C641. [DOI] [PubMed] [Google Scholar]
- 25.Yonezawa A, Inui K. Novel riboflavin transporter family RFVT/SLC52: Identification, functional characterization and genetic diseases of RFVT/SLC52. Mol Aspects Med. 2013; 34: 693–701. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian VS, Subramanya SB, Rapp L, et al. Differential expression of human riboflavin transporters −1, −2, and −3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta. 2011; 1808:3016–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanian VS, Lambrecht N, Lytle C, Said HM. Conditional (intestinal-specific) knockout of the riboflavin transporter-3 (RFVT-3) impairs riboflavin absorption. Am J Physiol Gastrointest Liver Physiol. 2016; 310: G285–G293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch AM, Stroek K, Abeling NG, et al. The Brown-Vialetto-Van Laere and Fazio Londe syndrome revised: natural history, genetics, treatment and future perspectives. Orphanet J Rare Dis. 2012; 7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981; 22:763–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 2013; 13:869–874. [DOI] [PubMed] [Google Scholar]
- 31. Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991; 70:443–459. [DOI] [PubMed] [Google Scholar]
- 32. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987; 28:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrong O, Metcalfe-gibson A, Morrison RB, Ng ST, Howard AV. In vivo dialysis of faeces as a method of stool analysis. i. technique and results in normal subjects. Clin Sci. 1965; 28:357375. [PubMed] [Google Scholar]
- 34.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980; 21:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalmasso G, Nguyen HT, Yan Y, et al. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One. 2008; 3:e2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musch MW, Bookstein C, Xie Y, Sellin JH, Chang EB. SCFA increase intestinal Na absorption by induction of NHE3 in rat colon and human intestinal C2/bbe cells. Am J Physiol Gastrointest Liver Physiol. 2001; 280: G687–G693. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, McCoy A, Li J, Zhao Y, Ghishan FK. Sodium butyrate stimulates NHE8 expression via its role on activating NHE8 basal promoter activity. Am J Physiol Gastrointest Liver Physiol. 2015; 309: G500–G505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangian HF, Tappenden KA. Butyrate increases GLUT2 mRNA abundance by initiating transcription in Caco2-BBe cells. JPEN J Parenter Enteral Nutr. 2009; 33:607–617. [DOI] [PubMed] [Google Scholar]
- 39.Zeissig S, Fromm A, Mankertz J, et al. Butyrate induces intestinal sodium absorption via Sp3-mediated transcriptional up-regulation of epithelial sodium channels. Gastroenterology. 2007; 132: 236–248. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian VS, Ghosal A, Kapadia R, Nabokina SM, Said HM. Molecular mechanisms mediating the adaptive regulation of intestinal riboflavin uptake process. PLoS One. 2015; 10:e0131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011; 141:1762–1772. [DOI] [PubMed] [Google Scholar]
- 42.Tsuruta T, Saito S, Osaki Y, et al. Organoids as an ex vivo model for studying the serotonin system in the murine small intestine and colon epithelium. Biochem Biophys Res Commun. 2016; 474:161–167. [DOI] [PubMed] [Google Scholar]
- 43.Anandam KY, Srinivasan P, Subramanian VS, Said HM. Molecular mechanisms involved in the adaptive regulation of the colonic thiamin pyrophosphate uptake process. Am J Physiol Cell Physiol. 2017; 313:C655–C663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Livek KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta DeltaC (T)) method. Methods. 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 45.Lakhan R, Subramanian VS, Said HM. Role of MicroRNA-423–5p in posttranscriptional regulation of the intestinal riboflavin transporter-3. Am J Physiol Gastrointest Liver Physiol. 2017; 313: G589–G598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian VS, Sabui S, Teafatiller T, Bohl JA, Said HM. Structure/functional aspects of the human riboflavin transporter-3 (SLC52A3): role of the predicted glycosylation and substrateinteracting sites. Am J Physiol Cell Physiol. 2017; 313: C228–C238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian VS, Srinivasan P, Said HM. Uptake of ascorbic acid by pancreatic acinar cells is negatively impacted by chronic alcohol exposure. Am J Physiol Cell Physiol. 2016; 311:C129C135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G275–G281. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Reinberg D. Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev. 2011; 21:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidali G, Boffa LC, Bradbury EM, Allfrey VG. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci USA. 1978; 75: 2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogliotti YS, Ross PJ. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian development. Epigenetics. 2012; 7: 976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabui S, Subramanian VS, Kapadia R, Said HM. Adaptive regulation of pancreatic acinar mitochondrial thiamin pyrophosphate uptake process: possible involvement of epigenetic mechanism(s). Am J Physiol Gastrointest Liver Physiol. 2017; 313: G448–G455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosal A, Sabui S, Said HM. Identification and characterization of the minimal 5’-regulatory region of the human riboflavin transporter-3 (SLC52A3) in intestinal epithelial cells. Am J Physiol Cell Physiol. 2015; 308: C189–C196. [DOI] [PMC free article] [PubMed] [Google Scholar]